Abstract
Impairment of olfaction is a characteristic and early feature of Parkinson's disease. Recent data indicate that >95% of patients with Parkinson's disease present with significant olfactory loss. Deficits in the sense of smell may precede clinical motor symptoms by years and can be used to assess the risk for developing Parkinson's disease in otherwise asymptomatic individuals. This paper summarizes the available information about olfactory function in Parkinson's disease, indicating the advantageous use of olfactory probes in early and differential diagnosis.
1. Prevalence and Character of Olfactory Loss in PD
According to a recent study by Politis et al. [1], olfactory loss belongs to the top-five most prevalent motor and nonmotor symptoms in early stage PD patients that have affected their quality of life. Only pain is referred to as a more prevalent troublesome nonmotor problem in this patient group.
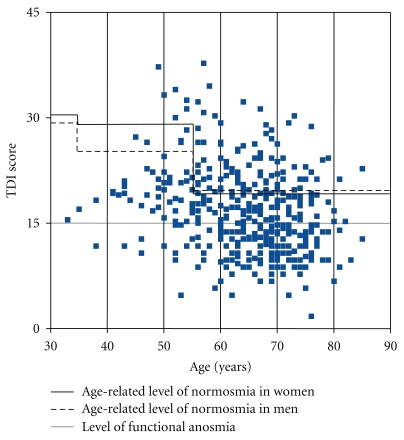
In line with this result, virtually all studies performed since the 1970s have shown olfactory disturbances in PD patients. Published data on the prevalence of olfactory dysfunction in PD range from 45% and 49% in the pioneering studies of Ansari and Johnson [2], and Ward et al. [3], respectively, up to 74% in the work of Hawkes et al. [4], or as high as 90% in a study published by Doty et al. [5]. In our recent multicentre study [6] using a comprehensive testing method (see chapter 2) in a large sample of PD patients (n = 400) from 3 independent populations, the prevalence of olfactory dysfunction in people with PD was greater than previously reported with regard to norms obtained in healthy young subjects. More than 96% of PD patients were found to present with olfactory dysfunction. When using age-dependent normative criteria, 74.5% of this study population was diagnosed with olfactory loss (Figure 1). Furthermore, more than 80% of PD patients with smell loss were functionally anosmic or severely hyposmic regardless of the olfactory test being used for diagnosis. Only very few patients present with accompanying parosmia, or phantosmia.
Figure 1.
Olfactory function of the total number of 400 PD patients. Results are shown as a composite TDI score (sum of odor threshold, odor discrimination, and odor identification score) adjusted to age-related norms [6].
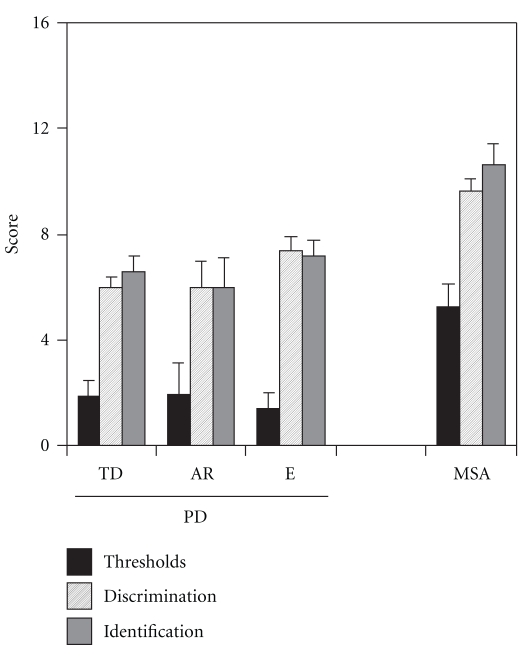
Our data also confirmed numerous previous studies with regard to the lack of olfactory improvement after therapy with dopaminergic agents [5, 7] and the missing correlation between olfactory loss and both duration of disease [4, 5, 8] and the clinical severity of PD as measured by means of the Hoehn and Yahr scale and the UPDRS (compare [9])—although some studies found a correlation between the severity of PD and certain measures of olfactory function, namely, latencies of olfactory event-related potentials [10] or results from an odor discrimination task [11]. With regard to olfactory function, we did not find major differences between subtypes of PD, namely, tremor-dominant PD, akinetic-rigid PD, and mixed-type PD. While this confirms previous observations in a small sample size of 37 patients [7] (Figure 2), the present findings are in contrast to reports by Stern and colleagues [8] who reported significantly better odor identification scores in patients with tremor-predominant PD than in cases with postural instability-gait disorder-predominant PD. While differences between studies may be due to the type of olfactory test used, sample size, normative data, and age distribution (which varied between these investigations), available data allow the conclusion that olfactory dysfunction is a highly reliable symptom of the disease. This concurs with the results of a case-control study on 90 PD patients and healthy controls by Bohnen et al. [12] who found that the accuracy of smell testing in PD diagnosis outweighs the accuracy of motor test batteries, and also other nonmotor tests of, for example, depression and anxiety.
Figure 2.
Olfactory function in PD subtypes (TD: tremor-dominant, AR: akinetic-rigid, e: equivalent type) and multiple system atrophy (MSA) [7].
2. Testing Methods
As the olfactory loss in PD has a general character, all three olfactory qualities (threshold, discrimination, identification) are involved. Therefore, different subtests of olfactory function may reflect smell loss in PD patients and may be used as single measurement. Only a comprehensive approach, however, allows a precise evaluation of olfactory function, that is, that it would be best to perform all 3 subtests to obtain a maximum of reliable information.
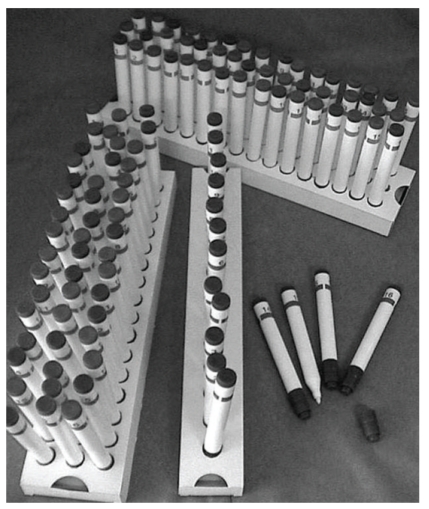
Psychophysical assessment of olfactory function is based on the presentation of odors and the recording of the subjects' response. Advantage of this “low-tech” approach include the speed of testing, allowing for rapid screening of olfactory function [13, 14]. While screening tests only differentiate between normal and pathologic states, more extensive tests allow for a reliable discrimination between anosmic, hyposmic, and normosmic subjects, respectively. Good tests have to be based (1) on normative data acquired and validated in (2) large samples (e.g., [15, 16]). Many tests are based on a forced choice verbal identification of odors while others also include results from odor discrimination and odor threshold measurements (comprehensive approach; Figure 3).
Figure 3.
“Sniffin' Sticks” test kit which is comprised of 3 individual tests of olfactory function (phenyl ethyl alcohol odor threshold, odor discrimination, and odor identification). The scores of the individual tests are summated to the so-called “TDI score” which is a reliable means to estimate the degree of olfactory function.
Most tests are based on the identification of odors. In odor identification tasks, an odorant is presented at a suprathreshold concentration and subjects are required to identify the odor from a list of descriptors. This forced-choice procedure controls the subjects' response bias. A major problem of odor identification is, however, that it strongly relies on the verbal abilities of the subject. Consequently, on average, this enables female subjects to outperform men [17]. In addition, odor identification tests have a strong cultural precondition as not all odors are known equally well in various cultural groups.
The concept embedded in threshold tests is that a subject is repeatedly exposed to ascending and descending concentrations of the same odorant and is required to identify the least detectable concentration for this individual odor ([18]; see also [19]).
Other measures assessing olfactory loss may include investigation of the patient's quality of life, for example, the “Questionnaire for Olfactory Dysfunction” [20], or the recording of olfactory event-related potentials (for review, see [21]).
In the near future, immunohistochemical, volumetric, and functional neuroimaging studies of the olfactory sytem might become relevant for PD diagnosis. There is still little information about PD-specific changes of the olfactory epithelium and their diagnostic use. In a recent study, we compared bioptic material from PD patients for histological/histochemical changes with that from patients with olfactory dysfunction due to other reasons [22]. However, we found no specific changes in the nasal mucosa of PD patients. Further, it could be assumed that loss of olfactory bulb volume would be a reliable finding in PD which, to some degree, might be helpful in differential and early diagnosis of PD. Results from a recent study [23] indicated that there is little if any difference in OB volume between PD patients with anosmia/hyposmia and healthy, normosmic controls. In another effort to identify brain structures responsible for smell loss in PD, functional magnetic resonance imaging (fMRI) was used to investigate brain activity related to olfactory processing in PD. Overall, the results of these studies [24, 25] indicate that neuronal activity in the amygdala and hippocampus is reduced in PD patients which may specifically impact on olfactory sensitivity. In addition, neuronal activity in components of corticostriatal loops appears to be upregulated indicating compensatory processes involving the dopaminergic system.
3. Olfactory Dysfunction as a Prodromal Symptom
Support for the existence of a prodromal phase comes from imaging, neuropathology, and various clinical or epidemiological surveys. The best evidence that derives from large prospective studies relates to disorders affecting olfaction, the enteric nervous system, and depression [26–28]. Estimates for the duration of the prodrome range from 2 to 50 years depending on the symptom, duration of followup, accuracy of diagnosis, and individual variation.
PD patients frequently report reduction in their sense of smell that occurs a few years prior to the onset of motor symptoms. However, patients' unawareness of smell deficits may account for the inconsistent results described in retrospective surveys. In a small study [29], upon questioning prior to olfactory testing, 9 out of 37 patients (24%) indicated an awareness of a decrease of olfactory function which actually preceded their diagnosis of PD.
A plethora of evidence from recent studies supports the view that deficits in the sense of smell may precede clinical motor symptoms by years. A study by Ponsen et al. [30] on 361 asymptomatic relatives of PD patients selected 40 relatives with the lowest olfactory performance. Within 2 years of followup, 10% of these first-degree relatives of PD patients with significant olfactory loss developed clinical PD. In a followup study, five years from baseline testing [31], five relatives had developed clinical PD as defined by the United Kingdom Parkinson's Disease Society Brain Bank Diagnostic Criteria for Parkinson's Disease. Initial clinical (motor) symptoms appeared 9 to 52 months (median 15 months) after baseline testing. Poorer performance on each of three olfactory tasks was associated with an increased risk of developing PD within 5 years. In 2007 [32], we published data on a clinical followup of a previous investigation [33], in which 30 patients diagnosed with idiopathic olfactory loss participated. Four years from baseline, 7% (n = 2) of the individuals with idiopathic olfactory loss who were available for followup examination (n = 24) had newly developed clinical PD symptoms. Altogether, 13% (n = 4) of the patients presented with PD-relevant abnormalities of the motor system. The results indicated that unexplained olfactory loss may be associated with an increased risk of developing PD-relevant motor symptoms.
This is in accord with the results of a large longitudinal study by Ross and colleagues [34]. They assessed olfactory function in 2267 elderly men in the Honolulu Heart Program and found an association between smell loss and future development of PD. They came to the conclusion that impaired olfaction can predate PD by at least 4 years and may be a useful screening tool to detect those at high risk for development of PD in later life. However, this relationship appears to weaken beyond the 4-year period.
Along with quantitative smell loss, such as hyposmia, idiopathic phantosmia has also been suggested to herald PD. A number of case reports could show that some patients have experienced phantosmia very early in the course of the disease [35–37]. According to a recent study by Landis et al. [38], however, idiopathic phantosmia as an early sign of PD remains probably a rather exceptional presentation whereas the overwhelming majority of people with idiopathic phantosmia will not develop PD.
Recent data on olfactory loss as a PD symptom that is present at the earliest stages of the disease are compatible with predictions made on the basis of neuropathological investigations. Braak et al. [39] describe involvement of olfactory pathways and lower brainstem before nigrostriatal pathways are affected which might cause early nonmotor symptoms. Huisman et al. [40] found an increase of (inhibitory) dopaminergic neurons in the olfactory bulb in PD patients. They interpreted their finding within the context of a possible compensatory mechanism in response to the loss of dopaminergic neurons in the basal ganglia. This concurs with their observation that dopaminergic neurogenesis in the glomerular layer tripled after nigrostriatal lesioning and, consistent with this finding, the total number of tyrosine hydroxylase- (TH-) positive cells increased [41]. However, results of a followup study [42] indicated a gender-related change, that is, that the number of dopaminergic cells in the olfactory bulbs of both male and female Parkinson's patients equals that of healthy males of the same age group. Authors, therefore, concluded that the hyposmia in Parkinson's disease patients cannot simply be ascribed to dopamine in the olfactory bulb.
Regardless the small number of prospective studies in this field, olfactory loss should be considered a promising contribution to the early diagnosis of PD. For instance, the current Parkinson's associated risk syndrome (PARS) study [43] will advance our understanding of early PD presentation.
4. Olfaction in Differential Diagnosis
Numerous studies suggest that olfactory disturbances in PD may have diagnostic utility for the differentiation of PD from other movement disorders. Wenning et al. [44] presented data suggesting that olfactory function is differentially impaired in distinct Parkinsonian syndromes. They reported a preserved or mildly impaired olfactory function to be more likely for atypical parkinsonism such as multiple system atrophy, progressive supranuclear palsy, or corticobasal degeneration whereas markedly pronounced olfactory loss appeared to suggest PD. Similar to the results of Wenning et al., in a study on 50 Parkinsonian patients [29], we also found evidence for olfactory loss in MSA, but little or no olfactory loss in (the few investigated) patients with PSP and CBD. With regard to the differentiation between MSA and PD (Figure 3) at a cutoff of a TDI score (combined results for odor thresholds, odor discrimination, and odor identification; see also [15]) of 19.5, psychophysical testing had a sensitivity of 78% and a specificity of 100%. When the cutoff TDI score was increased to 24.8, sensitivity in this sample was 100% while specificity fell to 63%. This moderate specificity seems to be the limiting parameter for diagnostic purposes. A recent American Academy of Neurology practice parameter on the diagnosis and prognosis of PD concluded that olfactory testing “should be considered” to differentiate PD from PSP and CBD but not from MSA [45]. Furthermore, Liberini et al. [46] reported a significant olfactory impairment in Lewy body disease (LBD) which does not allow differentiation from PD. In a sample of 116 patients with mild LBD, mild Alzheimer's disease, mild cognitive impairment, and controls, Williams et al. [47] describe even more marked olfactory impairment in patients with mild dementia with Lewy bodies than present in those with mild Alzheimer's disease. This lends significance to the role of Lewy body pathology in olfactory dysfunction [48] which would be in line with the observation that patients with nondegenerative causes of parkinsonism such as vascular parkinsonism [49] present with preserved smell function. There is also evidence for less olfactory disturbance in familial parkinsonism. In PARK2, the olfactory sense is relatively well preserved whereas PARK1 subjects are mildly hyposmic. Recent data [50] suggest that PARK 8 individuals present with impaired olfactory identification whilst asymptomatic carriers show normal olfactory performance.
In secondary parkinsonism, study results also indicate a relationship between Parkinsonian symptoms and olfactory dysfunction. We found an association between medication-induced parkinsonism and olfactory dysfunction in patients with psychotic depression treated with D2-blocking neuroleptic drugs [51]. Here, the severity of motor symptoms was positively correlated with the degree of olfactory dysfunction which might indicate patients with a latent basal ganglia dysfunction. Similar to the results seen in drug-induced parkinsonism, data from a recent study reveal that Wilson's disease patients with neurological symptoms show a significant olfactory dysfunction compared to hepatic-type patients [52]. Individuals who are more severely neurologically affected also present with more pronounced olfactory deficits. Based on these observations, olfactory testing should not be considered to differentiate PD from these specific conditions. However, olfactory testing has been shown to be important in cases where patients present with Parkinsonian features but with preserved olfaction. Here, it appears valid to question a diagnosis of PD.
5. Conclusions
Recent data suggest that inexpensive olfactory probes improve the diagnostic process in patients with PD. In contrast to imaging procedures, olfactory testing is quick and easy to perform. Validated tests can be used as reliable diagnostic tools even in nonspecialized centers. Deeb et al. [53] found that a basic smell test is as sensitive as a dopamine transporter scan. According to this study, the sensitivities of the University of Pennsylvania Smell Identification Test [54] and DaTSCAN are high at 86% and 92%, respectively. Although DaTSCAN is superior for “localization,” a smell test is considerably “cheaper,” and neither is disease specific. Consequently, structured and validated tests of olfactory function should be a mandatory part of the early and differential diagnosis of PD.
Our experience suggests that it only takes little time to follow up patients with a diagnosis of idiopathic smell loss neurologically as an essential part of their regularly scheduled visit to the Smell and Taste Clinic which is a time- and expense-efficient process well warranted. Such a comprehensive multidisciplinary approach might enable the physician to detect slight motor abnormalities in an at-risk population as early as possible. This may also give rise to clinical studies which allow administration of neuroprotective substances in individuals with, for example, unexplained smell loss. Up till now, therapeutic studies with neuroprotective agents in hyposmic PD patients are currently underway and may help us to evolve preventive strategies for PD in future.
References
- 1.Politis M, Wu K, Molloy S, Bain PG, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Movement Disorders. 2010;25(11):1646–1651. doi: 10.1002/mds.23135. [DOI] [PubMed] [Google Scholar]
- 2.Ansari KA, Johnson A. Olfactory function in patients with Parkinson’s disease. Journal of Chronic Diseases. 1975;28(9):493–497. doi: 10.1016/0021-9681(75)90058-2. [DOI] [PubMed] [Google Scholar]
- 3.Ward CD, Hess WA, Calne DB. Olfactory impairment in Parkinson’s disease. Neurology. 1983;33:943–946. doi: 10.1212/wnl.33.7.943. [DOI] [PubMed] [Google Scholar]
- 4.Hawkes CH, Shephard BC, Daniel SE. Olfactory dysfunction in Parkinson’s disease. Journal of Neurology Neurosurgery and Psychiatry. 1997;62:436–446. doi: 10.1136/jnnp.62.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 6.Haehner A, Boesveldt S, Berendse HW, et al. Prevalence of smell loss in Parkinson’s disease— a multicenter study. Parkinsonism and Related Disorders. 2009;15:490–494. doi: 10.1016/j.parkreldis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Muller A, Mungersdorf M, Reichmann H, Strehle G, Hummel T. Olfactory function in Parkinsonian syndromes. Journal of Clinical Neuroscience. 2002;9:521–524. doi: 10.1054/jocn.2001.1071. [DOI] [PubMed] [Google Scholar]
- 8.Stern MB, Doty RL, Dotti M, et al. Olfactory function in Parkinson’s disease subtypes. Neurology. 1994;44:266–268. doi: 10.1212/wnl.44.2.266. [DOI] [PubMed] [Google Scholar]
- 9.Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Movement Disorders. 2002;17:867–876. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- 10.Hummel T. Olfactory evoked potentials as a tool to measure progression of Parkinson’s disease. In: Chase T, Bedard P, editors. Focus on Medicine Vol. 14—New Developments in the Drug Therapy of Parkinson’s Disease. Oxford, UK: Blackwell Science; 1999. pp. 47–53. [Google Scholar]
- 11.Tissingh G, Berendse HW, Bergmans P, et al. Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Movement Disorders. 2001;16:41–46. doi: 10.1002/1531-8257(200101)16:1<41::aid-mds1017>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Bohnen NI, Studenski SA, Constantine GM, Moore RY. Diagnostic performance of clinical motor and non-motortests of Parkisnon’s disease: a matched case-control study. European Journal of Neurology. 2008;15(7):685–691. doi: 10.1111/j.1468-1331.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- 13.Davidson TM, Freed C, Healy MP, Murphy C. Rapid clinical evaluation of anosmia in children: the Alcohol Sniff Test. Annals of the New York Academy of Sciences. 1998;855:787–792. doi: 10.1111/j.1749-6632.1998.tb10659.x. [DOI] [PubMed] [Google Scholar]
- 14.Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Annals of Otology, Rhinology and Laryngology. 2001;110(10):976–981. doi: 10.1177/000348940111001015. [DOI] [PubMed] [Google Scholar]
- 15.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chemical Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 16.Kondo H, Matsuda T, Hashiba M, Baba S. A study of the relationship between the T&T olfactometer and the University of Pennsylvania Smell Identification Test in a Japanese population. American Journal of Rhinology. 1998;12(5):353–358. doi: 10.2500/105065898780182390. [DOI] [PubMed] [Google Scholar]
- 17.Larsson M, Nilsson LG, Olofsson JK, Nordin S. Demographic and cognitive predictors of cued odor identification: evidence from a population-based study. Chemical Senses. 2004;29:547–554. doi: 10.1093/chemse/bjh059. [DOI] [PubMed] [Google Scholar]
- 18.Ehrenstein WH, Ehrenstein A. Psychophysical methods. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Berlin, Germany: Springer; 1999. pp. 1211–1241. [Google Scholar]
- 19.Lötsch J, Lange C, Hummel T. A simple and reliable method for clinical assessment of odor thresholds. Chemical Senses. 2004;29(4):311–317. doi: 10.1093/chemse/bjh034. [DOI] [PubMed] [Google Scholar]
- 20.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. European Archives of Oto-Rhino-Laryngology. 2005;262(3):231–235. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 21.Hummel T, Kobal G. Olfactory event-related potentials. In: Simon SA, Nicolelis MAL, editors. Methods and Frontiers in Chemosensory Research. Boca Raton, Fla, USA: CRC Press; 2001. pp. 429–464. [Google Scholar]
- 22.Witt M, Hummel T. Vomeronasal versus olfactory epithelium: is there a cellular basis for human vomeronasal perception? International Review of Cytology. 2006;248:209–259. doi: 10.1016/S0074-7696(06)48004-9. [DOI] [PubMed] [Google Scholar]
- 23.Mueller A, Rodewald A, Reden J, Gerber J, Von Kummer R, Hummel T. Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. NeuroReport. 2005;16(5):475–478. doi: 10.1097/00001756-200504040-00011. [DOI] [PubMed] [Google Scholar]
- 24.Westermann B, Wattendorf E, Schwerdtfeger U, et al. Functional modulation of cortical olfactory pathways in Parkinson’s disease: an fMRI study. Journal of Neurology Neurosurgery and Psychiatry. 2008;79:19–24. doi: 10.1136/jnnp.2006.113860. [DOI] [PubMed] [Google Scholar]
- 25.Hummel T, Fliessbach K, Abele M, et al. Olfactory fMRI in patients with Parkinson’s disease. Frontiers in Integrative Neuroscience. 2010;4:p. 125. doi: 10.3389/fnint.2010.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism and Related Disorders. 2010;16(2):79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara-Paul L, Wainwright NW, Khaw KT, et al. Prospective association between emotional health and clinical evidence of Parkinson’s disease. European Journal of Neurology. 2008;15(11):1148–1154. doi: 10.1111/j.1468-1331.2008.02299.x. [DOI] [PubMed] [Google Scholar]
- 28.Leentjens AF, Van den Akker M, Metsemakers JF, Lousberg R, Verhey FR. Higher incidence of depression preceding the onset of Parkinson’s disease: a register study. Movement Disorders. 2003;18(4):414–418. doi: 10.1002/mds.10387. [DOI] [PubMed] [Google Scholar]
- 29.Müller A, Reichmann H, Livermore A, Hummel T. Olfactory function in idiopathic Parkinson’s disease (IPD): results from cross-sectional studies in IPD patients and long-term follow-up of de-novo IPD patients. Journal of Neural Transmission. 2002;109(5-6):805–811. doi: 10.1007/s007020200067. [DOI] [PubMed] [Google Scholar]
- 30.Ponsen MM, Stoffers D, Booij J, et al. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Annals of Neurology. 2004;56(2):173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- 31.Ponsen MM, Stoffers D, Twisk JW, Wolters ECh, Berendse HW. Hyposmia and executive dysfunction as predictors of future Parkinson’s disease: a prospective study. Movement Disorders. 2009;24(7):1060–1065. doi: 10.1002/mds.22534. [DOI] [PubMed] [Google Scholar]
- 32.Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Movement Disorders. 2007;22(6):839–842. doi: 10.1002/mds.21413. [DOI] [PubMed] [Google Scholar]
- 33.Sommer U, Hummel T, Cormann K, et al. Detection of presymptomatic Parkinson’s disease: combining smell tests, transcranial sonography, and SPECT. Movement Disorders. 2004;19(10):1196–1202. doi: 10.1002/mds.20141. [DOI] [PubMed] [Google Scholar]
- 34.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Annals of Neurology. 2008;63(2):167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 35.Landis BN, Burkhard PR. Phantosmias and Parkinson disease. Archives of Neurology. 2008;65(9):1237–1239. doi: 10.1001/archneur.65.9.1237. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch AR. Parkinsonism: the hyposmia and phantosmia connection. Archives of Neurology. 2009;66(4):538–539. doi: 10.1001/archneurol.2009.38. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Schwankhaus J. Olfactory disturbance in Parkinson disease. Archives of Neurology. 2009;66(6):p. 805. doi: 10.1001/archneurol.2009.87. [DOI] [PubMed] [Google Scholar]
- 38.Landis BN, Reden J, Haehner A. Idiopathic phantosmia: outcome and clinical significance. Journal of Oto-Rhino-Laryngology and its Related Specialties. 2010;72(5):252–255. doi: 10.1159/000317024. [DOI] [PubMed] [Google Scholar]
- 39.Braak H, Del Tredici K, Rub U, De Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 40.Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in parkinson’s disease. Movement Disorders. 2004;19(6):687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 41.Winner B, Geyer M, Couillard-Despres S, et al. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Experimental Neurology. 2006;197(1):113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Huisman E, Uylings HB, Hoogland PV. Gender-related changes in increase of dopaminergic neurons in the olfactory bulb of Parkinson’s disease patients. Movement Disorders. 2008;23(10):1407–1413. doi: 10.1002/mds.22009. [DOI] [PubMed] [Google Scholar]
- 43.Stern MB, Siderowf A. Parkinson’s at risk syndrome: can Parkinson’s disease be predicted? Movement Disorders. 2010;25(supplement 1):S89–S93. doi: 10.1002/mds.22719. [DOI] [PubMed] [Google Scholar]
- 44.Wenning GK, Shephard B, Hawkes C, Petruckevitch A, Less A, Quinn N. Olfactory function in atypical parkinsonian syndromes. Acta Neurologica Scandinavica. 1995;91(4):247–250. doi: 10.1111/j.1600-0404.1995.tb06998.x. [DOI] [PubMed] [Google Scholar]
- 45.McKinnon JH, Demaerschalk BM, Caviness JN, Wellik KE, Adler CH, Wingerchuk DM. Sniffing out Parkinson disease: can olfactory testing differentiate parkinsonian disorders? Neurologist. 2007;13(6):382–385. doi: 10.1097/NRL.0b013e31815a351a. [DOI] [PubMed] [Google Scholar]
- 46.Liberini P, Parola S, Spano PF, Antonini L. Olfactory dysfunction in dementia associated with Lewy bodies. Parkinsonism and Related Disorders. 1999;5(30) [Google Scholar]
- 47.Williams SS, Williams J, Combrinck M, Christie S, Smith AD, McShane R. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80(6):667–670. doi: 10.1136/jnnp.2008.155895. [DOI] [PubMed] [Google Scholar]
- 48.Hawkes C. Olfaction in neurodegenerative disorder. Movement Disorders. 2003;18(4):364–372. doi: 10.1002/mds.10379. [DOI] [PubMed] [Google Scholar]
- 49.Katzenschlager R, Lees AJ. Olfaction and Parkinson’s syndromes: its role in differential diagnosis. Current Opinion in Neurology. 2004;17(4):417–423. doi: 10.1097/01.wco.0000137531.76491.c2. [DOI] [PubMed] [Google Scholar]
- 50.Silveira-Moriyama L, Guedes LC, Kingsbury A, et al. Hyposmia in G2019S LRRK2-related parkinsonism: clinical and pathologic data. Neurology. 2008;71(13):1021–1026. doi: 10.1212/01.wnl.0000326575.20829.45. [DOI] [PubMed] [Google Scholar]
- 51.Krüger S, Haehner A, Thiem C, Hummel T. Neuroleptic-induced parkinsonism is associated with olfactory dysfunction. Journal of Neurology. 2008;255(10):1574–1579. doi: 10.1007/s00415-008-0993-5. [DOI] [PubMed] [Google Scholar]
- 52.Müeller A, Reuner U, Landis B, Kitzler H, Reichmann H, Hummel T. Extrapyramidal symptoms in Wilson’s disease are associated with olfactory dysfunction. Movement Disorders. 2006;21(9):1311–1316. doi: 10.1002/mds.20989. [DOI] [PubMed] [Google Scholar]
- 53.Deeb J, Shah M, Muhammed N, et al. A basic smell test is as sensitive as a dopamine transporter scan: comparison of olfaction, taste and DaTSCAN in the diagnosis of Parkinson’s disease. QJM. 2010;103(12):941–952. doi: 10.1093/qjmed/hcq142. [DOI] [PubMed] [Google Scholar]
- 54.Doty RL. The Smell Idenfication Test9 Administration Manual. 2nd edition. Haddonfield, NJ, USA: Sensonics; 1989. [Google Scholar]