Abstract
Objectives
To investigate expressions of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in squamous cell carcinoma of the tonsil and to correlate expression profiles with clinicopathological characteristics.
Methods
Paraffin blocks were obtained from 45 tonsil squamous cell carcinoma (SCC) patients, who underwent surgery as an initial treatment between 1994 and 2004, and from 20 normal controls. Expressions of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 were investigated immunohistochemically.
Results
The expressions of MMPs (except MMP-2) and TIMPs were found to be significantly different in tonsil SCC and normal control tissues. Furthermore, MMP-13 expression was found to be correlated with tumor invasion (P=0.05), and the expressions of MMP-9 and TIMP-1 with nodal metastasis (P=0.048, 0.031). No relation was found between MMP or TIMP expression and recurrence. However, MMP-9 expression was found to be significantly associated with 5-year survival in tonsil SCC patients by multivariate analysis (hazard ratio, 3.853; P=0.013).
Conclusion
Significant overexpressions of multiple MMPs and TIMPs were found in tonsil SCC tissues. Furthermore, our findings suggest that MMP-9 expression might be a useful prognostic factor.
Keywords: MMP, TIMP, Tonsil, Squamous cell carcinoma, Prognosis
INTRODUCTION
The tonsil is the most common oropharyngeal site of head and neck squamous cell carcinoma (SCC). Tonsil SCC mainly affects 40 to 60 year olds, and appears to be strongly associated with alcohol, cigarette smoking, and human papillomavirus. The clinical course of tonsil SCC is known to be aggressive, with frequent local or regional recurrence, and early dissemination. In fact, 60 to 80% of tonsil SCC patients have cervical lymph node metastasis at initial diagnosis. Surgery or radiation therapy alone is used to treat early tumors, whereas combined therapy is used for advanced tumors. However, it is difficult to determine the characteristics of tumors by TNM staging alone, and therefore, treatment modality selection and predicting prognosis are problematic. Accordingly, if a reliable method was devised to predict prognosis, cases with a poor prognosis could be aggressively treated, which would influence treatment success rates in this patient population (1).
The matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases, proteolytic enzymes that can decompose extracellular matrix components, like collagen, gelatin, elastin, fibronectin, and the proteoglycans. They also breakdown basement membranes around transformed keratinocytes and vessel or lymphatic duct epithelium, and thus, help tumor local invasion and metastasis. Several previous studies have concluded that the MMPs play an important role during the course of head and neck SCC. However, the nature of their roles in each head and neck primary site remains unresolved due to methodological differences between studies in terms of MMP detection and the relatively small sample sizes used. Tonsil SCC is characterized by a rapid rate of local growth and a high propensity of early nodal metastasis, and therefore, it is expected that the roles played by MMPs may be important for tonsil SCC progression. However, no study has been performed on the role of MMPs in tonsil SCC (2, 3).
In this study, we studied the expressions of MMP-2, 9, 13 and of their inhibitors tissue inhibitors of metalloproteinase (TIMP)-1 and 2 in patients with tonsil SCC that underwent surgery as an initial treatment, and we searched for correlations between these expressions and clinical features, which including stage and treatment outcome. We also investigated whether these MMPs and TIMPs predict the course and prognosis of SCC of the tonsils.
MATERIALS AND METHODS
Selection of patients and tissue samples
Forty-five patients diagnosed as tonsil SCC and that underwent surgery as primary treatment between September 1994 and March 2004, with available paraffin blocks were recruited. The patients consisted of 41 males and 4 females, with a mean age of 56.6 years (range, 32 to 78 years) and smoking history was found in 38 patients (84.4%). Of these 45 patients, 9 received only surgical treatment, and 36 underwent surgery and postoperative radiotherapy. Follow-up periods ranged from 10 to 152 months (average 74.1 months), and 44 of the 45 patients were followed. Disease stages were evaluated using the AJCC TNM system (2002). In the 45 cases stages were distributed as follows: 9 patients were of T1, 27 of T2, 7 of T3, 2 of T4, 12 of N0, 6 of N1, 25 of N2, and 2 were of N3. The control group was composed of 20 healthy age and gender matched individuals who underwent tonsillectomy due to chronic follicular tonsillitis.
Immunohistochemical staining
Paraffin-embedded tissue blocks were sectioned at 4-5 µm, mounted on superfrost glass slides, deparaffinized with xylene, and rehydrated in a graded ethanol series. Endogenous peroxidase activity was inactivated by incubating slides for 10 minutes in 0.3% hydrogen peroxide in methanol. After rinsing sections in Tris buffered saline, non-specific binding was blocked by incubation with goat serum for 30 minutes. Tissue sections were then incubated with the primary antibodies for: MMP-2 Ab-4 (Monoclonal, Clone A-Gel VC2), MMP-9 Ab-9 (Polyclonal), MMP-13 Ab-1 (Monoclonal, Clone VIIIA2), TIMP-1 Ab-5 (Polyclonal), TIMP-2 Ab-5 (Monoclonal, Clone 3a4) (all were purchased from Neomarkers, Fremont, CA, USA) for 2 hours at room temperature. After incubation with biotinylated secondary antibodies (Zymed Co., South San Francisco, CA, USA), sections were incubated with avidin-biotinylated peroxidase complex for 20 minutes at room temperature. Antigen visualization was achieved using 3-amino-9-ethyl carbazole (AEC), and counter staining was performed using Mayer's hematoxylin.
Interpretation
Optical microscopic examinations (×400) were performed at 3 sites per section with high tumor cell densities and showing local invasiveness as determined by two pathologists. It was regarded as positive reaction when more than 10% of tumor cells have brownish granules in the cytoplasm or when stroma is stained brownish.
Statistical analysis
Data were analyzed using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). The chi-square test and Spearman's correlational analysis were used to investigate the association between immunohistochemical staining findings and clinical data. Survival was estimated using the Kaplan-Meier method, and the differences between curves were assessed with the log-rank test. Cox's regression test was used to identify factors that influence survival. Statistical significance was defined as P<0.05.
RESULTS
Expression patterns of MMPs and TIMPs
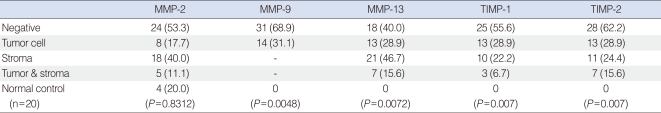
The expression patterns of MMPs and TIMPs as determined by immunohistochemical staining are summarized in Table 1. MMP-2, MMP-13, TIMP-1, and TIMP-2 were detected in tumor cells and/or stroma, whereas MMP-9 was detected in tumor cells alone (Fig. 1). The expression rates of MMP-9, MMP-13, TIMP-1, and TIMP-2 were significantly higher in tumor cells than in control cells, but MMP-2 expression rate was not. Furthermore, a significant association was observed between TIMP-1 expression in stroma and MMP-13 expression in tumor cells (P=0.013).
Table 1.
Expression rates of MMPs and TIMPs by immunohistochemical staining in squamous cell carcinoma of the tonsil and normal control tissues (n=45)
Values are presented as number (%). Chi-square test.
MMP: matrix metalloproteinase; TIMP: tissue inhibitors of metalloproteinases.
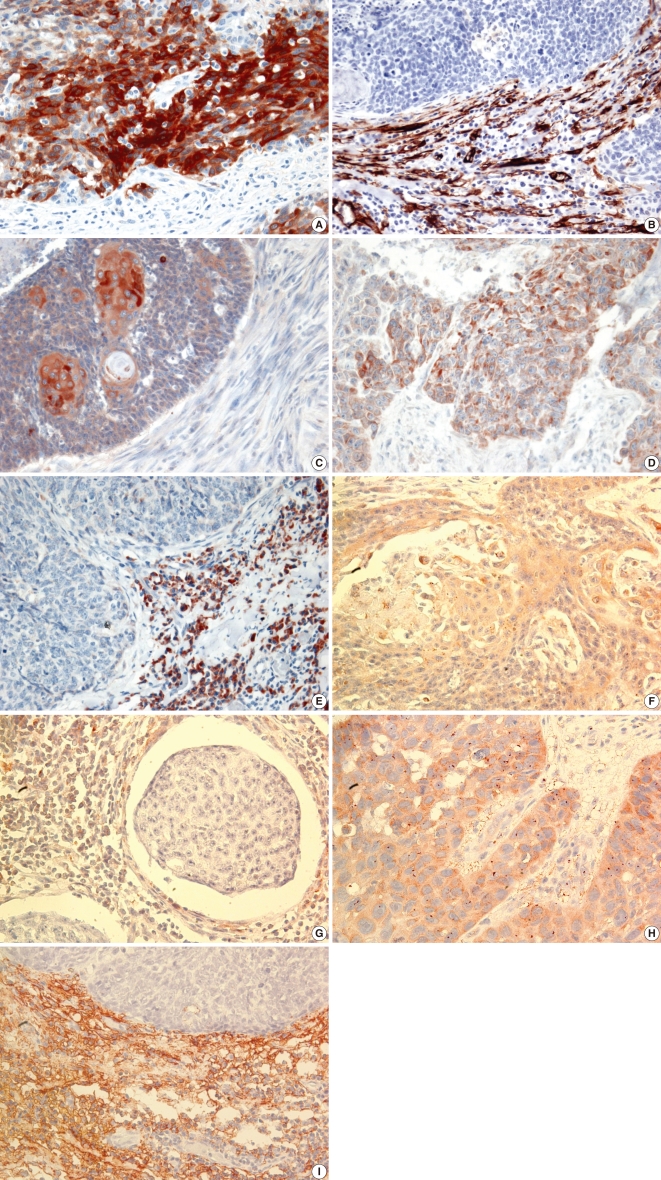
Fig. 1.
Immunohistochemistry in squamous cell carcinoma of the tonsil. Matrix metalloproteinase (MMP)-2 and 13 and tissue inhibitors of metalloproteinase (TIMP)-1 and 2 immunostaining were observed in tumor cells and/or stroma but MMP-9 immunostaining was observed in tumor cells only (×400). (A) MMP-2 immunostaining in tumor cells. (B) MMP-2 immunostaining in stroma. (C) MMP-9 immunostaining in tumor cells. (D) MMP-13 immunostaining in tumor cells. (E) MMP-13 immunostaining in stroma. (F) TIMP-1 immunostaining in tumor cells. (G) TIMP-1 immunostaining in stroma. (H) TIMP-2 immunostaining in tumor cells. (I) TIMP-2 immunostaining in stroma.
Pathologic findings and the expression of MMPs and TIMPs
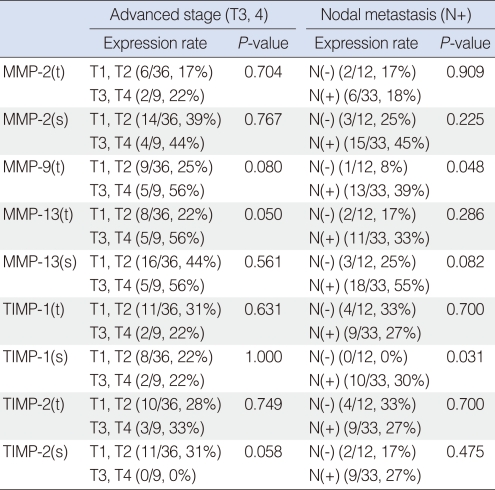
Patients were divided by T-stage into early (T1, T2) and advanced (T3, T4) stages, and by N-stage into N0 and N+ stages. Relationships between MMP and TIMP expressions in these groups were analyzed. It was found that MMP-13 expression in tumor cells was significantly correlated with advanced T stage, and MMP-9 expression in tumor cells and TIMP-1 expression in stroma were found to be significantly related to the presence of cervical nodal metastasis (Table 2).
Table 2.
Comparative analysis findings for correlations between expression profiles of MMPs and TIMPs and clinical parameters
Spearman's correlation analysis.
MMP: matrix metalloproteinase; TIMP: tissue inhibitors of metalloproteinase; t: tumor; s: stroma.
However, the expressional patterns of MMPs and TIMPs showed no significant correlation with tumor grade, perineural invasion, lymphovascular tumor emboli, or extracapsular extension of metastatic lymph nodes.
Recurrence and the expressions of MMPs and TIMPs
Clinical follow-up was performed on all 44 patients; one patient was lost to follow-up. Three cases (7%) developed local recurrence, 1 (2%) developed cervical nodal recurrence, and in 7 (16%) cases developed distant metastasis. 33 of the 44 cases (75%) did not recur over >5 years of follow-up. MMP and TIMP expressions showed no significant correlation with primary or regional recurrence or distant metastasis.
Survival and the expressions of MMPs and TIMPs
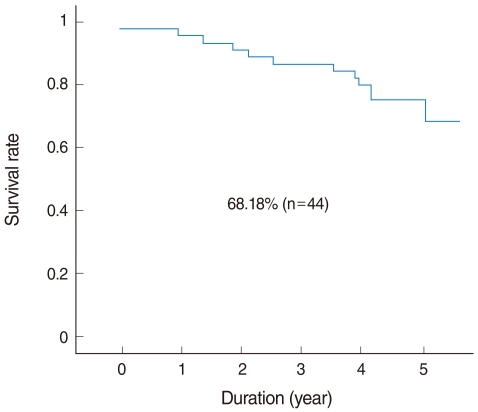
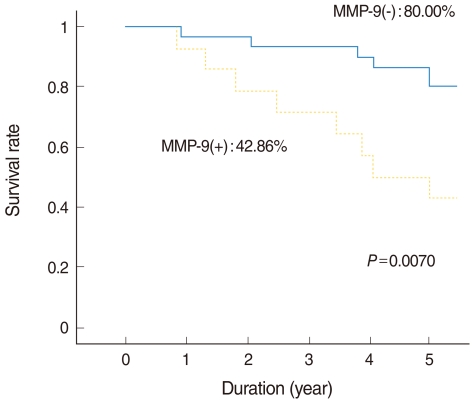
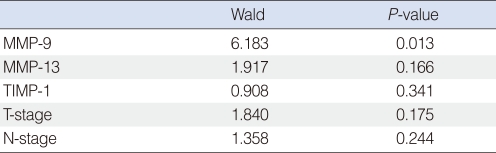
Of the 44 patients, 31 (70%) remained alive with no evidence of disease at the end of the study. Eight patients (18%) died due to treatment failure and 5 (11%) died due to other disease without recurrence. The 5-year overall survival rate as determined by Kaplan-Meier analysis was 68.18% (Fig. 2). Those expressing MMP-9 in tumor cells had significantly poorer survival (Fig. 3), but other MMPs or TIMPs positivity in tumor cells was not found to be significantly associated with survival. Multivariate analysis performed using Cox's regression test showed that MMP-9 expression was significantly associated with survival of tonsil SCC patients (hazards ratio, 3.853; confidence interval, 1.331 to 11.156; P=0.013) (Table 3).
Fig. 2.
Overall 5-year survival of tonsillar squamous cell carcinoma patients (n=44).
Fig. 3.
Kaplan-Meier curves for tonsillar squamous cell carcinoma with respect to matrix metalloproteinase (MMP)-9 expression in tissues.
Table 3.
Multivariate analysis for factors influencing survival using Cox's regression test
MMP: matrix metalloproteinase; TIMP: tissue inhibitors of metalloproteinase.
DISCUSSION
Metastases from head and neck squamous cell carcinoma develop in a step-wise manner, that is, transformed keratinocytes adhere to and breakdown basement membrane (BM), and then tumor cells infiltrate peripheral tissues. When primary tumor grows to a certain size, their survival is threatened because nutrient supply by diffusion process alone is inadequate. Thus, tumors may utilize cytokines and other factors to induce angiogenesis to secure a nutrient supply. Metastasis is another mechanism that allows tumor cells to proliferate, after breaching the BM, tumor cells enter into vessels and lymphatic ducts and adhere to the endothelial surfaces of other organs. Accordingly, proteolytic enzymes probably play a key role in the process that leads to metastasis, and the matrix metalloproteinases (MMPs) are archetypical proteolytic enzymes (4-6).
More than 30 types of MMPs have been identified and classified as: collagenases, gelatinases, stromelysines, stromelysine-like MMPs, membranous MMPs, and TIMPs. Furthermore, several studies have reported that MMP-2, 3, 7, 9, 11, and 13, MT1-MMP, and TIMP-1 contributes functionally to squamous cell carcinoma of the head and neck (2).
MMP-2 is a gelatinase, and has been associated with local invasiveness, cervical nodal metastasis, and the prognosis of oral HNSCC. Patel et al. (7) reported that tumor cells obtained from 39 oral cancer patients showed higher MMP-2 activity than normal peripheral cells by zymography, and that MMP-2 activity was higher in patients with cervical nodal metastasis than in those without. They also reported that MMP-2 activity could be used as a predictor future cervical nodal metastasis. Xie et al. (8) reported that the tumors of 32 supraglottic cancer patients showed more MMP-2 expression than normal tissues by RT-PCR and immunohistochemistry (IHC), and that MMP-2 was associated with the development of cervical nodal metastasis. In addition to those of MMP-7 and 9, MMP-2 expression was also found to be an indicator of future occult metastasis. Gorogh et al. (9) after performing RT-PCR, IHC, and zymography on tumor cells from 48 laryngeal cancer patients and on 10 types of head and neck squamous cell carcinomas (HNSCC) cell lines, concluded that MMP-2 expression is associated with the development of cervical nodal metastasis. However, Charous et al. (3) found no relationship between MMP-2, 9 expression (as determined by in situ hybridization) and stage or prognosis among 27 HNSCC patients. Furthermore, Burian et al. (10) found no association between MMP-2 expression (by IHC) and tumor stage or survival among 41 patients with laryngeal cancer. In the present study, no significant difference was found between tumor and normal cells in terms of MMP-2 expression, and no relationship between MMP-2 expression and local invasiveness, cervical nodal metastasis, recurrence, or survival. Thus, it would be expected that the role of MMP-2 in tonsil SCC is minimal. However, more zymographic studies are required to prove this expectation. Furthermore, it has not been determined whether MMP-2 expression originates from tumor cells or peripheral tissues (11, 12). In the present study, cases were observed that expressed MMP-2 in tumor cells alone or in stromal cells.
MMP-9 is a gelatinase like MMP-2, but unlike MMP-2, it is known that MMP-9 is produced by tumor cells and that its expression is related to tumor progression and prognosis in several HNSCCs. Katayama et al. (13) found that MMP-9 expression in tumor cells (as determined by IHC) was significantly associated with cervical nodal metastasis and distant metastasis among 53 patients with early oral cancer, and they also found a significant relationship between MMP-9 expression and survival by univariate analysis. O-Charoenrat et al. (14) described that tumor tissues from 54 HNSCC cases showed more MMP-9 expression than normal tissues by RT-PCR, Western blot, and zymography and that MMP-9 expression was found to be related to T-stage, and the presence of cervical nodal metastasis and tumor invasion. Dunne et al. (15) reported that MMP-9 expression in tumor cells (determined by IHC) is statistically associated with T stage and cervical nodal metastasis in 105 patients with oropharyngeal SCC. However, Guttman et al. (16) reported that in 23 patients with tongue SCC, MMP-9 expression in tumor cells (determined by IHC) was not related to local invasion, cervical nodal metastasis, or survival. In the present study, MMP-9 expression was detected in tumor cells alone, which suggests that MMP-9 is produced by tumor cells. However, we found no significant association between MMP-9 expression and T-stage. Nevertheless, MMP-9 tended to be expressed in advanced T-stage tumors, and cases with cervical nodal metastasis showed significantly more MMP-9 expression than cases without cervical nodal metastasis. Furthermore, multivariate analysis showed that MMP-9 expression was associated with a poor prognosis, which suggests that MMP-9 plays an important role during the tumor progression and that its expression likely to be associated with survival in tonsil SCC patients who received surgery as initial treatment. Multivariate analysis did not show the relationship between tumor stage and survival. We thought that the reason for this might be due to a relatively small sample size.
The study of MMP-13 (a collagenase) began only comparatively recently, but nevertheless, considerable work has been conducted on the involvement of MMP-13 in HNSCC. Cazorla et al. (17) reported detecting MMP-13 in only tumor tissues by Northern and Western blotting in 35 cases with SCC of the larynx. In addition, they found that MMP-13 expression was associated with tumor invasion and differentiation, and that was related to the overexpressions of MMP-2 and MT1-MMP. Johasson et al. (18) in an in situ hybridization study, found that MMP-13 expression was mainly detected in tumor cells, but that MMP-13 was also detected in stroma. Furthermore, they reported an association between MMP-13 expression and tumor invasion. According to our findings, MMP-13, like MMP-2, was detected in stroma and in tumor cells. Although no association was found between MMP-13 expression and cervical nodal metastasis or between it and survival, MMP-13 expression was detected significantly more in those with an advanced T-stage. These results suggest that MMP-13 may participate in tumor invasion.
The role of TIMPs in HNSCC is uncertain, though it is assumed that TIMPs inhibit the progression of head and neck cancer, because they suppress MMP (2). Ikebe et al. (19) found that TIMP-1 levels were high in oral cancer patients without metastasis in a 57 case study. Gorogh et al. (9) reported that TIMP-1 and 2 levels were inversely related to the occurrence of cervical nodal metastasis. However, O-Charoenrat et al. (14) found that TIMP-1 expression was significantly associated advanced stage of primary tumor lesions. In the present study, expressions of TIMP-1 and TIMP-2 were found in tumor tissue alone, and mixed patterns of expression were observed in tumor cell and stroma in tumor tissue. Furthermore, TIMP-1 expression in stroma was found to be significantly associated with MMP-13 expression in tumor cells, and TIMP-1 expression was significantly higher expression in those with cervical nodal metastasis. However, no relationship was found between TIMP expression and recurrence or prognosis.
Studies that have addressed the expressions of MMPs in HNSCC have produced inconsistent results, presumably because of the various primary sites examined and the many methods used. Furthermore, these discrepancies are probably responsible for the different opinions expressed regarding the role of MMPs. The present study is the first to examine the expressions of MMPs in SCC of the tonsil received surgery as initial treatment. We chose to use IHC because it has been used most frequently in similar studies. In the present study, MMP-9, MMP-13, and TIMP-1 were found to be associated with tumor invasion and cervical nodal metastasis. Furthermore, multivariate analysis showed that MMP-9 is an independent factor that influences survival rate. These results suggest that MMP-9 could be used as a prognostic indicator of tonsil cancer patients who received surgery as initial treatment.
ACKNOWLEDGEMENT
This Research was supported by the Chung-Ang University Research Grant in 2011.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Poulsen M, Porceddu SV, Kingsley PA, Tripcony L, Coman W. Locally advanced tonsillar squamous cell carcinoma: treatment approach revisited. Laryngoscope. 2007 Jan;117(1):45–50. doi: 10.1097/01.mlg.0000243044.91193.32. [DOI] [PubMed] [Google Scholar]
- 2.Werner JA, Rathcke IO, Mandic R. The role of matrix metalloproteinases in squamous cell carcinomas of the head and neck. Clin Exp Metastasis. 2002;19(4):275–282. doi: 10.1023/a:1015531319087. [DOI] [PubMed] [Google Scholar]
- 3.Charous SJ, Stricklin GP, Nanney LB, Netterville JL, Burkey BB. Expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases in head and neck squamous cell carcinoma. Ann Otol Rhinol Laryngol. 1997 Apr;106(4):271–278. doi: 10.1177/000348949710600402. [DOI] [PubMed] [Google Scholar]
- 4.Liotta LA, Rao CN, Wewer UM. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- 5.MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995 Dec;14(4):351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- 6.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997 Oct 15;80(8 Suppl):1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Patel BP, Shah PM, Rawal UM, Desai AA, Shah SV, Rawal RM, et al. Activation of MMP-2 and MMP-9 in patients with oral squamous cell carcinoma. J Surg Oncol. 2005 May 01;90(2):81–88. doi: 10.1002/jso.20240. [DOI] [PubMed] [Google Scholar]
- 8.Xie M, Sun Y, Li Y. Expression of matrix metalloproteinases in supraglottic carcinoma and its clinical implication for estimating lymph node metastases. Laryngoscope. 2004 Dec;114(12):2243–2248. doi: 10.1097/01.mlg.0000149467.18822.59. [DOI] [PubMed] [Google Scholar]
- 9.Gorogh T, Beier UH, Baumken J, Meyer JE, Hoffmann M, Gottschlich S, et al. Metalloproteinases and their inhibitors: influence on tumor invasiveness and metastasis formation in head and neck squamous cell carcinomas. Head Neck. 2006 Jan;28(1):31–39. doi: 10.1002/hed.20298. [DOI] [PubMed] [Google Scholar]
- 10.Burian M, Quint C, Neuchrist C. Angiogenic factors in laryngeal carcinomas: do they have prognostic relevance? Acta Otolaryngol. 1999 Mar;119(2):289–292. doi: 10.1080/00016489950181846. [DOI] [PubMed] [Google Scholar]
- 11.Miyajima Y, Nakano R, Morimatsu M. Analysis of expression of matrix metalloproteinases-2 and -9 in hypopharyngeal squamous cell carcinoma by in situ hybridization. Ann Otol Rhinol Laryngol. 1995 Sep;104(9 Pt 1):678–684. doi: 10.1177/000348949510400902. [DOI] [PubMed] [Google Scholar]
- 12.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, et al. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katayama A, Bandoh N, Kishibe K, Takahara M, Ogino T, Nonaka S, et al. Expressions of matrix metalloproteinases in early-stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Cancer Res. 2004 Jan 15;10(2):634–640. doi: 10.1158/1078-0432.ccr-0864-02. [DOI] [PubMed] [Google Scholar]
- 14.O-Charoenrat P, Rhys-Evans PH, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2001 Jul;127(7):813–820. [PubMed] [Google Scholar]
- 15.Dunne AA, Grobe A, Sesterhenn AM, Barth P, Dalchow C, Werner JA. Influence of matrix metalloproteinase 9 (MMP-9) on the metastatic behavior of oropharyngeal cancer. Anticancer Res. 2005 Nov-Dec;25(6B):4129–4134. [PubMed] [Google Scholar]
- 16.Guttman D, Stern Y, Shpitzer T, Ulanovski D, Druzd T, Feinmesser R. Expression of MMP-9, TIMP-1, CD-34 and factor-8 as prognostic markers for squamous cell carcinoma of the tongue. Oral Oncol. 2004 Sep;40(8):798–803. doi: 10.1016/j.oraloncology.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Cazorla M, Hernandez L, Nadal A, Balbin M, Lopez JM, Vizoso F, et al. Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J Pathol. 1998 Oct;186(2):144–150. doi: 10.1002/(SICI)1096-9896(1998100)186:2<144::AID-PATH147>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Johansson N, Airola K, Grenman R, Kariniemi AL, Saarialho-Kere U, Kahari VM. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol. 1997 Aug;151(2):499–508. [PMC free article] [PubMed] [Google Scholar]
- 19.Ikebe T, Shinohara M, Takeuchi H, Beppu M, Kurahara S, Nakamura S, et al. Gelatinolytic activity of matrix metalloproteinase in tumor tissues correlates with the invasiveness of oral cancer. Clin Exp Metastasis. 1999 Jun;17(4):315–323. doi: 10.1023/a:1006642428826. [DOI] [PubMed] [Google Scholar]