Abstract
INTRODUCTION AND OBJECTIVE:
Kidney disorders can cause essential hypertension, which can subsequently cause renal disease. High blood pressure is also common among those with chronic kidney disease; moreover, it is a well-known risk factor for a more rapid progression to kidney failure. Because hypertension and kidney function are closely linked, the present study aimed to observe the beneficial effects of low-intensity physical activity on structural and ultrastructural renal morphology and blood pressure in normotensive and spontaneously hypertensive rats.
METHOD:
Male Wistar-Kyoto rats and spontaneously hypertensive rats were randomly allocated into four groups: sedentary or exercised Wistar-Kyoto and sedentary or exercised spontaneously hypertensive rats. The exercise lasted 20 weeks and consisted of treadmill training for 1 hour/day, 5 days/week.
RESULTS:
The exercised, spontaneously hypertensive rats showed a significant blood pressure reduction of 26%. The body masses of the Wistar-Kyoto and spontaneously hypertensive strains were significantly different. There were improvements in some of the renal structures of the animals treated with physical activity: (i) the interdigitations of the proximal and distal convoluted tubules; (ii) the basal membrane of the proximal and distal convoluted tubules; and (iii) in the basal membrane, slit diaphragm and pedicels of the glomerular filtration barrier. The spontaneously hypertensive rats also showed a decreased expression of connexin-43.
CONCLUSION:
Physical exercise could be a therapeutic tool for improving kidney ultrastructure and, consequently, renal function in hypertensive individuals.
Keywords: Hypertension, Physical activity, Kidney, SHR, Ultrastructure
INTRODUCTION
Kidney disorders can cause essential hypertension, which can subsequently cause renal disease. High blood pressure is also common in chronic kidney disease; moreover, it is a well-known risk factor for a more rapid progression to kidney failure. Hypertension and kidney function are therefore closely linked.1-3
Substantial evidence supports the notion that elevated blood pressure (BP) is the most significant risk factor for developing chronic kidney disease.1 Hypertensive factors related to the progression of renal damage include the magnitude of the increase in systemic BP and the degree to which the systemic BP elevation is transmitted to the renal microvasculature (i.e., the degree of renal autoregulation impairment). In a healthy kidney, renal autoregulation mechanisms maintain a constant level of renal blood flow and intraglomerular capillary pressure, despite fluctuations in a systemic blood pressure that can vary between 80 and 170 mm Hg. This regulation is accomplished through a myogenic reflex that is inherent to the kidney, in which BP increases, whether episodic or sustained, result in proportionate increases in renal vascular resistance, so that renal blood flow is unchanged. Because these resistance changes are confined to the preglomerular resistance vessels, primarily the afferent arteriole, glomerular capillary pressures also remain relatively constant. Thus, the glomerular capillaries are protected from barotrauma as long as the autoregulatory mechanisms are intact and the BP remains within the autoregulatory range. When systemic BP increases, the afferent arteriole constricts, thereby limiting the transmission of the increased pressure to the glomerular capillaries.4,5
It is well established that a daily routine of physical exercise aids the prevention of and recover from cardiovascular diseases because of its beneficial effects on the cardiovascular system and the associated risk indicators.6,7 There is overwhelming evidence from a number of sources, including epidemiological, prospective cohort and intervention studies, suggesting that cardiovascular disease is mainly associated with physical inactivity.8 Thus, regular aerobic exercise has been shown to be an important therapeutic tool that produces a significant reduction in BP in both hypertensive patients and animal models.6,7,9
Exercise-induced reductions in vascular resistance,10-12 insulin resistance13 and sympathetic activity14 have been associated with reductions in BP.9
Rats that were subjected to low-intensity exercise have blood pressure values that are significantly lower than those of sedentary rats and rats subjected to high-intensity exercise. Low-intensity exercise decreases heart rate and cardiac output and, consequently, attenuates hypertension in spontaneously hypertensive rats (SHR).7,15,16
It has been demonstrated that physical activity improves renal function in elderly individuals.17,18 Moreover, physical activity is effective at reducing fatigue and improving the physical capacity to control hypertension in patients who already have chronic kidney disease, and it reduces fatigue in patients who were originally classified as sedentary.19,20 Renal function seems to be associated with lifestyle and biological cardiovascular disease risk factors, and it contributes to the long-term incidence of cardiac events.21
If there is relationship between physical activity and renal function, the mechanisms by which physical activity improves renal function, and consequently renal morphology, are still unknown. Therefore, the present study aimed to observe the beneficial effects of low-intensity physical activity on BP and structural and ultrastructural renal morphology in normotensive rats and SHR.
MATERIALS AND METHODS
Animals and treatments
This study was performed in accordance with the “Care and Use of Laboratory Animals” guidelines (U.S. National Institutes of Health 85-23, revised 1996). The handling and experimentation protocols were approved by the local Ethics Committee for the Use and Care of Experimental Animals. The Wistar-Kyoto rats (WKY) and SHR (lzm strain) were kept under standard conditions (12 h light/dark cycle, 21±2°C, 60±10% humidity) and received water and standard chow ad libitum (Nuvilab, Parana, Brazil). Two-month-old rats were purchased from the Center of Experimental Models for Medicine and Biology, Universidade Federal de São Paulo (www.unifesp.br/centros/cedeme), and the experiment began when the rats were 3 months old. The rats were randomly allocated into four groups (n = 8 each): sedentary WKY (SED-WKY), exercised WKY (EX-WKY), sedentary SHR (SED-SHR), and exercised SHR (EX-SHR). The sedentary rats were handled daily, and three days a week they were submitted to a 5-min period of exercise on a motorized treadmill (0.5 m/h) to become accustomed to the experimental procedures. The exercised rats underwent 20 weeks of progressive exercise training on a motorized treadmill (1 h/day, 5 days/week). The desired final training intensity and duration (16 m/min, 0% grade, 60 min/day) were reached at the end of Week 20, according to a previously established protocol. The exercise training was characterized as low intensity because the O2 consumption did not exceed 55% of the O2max throughout the experiment.16 BP and body mass (BM) were measured weekly. The BP was measured in conscious rats, using the noninvasive tail-cuff plethysmography method (Letica LE 5100, Panlab, Spain).
Euthanasia
At Week 20, the rats were deeply anesthetized with sodium pentobarbital (i.p., 150 mg/kg). After the procedure, the animals were euthanized by excess anesthetic. The abdomen was opened, and the kidneys were removed.
Transmission electron microscopy
Fragments of the kidneys were immediately fixed in 2.5% glutaraldehyde (Sigma-Aldrich Laborchemikalien GmbH, Seelze, Germany) in a 0.1 M cacodylate buffer (pH 7.2) and 0.25% tannic acid (Merck KGaA, Darmstadt, Germany), postfixed in 1% osmium tetroxide (Sigma-Aldrich, Saint Louis, U.S.A.), and embedded in Epon (Embed-812, EMS, Hatfield, PA, USA). Ultrathin sections (60 to 70 nm) were obtained from selected areas using an ultramicrotome (Leica ULTRA-CUT; Leica Aktiengesellschaft, Wien, Austria), stained with uranyl acetate and lead citrate, and examined with a Zeiss EM 906 transmission electron microscope (TEM) (Carl Zeiss EM 906, Oberköchen, Germany) at 80 kV.
Scanning electron microscopy
Other fragments of the kidneys were fixed in 2.5% glutaraldehyde (Sigma-Aldrich Laborchemikalien GmbH, Seelze, Germany) in a 0.1 M cacodylate buffer (pH 7.2) and postfixed for 30 min in 1% osmium tetroxide and dehydrated acetone. The samples were then critical point dried with CO2, coated with gold, and examined with a scanning electron microscope (SEM; Carl Zeiss LEO 1450 VP, Oberköchen, Germany) at 15 kV.
Ultrastructural immunohistochemistry
Ultrathin sections (LR White Resin embedded material, hard grade acrylic resin; London Resin Company, England) were obtained from selected areas and collected on 300-mesh nickel grids. The sections were incubated with anti-connexin-43 (anti-Cx-43) (1∶20, MAB3067, Chemicon). They were then incubated with secondary antibody protein-A gold-labeled 10-nm colloidal (1∶50, P-1039, Sigma-Aldrich, Saint Louis, USA). Finally, the grids were counterstained with uranyl acetate and lead citrate and examined with a Zeiss EM 906 transmission electron microscope (TEM) (Carl Zeiss EM 906, Oberköchen, Germany) at 80 kV.
Data analysis
Data are shown as the mean and standard error of the mean. The data were tested for normality and homogeneity of variances, and the differences between groups were analyzed using a one-way ANOVA model and a post-hoc Tukey's test. A two-way analysis of variance assessing the effects of genotype (WKY and SHR) and exercise training (sedentary and exercised) was also performed. P-values ≤0.05 were considered statistically significant (Graph-Pad Prism version 5.03, San Diego, U.S.A.).
RESULTS
Blood pressure and body mass
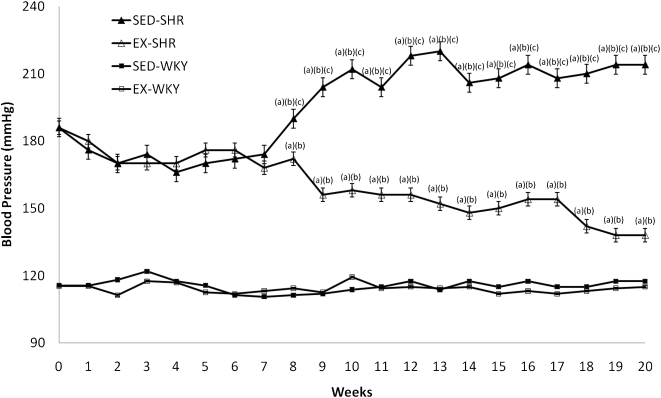
The BP of the SED-WKY and EX-WKY groups did not differ significantly (P>0.05) throughout the experiment (Figure 1). The EX-SHR group showed a BP reduction of 26% (from 186±5.1 to 138±3.7) and arriving BP levels similar to the SED-WKY (116±1.5 to 118±0.9) (P<0.001) and EX-WKY (116±1.5 to 115±0.9) (P<0.001) control groups. The SED-SHR group had increased BP levels (186±5 to 214±9) (P<0.001) after 20 weeks of experimentation. These changes became significant at the 8th week.
Figure 1.
Blood pressure before and during twenty weeks of treatment. Abbreviations: EX, exercised; SHR, spontaneously hypertensive rats; SED, sedentary; WKY, Wistar-Kyoto rats. One-way ANOVA and post-hoc Tukey's test; P<0.001 for (a) vs. SED-WKY, (b) vs. EX-WKY, (c) vs. EX-SHR.
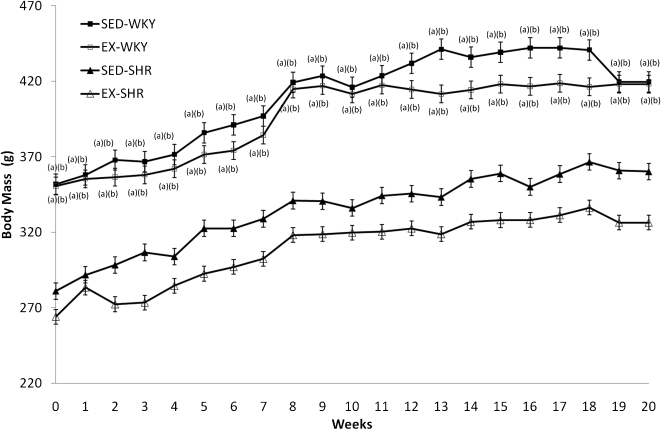
The BM of the SED-WKY and EX-WKY groups did not differ significantly (P>0.05) throughout the experiment, nor were there significant differences between the SED-SHR and EX-SHR groups (Figure 2). However, there was a significant difference between the WKY and SHR strains before training, and this difference persisted after twenty weeks of exercise training (P<0.001). In general, the animals showed a gradual increase in BM.
Figure 2.
Body mass before and during twenty weeks of treatment. Abbreviations: EX, exercised; SHR, spontaneously hypertensive rats; SED, sedentary; WKY, Wistar-Kyoto rats. One-way ANOVA and post-hoc Tukey's test; P<0.001 for (a) vs. SED-WKY, (b) vs. EX-WKY.
Transmission electron microscopy (TEM)
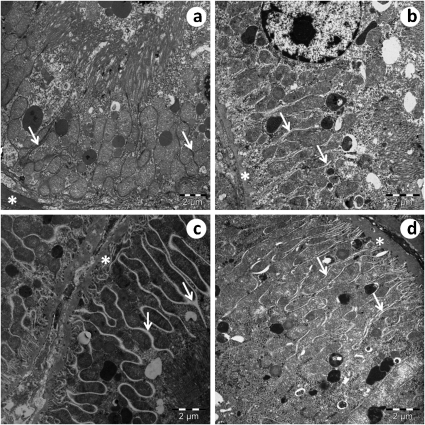
There was an increase in the spaces between the interdigitations of the convoluted proximal tubule (Figure 3c) and the convoluted distal tubule (Figure 4c). The basal membrane of the tubules (Figures 3 and 4) exhibited a thickened appearance in SED-SHR group (Figures 3c and 4c) compared to the EX-SHR group (Figures 3d and 4d) and the SED-WKY (Figures 3a and 4a) and EX-WKY (Figures 3b and 4b) control groups.
Figure 3.
An electron micrograph of a convoluted proximal tubule. In the SED-SHR (c), there is an increase in the space between the interdigitations (arrow), and the basal membrane (*) of the tubules exhibit a thickened appearance compared to the EX-SHR (d) and the SED-WKY (a) and EX-WKY (b) control groups.
Figure 4.
An electron micrograph of a convoluted distal tubule. In the SED-SHR (c), there is an increase in the space between the interdigitations (arrow), and the basal membrane (*) of the tubules exhibits a thickened appearance compared to the EX-SHR (d) and the SED-WKY (a) and EX-WKY (b) control groups. Abbreviations: EX, exercised; SHR, spontaneously hypertensive rats; SED, sedentary; WKY, Wistar-Kyoto rats.
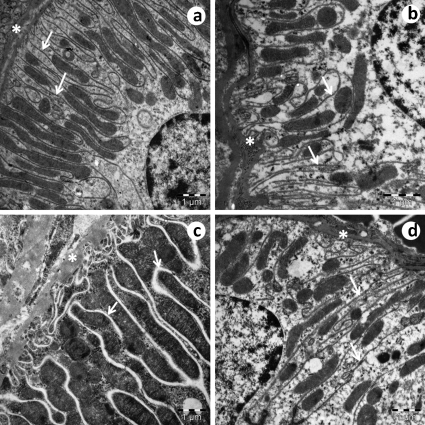
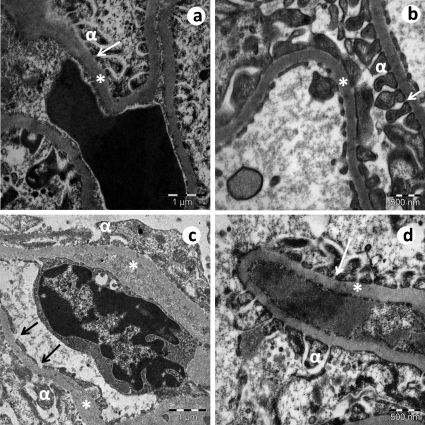
The basal membranes of the glomeruli exhibited a thickened appearance in the SED-SHR group (Figure 5c) compared to the EX-SHR group (Figure 5d) and the SED-WKY (Figure 5a) and EX-WKY (Figure 5b) control groups. In the glomerular filtration barrier, the slit diaphragm and the podocyte foot processes (pedicels) were more preserved in the SED-WKY (Figure 5a), EX-WKY (Figure 5b) and EX-SHR (Figure 5d) groups compared to the SED-SHR group (Figure 5c), in which the pedicels were shorter.
Figure 5.
An electron micrograph of a glomerulus. In the SED-SHR (c), the basal membrane (*) of the glomeruli exhibits a thickened appearance compared to the EX-SHR (d) and the control groups SED-WKY (a) and EX-WKY (b). In the glomerular filtration barrier, the slit diaphragm (arrow) and the pedicels (α) were more preserved in the SED-WKY (a), EX-WKY (b) and EX-SHR (d) compared to the SED-SHR (c). Abbreviations: EX, exercised; SHR, spontaneously hypertensive rats; SED, sedentary; WKY, Wistar-Kyoto rats.
Scanning electron microscopy (SEM)
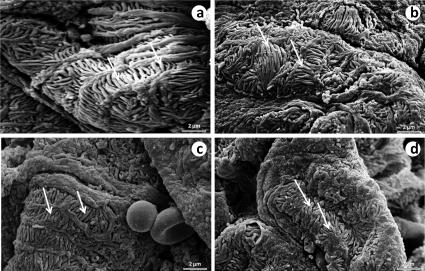
The pedicels were more preserved in the SED-WKY (Figure 6a), EX-WKY (Figure 6b) and EX-SHR (Figure 6d) groups compared to the SED-SHR group (figure 6c), which showed thinner, shorter, and more tortuous pedicels. Despite the improvement in the EX-SHR pedicels (Figure 6d), their morphology was not as preserved as that of the pedicels of the SED-WKY (Figure 6a) and EX-WKY (Figure 6b) control groups.
Figure 6.
An electron micrograph of a podocyte foot process. The pedicels (arrow) were more preserved in the SED-WKY (a), EX-WKY (b) and EX-SHR (d) than in the SED-SHR (c), which showed thinner, shorter and more tortuous pedicels. Although the EX-SHR showed pedicel improvement, their morphology was not as well-preserved as in the SED-WKY (a) and EX-WKY (b) control groups. Abbreviations: EX, exercised; SHR, spontaneously hypertensive rats; SED, sedentary; WKY, Wistar-Kyoto rats.
Ultrastructural immunohistochemistry
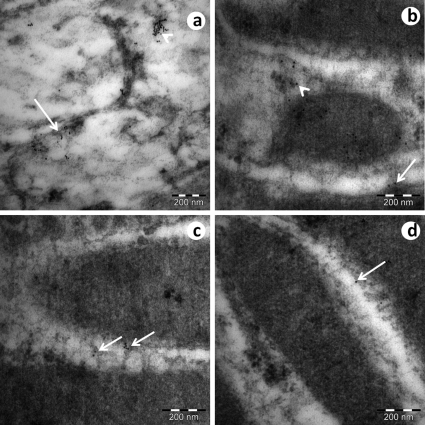
Connexin-43 (Cx-43) immunostaining was observed in the spaces between the interdigitations and in the cytoplasmic compartments of the endothelial cells of the convoluted proximal tubules in the SED-WKY (Figure 7a) and EX-WKY (Figure 7b) control groups. The SED-SHR (Figure 7c) and EX-SHR (Figure 7d) groups showed a decreased expression of Cx-43.
Figure 7.
The connexin-43 ultrastructural immunohistochemistry of a convoluted proximal tubule. Connexin-43 (Cx-43) immunostaining was observed in the space between the interdigitations (arrow) and in the cytoplasmic compartments of the endothelial cells (arrowheads) in the convoluted proximal tubules of the SED-WKY (a) and EX-WKY (b) control groups. The SED-SHR (c) and EX-SHR (d) groups showed decreased Cx-43 expression. Abbreviations: EX, exercised; SHR, spontaneously hypertensive rats; SED, sedentary; WKY, Wistar-Kyoto rats.
DISCUSSION
The aim of the present study was to provide new insight into the development of kidney damage in hypertensive animals and into the benefits of low-intensity physical activity in these animals.
Palmer & Fenves5 have suggested that in damaged kidneys, the myogenic reflex is blunted, renal autoregulation becomes impaired, and the ability to prevent the transmission of systemic BP changes into the glomerular circulation is partially or totally lost. Consequently, the intraglomerular pressure begins to change in direct proportion to the changes in systemic arterial pressure. Bidani et al.4 hypothesized that the remodeling changes in the resistance vessels exposed to increased pressures causes them to develop benign nephrosclerosis over time. When the BP exceeds the threshold for autoregulation, however, the vascular injury risk increases, acute malignant nephrosclerosis ensues, and the preglomerular vasculature's autoregulatory ability to protect the glomerular capillaries is breached. In SHR, smaller afferent arterioles are a possible cause of increased renal vascular resistance. Renal vascular resistance thus increases in SHR, possibly as a result of these smaller arterioles and, after the onset of hypertension, probably as a consequence of physiological autoregulation as well.22,23 The TEM revealed that the basal membranes in the SED-SHR had a thickened appearance. The basal membrane, the slit diaphragm and pedicel were more preserved in the SED-WKY, EX-WKY and EX-SHR compared to the SED-SHR. Increasing intraglomerular pressure is a possible explanation for how hypertension affects the glomerular ultrastructure of the kidneys in the SED-SHR.
To better understand the pedicel alterations, we examined this structure using SEM. We found that the pedicels were more preserved in the SED-WKY, EX-WKY, and EX-SHR compared to the SED-SHR, whose kidneys showed thinner, shorter and more tortuous pedicels. Although the EX-SHR exhibited pedicel improvement, pedicel morphology was not as well-preserved in this group as in the SED-WKY and EX-WKY control groups.
Alterations of the glomerular filtration barrier can cause proteinuria.24 This barrier has three layers: the fenestrated endothelium, the glomerular basement membrane, and the podocytes. The filtration barrier is believed to be size-selective and charge-selective. The podocyte slit diaphragm has an important and direct role in glomerular filtration. Some of its protein components are involved in the mechanisms of proteinuria.25-28 In our experiments, we observed that some components of the glomerular filtration barrier, such as the slit diaphragm and the pedicels, were altered in the SED-SHR. It is possible that these animals were susceptible to developing proteinuria.
Proteinuria, a useful marker for kidney damage associated with hypertension, is itself a risk factor for the progression of renal disease. 29,30 The accumulation of filtered proteins in the proximal tubular cells triggers proinflammatory, profibrogenic, and cytotoxic pathways that contribute to tubulointerstitial injury and renal scarring.31 In our experiments, the TEM revealed an increase in the spaces between the interdigitations in the convoluted proximal tubule and in the convoluted distal tubule, and the basal membranes of the tubules exhibited a thickened appearance in the SED-SHR compared to the EX-SHR and the SED-WKY and EX-WKY control groups. It is possible that these alterations were stimulated by these proinflammatory, profibrogenic, and cytotoxic signals.
Thus, hypertension promotes the progression of renal disease by worsening glomerular injury and increasing proteinuria, and proteinuria in turn further promotes renal damage. The benefits of appropriate BP control include a reduction in proteinuria levels and a possible slowing of the progressive loss of kidney function.30
Gap junctions represent one way in which vertebrate cells communicate (in the case of gap junctions, by sharing ions, second messengers, small metabolites, and other signaling molecules). This type of intercellular communication permits coordinated cellular activity, including secretion, by allowing cells to review the functional state of their neighbors, a critical feature for the homeostasis of multicellular systems. Intercellular gap junctions result from the association of 2 half channels called connexons. Each connexon is an assembly of 6 membrane proteins called connexins. Although connexins are expressed in tubular cells, almost nothing is known of their physiological role in the tubular system. Morphological gap junctions are only found between the proximal tubule cells, despite all cells of the tubular system having been found to express connexins to some extent.32 Dlugosova et al.33 have demonstrated that hypertension alters Cx-43 expression in the aorta of SHR. Connexin-43 immunostaining was observed in the space between the interdigitations and in the cytoplasmic compartments of the endothelial cells of the convoluted proximal tubules of the SED-WKY and EX-WKY control groups. The SED-SHR and EX-SHR exhibited decreased Cx-43 expression. The decreased Cx-43 expression in the SED-SHR and EX-SHR indicates the possibility that hypertension affected the Cx-43 expression in the tubular endothelial cells; furthermore, the decreased Cx-43 expression in the EX-SHR indicates that the low-intensity physical activity probably did not exert any effect during the experimental period other than changing the Cx-43 expression in the hypertensive animals.
As previous studies have shown, physical activity causes a significant BP drop in hypertensive humans and animal models.6,7 Robinson et al.34 have described an association between physical activity and lower urinary albumin excretion in nondiabetic women. This simple fact could explain why the kidney ultrastructure was more preserved in the EX-SHR than in the SED-SHR. Lehnen et al.35 demonstrated that even in detrained SHR, the cardiorespiratory and metabolic benefits of exercise are preserved, which reinforces the benefits of physical activity.
Mustata et al.36 has suggested that long-term exercise training improves physical impairment, arterial stiffness and health-related quality of life in patients with pre-dialysis chronic kidney disease. Toyama et al.37 observed that a physical exercise intervention resulted in a significant improvement in renal function in patients with both cardiovascular disease and chronic kidney disease. This improvement was partially correlated with modified lipid metabolism and cardiopulmonary function.
Despite the results of this work, some limitations must be considered. The exercise training protocol used in this study offers important insight into its effects on high blood pressure, but it does not determine the best exercise training modality, intensity or frequency for treating essential hypertension. Other possible directions for future research include using a group of older animals with established renal injuries and using antihypertensive drugs to see whether the results found in this study were due solely to the reduced hypertension caused by the physical activity.
Our results provided insight into the effects of hypertension on the kidney ultrastructure and the benefits of physical exercise for hypertensive individuals. We recognize that physical exercise can improve health-related quality of life in patients with kidney disease; however, we still do not know how physical exercise improves kidney morphology, and more research is needed. In conclusion, we hypothesize that physical exercise can be a therapeutic tool for improving kidney ultrastructure and, consequently, renal function in hypertensive individuals.
ACKNOWLEDGMENTS
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de nível Superior (Capes) and Universidade do Estado do Rio de Janeiro (UERJ).
REFERENCES
- 1.Barri YM. Hypertension and kidney disease: a deadly connection. Curr Hypertens Rep. 2008;10:39–45. doi: 10.1007/s11906-008-0009-y. 10.1007/s11906-008-0009-y [DOI] [PubMed] [Google Scholar]
- 2.Krzesinski JM, Cohen EP. Hypertension and the kidney. Acta Clin Belg. 2007;62:5–14. doi: 10.1179/acb.2007.002. [DOI] [PubMed] [Google Scholar]
- 3.Zamo FS, Lacchini S, Mostarda C, Chiavegatto S, Silva IC, Oliveira EM, et al. Hemodynamic, morphometric and autonomic patterns in hypertensive rats - Renin-Angiotensin system modulation. Clinics (Sao Paulo) 2010;65:85–92. doi: 10.1590/S1807-59322010000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393–8. doi: 10.1161/HYPERTENSIONAHA.109.133777. 10.1161/HYPERTENSIONAHA.109.133777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer BF, Fenves AZ. Optimizing blood pressure control in patients with chronic kidney disease. Proc (Bayl Univ Med Cent) 2010;23:239–45. doi: 10.1080/08998280.2010.11928626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moraes-Teixeira JD, Félix A, Fernandes-Santos C, Moura AS, Mandarim-de- Lacerda CA, de Carvalho JJ. Exp Mol Pathol. 2010 Aug 26 [Epub ahead of print]; Exercise training enhances elastin, fibrillin and nitric oxide in the aorta wall of spontaneously hypertensive rats. [DOI] [PubMed] [Google Scholar]
- 7.Lee LL, Watson MC, Mulvaney CA, Tsai CC, Lo SF. The effect of walking intervention on blood pressure control: A systematic review. Int J Nurs Stud. 2010;47:1545–61. doi: 10.1016/j.ijnurstu.2010.08.008. 10.1016/j.ijnurstu.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 8.Leung FP, Yung LM, Laher I, Yao X, Chen ZY, Huang Y. Exercise, vascular wall and cardiovascular diseases: an update (Part 1) Sports Med. 2008;38:1009–24. doi: 10.2165/00007256-200838120-00005. 10.2165/00007256-200838120-00005 [DOI] [PubMed] [Google Scholar]
- 9.Coimbra R, Sanchez LS, Potenza JM, Rossoni LV, Amaral SL, Michelini LC. Is gender crucial for cardiovascular adjustments induced by exercise training in female spontaneously hypertensive rats. Hypertension. 2008;2:514–21. doi: 10.1161/HYPERTENSIONAHA.108.114744. 10.1161/HYPERTENSIONAHA.108.114744 [DOI] [PubMed] [Google Scholar]
- 10.Gando Y, Yamamoto K, Murakami H, Ohmori Y, Kawakami R, Sanada K, et al. Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension. 2010;56:540–6. doi: 10.1161/HYPERTENSIONAHA.110.156331. 10.1161/HYPERTENSIONAHA.110.156331 [DOI] [PubMed] [Google Scholar]
- 11.Thijssen DH, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010;108:845–75. doi: 10.1007/s00421-009-1260-x. 10.1007/s00421-009-1260-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan TS. Exercise-induced reduction in systemic vascular resistance: a covert killer and an unrecognised resuscitation challenge. Med Hypotheses. 2009;73:479–84. doi: 10.1016/j.mehy.2009.06.021. 10.1016/j.mehy.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 13.Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes. 2010:1–9. doi: 10.1155/2011/482564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol. 2009;587:2049–57. doi: 10.1113/jphysiol.2009.170134. 10.1113/jphysiol.2009.170134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. 10.1016/j.arr.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Véras-Silva AS, Mattos KC, Gava NS, Brum PC, Negrão CE, Krieger EM. Low-intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats. Am J Physiol. 1997;273:H2627–H2631. doi: 10.1152/ajpheart.1997.273.6.H2627. [DOI] [PubMed] [Google Scholar]
- 17.Odden MC. Physical functioning in elderly persons with kidney disease. Adv Chronic Kidney Dis. 2010;17:348–57. doi: 10.1053/j.ackd.2010.02.002. 10.1053/j.ackd.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Westhoff TH, Franke N, Schmidt S, Vallbracht-Israng K, Meissner R, Yildirim H, et al. Too old to benefit from sports. The cardiovascular effects of exercise training in elderly subjects treated for isolated systolic hypertension. Kidney Blood Press Res. 2007;30:240–7. doi: 10.1159/000104093. [DOI] [PubMed] [Google Scholar]
- 19.Chang Y, Cheng SY, Lin M, Gau FY, Chao YF. The effectiveness of intradialytic leg ergometry exercise for improving sedentary life style and fatigue among patients with chronic kidney disease: a randomized clinical trial. Int J Nurs Stud. 2010;47:1383–8. doi: 10.1016/j.ijnurstu.2010.05.002. 10.1016/j.ijnurstu.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 20.Henrique DM, Reboredo Mde M, Chaoubah A, Paula RB. Aerobic exercise improves physical capacity in patients under chronic hemodialysis. Arq Bras Cardiol. 2010;94:823–8. doi: 10.1590/s0066-782x2010005000043. 10.1590/S0066-782X2010005000043 [DOI] [PubMed] [Google Scholar]
- 21.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Toutouza M, Papaioannou I, et al. Renal function, cardiovascular disease risk factors' prevalence and 5-year disease incidence; the role of diet, exercise, lipids and inflammation markers: the ATTICA study. QJM. 2010;103:413–22. doi: 10.1093/qjmed/hcq045. 10.1093/qjmed/hcq045 [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;298:H1769–75. doi: 10.1152/ajpheart.00537.2009. 10.1152/ajpheart.00537.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ofstad J, Iversen BM. Glomerular and tubular damage in normotensive and hypertensive rats. Am J Physiol Renal Physiol. 2005;288:F665–72. doi: 10.1152/ajprenal.00226.2004. 10.1152/ajprenal.00226.2004 [DOI] [PubMed] [Google Scholar]
- 24.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–401. doi: 10.1056/NEJMra052131. 10.1056/NEJMra052131 [DOI] [PubMed] [Google Scholar]
- 25.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. 10.1093/hmg/10.1.1 [DOI] [PubMed] [Google Scholar]
- 26.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829–36. doi: 10.1128/MCB.21.14.4829-4836.2001. 10.1128/MCB.21.14.4829-4836.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciani L, Patel A, Allen ND, ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23:3575–82. doi: 10.1128/MCB.23.10.3575-3582.2003. 10.1128/MCB.23.10.3575-3582.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004;24:550–60. doi: 10.1128/MCB.24.2.550-560.2004. 10.1128/MCB.24.2.550-560.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45:281–7. doi: 10.1053/j.ajkd.2004.10.019. 10.1053/j.ajkd.2004.10.019 [DOI] [PubMed] [Google Scholar]
- 30.Flack JM, Ferdinand KC, Nasser SA, Rossi NF. Hypertension in special populations: chronic kidney disease, organ transplant recipients, pregnancy, autonomic dysfunction, racial and ethnic populations. Cardiol Clin. 2010;28:623–38. doi: 10.1016/j.ccl.2010.07.007. 10.1016/j.ccl.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 31.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage. J Am Soc Nephrol. 2006;17:2974–84. doi: 10.1681/ASN.2006040377. 10.1681/ASN.2006040377 [DOI] [PubMed] [Google Scholar]
- 32.Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1143–55. doi: 10.1152/ajpregu.00808.2009. 10.1152/ajpregu.00808.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dlugosova K, Okruhlicova L, Mitasikova M, Sotnikova R, Bernatova I, Weismann P, et al. Modulation of connexin-43 by omega-3 fatty acids in the aorta of old spontaneously hypertensive rats. J Physiol Pharmacol. 2009;60:63–9. [PubMed] [Google Scholar]
- 34.Robinson ES, Fisher ND, Forman JP, Curhan GC. Physical activity and albuminuria. Am J Epidemiol. 2010;171:515–21. doi: 10.1093/aje/kwp442. 10.1093/aje/kwp442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehnen AM, Leguisamo NM, Pinto GH, Markoski MM, De Angelis K, Machado UF, et al. The beneficial effects of exercise in rodents are preserved after detraining: a phenomenon unrelated to GLUT4 expression. Cardiovasc Diabetol. 2010;9:67. doi: 10.1186/1475-2840-9-67. 10.1186/1475-2840-9-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mustata S, Groeneveld S, Davidson W, Ford G, Kiland K, Manns B. Int Urol Nephrol. 2010 [Epub ahead of print]; Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. [DOI] [PubMed] [Google Scholar]
- 37.Toyama K, Sugiyama S, Oka H, Sumida H, Ogawa H. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol. 2010;56:142–6. doi: 10.1016/j.jjcc.2010.06.007. 10.1016/j.jjcc.2010.06.007 [DOI] [PubMed] [Google Scholar]