Abstract
INTRODUCTION:
Osteoporotic fractures are common during osteoporotic states. Piper sarmentosum extract is known to possess antioxidant and anti-inflammatory properties.
OBJECTIVES:
To observe the radiological changes in fracture calluses following administration of a Piper sarmentosum extract during an estrogen-deficient state.
METHODS:
A total of 24 female Sprague-Dawley rats (200-250 g) were randomly divided into 4 groups: (i) the sham-operated group; (ii) the ovariectomized-control group; (iii) the ovariectomized + estrogen-replacement therapy (ovariectomized-control + estrogen replacement therapy) group, which was supplemented with estrogen (100 µg/kg/day); and (iv) the ovariectomized + Piper sarmentosum (ovariectomized + Piper sarmentosum) group, which was supplemented with a water-based Piper sarmentosum extract (125 mg/kg). Six weeks after an ovariectomy, the right femora were fractured at the mid-diaphysis, and a K-wire was inserted. Each group of rats received their respective treatment for 6 weeks. Following sacrifice, the right femora were subjected to radiological assessment.
RESULTS:
The mean axial callus volume was significantly higher in the ovariectomized-control group (68.2±11.74 mm3) than in the sham-operated, estrogen-replacement-therapy and Piper sarmentosum groups (20.4±4.05, 22.4±4.14 and 17.5±3.68 mm3, respectively). The median callus scores for the sham-operated, estrogen-replacement-therapy and Piper sarmentosum groups had median (range, minimum - maximum value) as 1.0 (0 - 2), 1.0 (1 - 2) and 1.0 (1 - 2), respectively, which were significantly lower than the ovariectomized-control group score of 2.0 (2 - 3). The median fracture scores for the sham-operated, estrogen-replacement-therapy and Piper sarmentosum groups were 3.0 (3 - 4), 3.0 (2 - 3) and 3.0 (2 - 3), respectively, which were significantly higher than the ovariectomized-control group score of 2.0 (1 - 2) (p<0.05).
CONCLUSION:
The Piper sarmentosum extract improved fracture healing, as assessed by the reduced callus volumes and reduced callus scores. This extract is beneficial for fractures in osteoporotic states.
Keywords: Piper sarmentosum, Antioxidant, Fracture: Healing, Osteoporosis, Ovariectomy
INTRODUCTION
Osteoporotic fractures are common during osteoporotic states and affect a patient's quality of life.1 Osteoporosis is a metabolic disorder that mainly affects postmenopausal women. It is characterized by decreased bone mineral density (BMD), which leads to bone fragility and an increase in fracture incidence.2 Ovariectomized rats are a useful model for postmenopausal osteoporosis because the pathogenic process is similar to that occurring in osteoporotic women.3
There is increased concern about the effects of osteoporosis on fracture healing. Previous studies on animals have reported that osteoporosis is associated with a delayed fracture healing process, although there is a lack of substantial clinical evidence.4,5 The pathogenesis of postmenopausal osteoporosis resulting from estrogen loss involves an imbalance in the bone remodeling process. The resorption of bone increases without adequate new bone formation due to the activation of bone multicellular units (BMUs), apoptosis of osteoblasts and suppression of osteoclast apoptosis.6 Bone is the only tissue that can regenerate without leaving a scar.
Oxidative stress refers to the oxidative damage in a tissue induced by reactive oxygen species (ROS).7 The relationship between estrogen and oxidative stress is complex. Previous research studies have concluded that an estrogen-deficient state is associated with the over-production of ROS, which induces the production of cytokines involved in osteoclastogenesis, such as tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6).8 According to earlier research reports, estrogen deficiency leads to decreased levels of thiol antioxidants in rodent bone cells.9 Ovariectomies induce oxidative stress, which results in bone loss by increasing the levels of hydrogen peroxide (H2O2) in rats.10 It has been reported that the ROS H2O2 has an important role in osteoclastogenesis and signals bone loss in estrogen-deficient states.11 Osteoclastogenesis results in the increased activation of osteoclasts and subsequent increased bone resorption.
The use of herbal medicines has been gaining in popularity worldwide. In Malaysia, the annual sales of traditional medicines have increased considerably recently, without the knowledge of side-effects.12 Piper sarmentosum (P.s.) is an herb commonly used in Malaysia as a traditional medicine for treating diabetes, hypertension and joint aches.13 A previous phytochemical investigation of P.s. extract revealed various active compounds, such as alkaloids, amides, flavonoids, lignans and phenylpropanoids.14 A methanolic extract of P.s. contains a highly active natural antioxidant scavenger, naringenin, which belongs to the flavonoid group.13 Flavonoids are a group of naturally occurring phenolic compounds and are widely distributed throughout the plant kingdom.15 The flavonoid hesperidin has been found to inhibit ovariectomy-induced osteopenia and increase bone strength in ovariectomized rats.16 Researchers have reported that flavonoids can replace α-tocopherol as a chain-breaking antioxidant.17
It has been reported that extracts derived from different parts of the P.s. plant possess antioxidative, antimicrobial, anti-inflammatory, anticarcinogenic and hypoglycemic properties.18 It has been found that a water-based P.s. extract reduces bone resorption by decreasing cortisol levels in rats.19 Administration of antioxidants may prevent bone loss and help accelerate fracture healing in osteoporotic patients.20 The main aim of the present study was to observe the effects of supplementation with a P.s. extract on osteoporotic fracture healing in estrogen-deficient rats.
MATERIALS AND METHODS
Preparation of the P.s. extract
Fresh P.s. leaves (5 kg) were obtained from a supplier after being identified by a plant botanist from Furley Marketing Sdn. Bhd, Malaysia. The entire P.s. extraction process was performed by Furley Marketing Sdn. Bhd, Malaysia. The freeze-drying process (Freeze Dryer, Labconco, Italy) was performed at the Biotechnology Science Faculty, Universiti Kebangsaan Malaysia. The powdered extract was kept in a dark bottle at 4°C until used. The freeze-dried P.s. was dissolved in normal saline and administered orally.
Animals and study design
A total of 24 female Sprague-Dawley rats (200-250 g) were obtained from the Laboratory Animal Resource Unit, University of Kebangsaan Malaysia. Prior ethical approval was obtained from the Institutional Animal Ethics Committee. The rats were housed individually in clean cages at a normal temperature with adequate ventilation, a normal 12-hour light-dark cycle, and free access to water and rat chow. They were acclimatized for 1 week before commencing the study. The rats were randomly allocated into sham-operated (SO) (n = 6) and ovariectomy-operated (OVX) groups (n = 18). The rats underwent a sham operation or a bilateral ovariectomy at the beginning of the study. Six weeks after the ovariectomy, structural histomorphometry was performed to confirm the development of osteoporosis in the rats.
The right femora of the rats were subjected to a closed fracture. All of the rats were anaesthetized using a combination of Xylazil and ketamine (1∶1) at an intramuscular dose of 0.1 ml/100 g (Troy Laboratories, Australia). A transverse incision was made. Using a standard medial parapatellar approach, the anterior intercondylar notch was appreciated. A 1.0 mm Kirschner wire (K-wire) (Jorgensen laboratories, USA) was inserted into the right femoral medullary canal until the canal was filled and the K-wire eventually emerged from the femoral trochanter. The ends of the wire were then cut. After the insertion and while the rats were still under anesthesia, a 500 g blunt guillotine-like blade device was released on the mid-diaphysis of the rat femur to generate a transverse mid-femoral closed fracture, as described in a previous protocol21 (Fig 1). Following the fracture, the soft tissues were re-approximated, and the incision was closed with 4/0 Serasynth and 2/0 Seralon sutures.
Figure 1.
A photograph of the guillotine fracture device.
Using an X-ray machine (Proteus XR/a, GE, UK), radiographic images were immediately obtained to confirm both the fracture and the intramedullary placement of the K-wire. The rats were then individually housed in clean cages and the beddings were changed weekly. The antibiotic Baytril (5%) (Bayer, Thailand) was administered intramuscularly at a dose of 10 mg/kg daily for 7 days, and a daily dressing with a povidone-iodine solution was applied to prevent wound infection. The next day, the post-fracture, ovariectomized rats were randomly divided into 3 groups: (i) the ovariectomized-control (OVXC) group (n = 6), which was supplemented with the normal saline vehicle; (ii) the ovariectomized + estrogen-replacement therapy (OVX+ERT) group (n = 6), which was supplemented with estrogen (100 µg/kg/day); and (iii) the ovariectomized + P.s. (OVX+P.s.) group (n = 6), which was supplemented with the water-based P.s. extract. The sham (SO) group (n = 6) was also supplemented with the normal saline vehicle. All of the rats received treatment for 6 weeks following the fracture. After the treatment was completed, the rats were sacrificed. The right femora were dissected from the hind limb, cleaned of soft tissues and wrapped with sterile gauze soaked in a PBS solution to keep the bone sample in a moist state and prevent it from drying. The femora were then wrapped with aluminum foil and stored at -70°C until they were used in the radiological assessment.
Treatment
Based on previous studies, the P.s. extract dose used in this study was 125 mg/kg/day.18,19 The extract was mixed with normal saline before being orally administered to the rats. The estrogen replacement therapy (ERT) consisted of estrogen (conjugated estrogen, Premarin, Wyeth, Canada) administered at a dose of 100 µg/kg/day. It is pertinent to mention that the ERT dose in this study was based on a previous study22 and we calculated the equivalent human dose of 0.625 mg/kg/day. The Premarin tablets were crushed and dissolved in normal saline to achieve the desired concentration. The treatment was administered by daily oral gavage for 6 weeks, beginning immediately after the right femur was fractured.
Radiological studies
X-rays
The frozen femora were thawed at room temperature for the radiological assessment. The X-rays were performed on the dissected femora in the anteroposterior and mediolateral planes using a high-resolution digital radiography system (Philips Digital Diagnostic/Optimus 80 system) at 46 kV, 2.5 mA and 10.6 ms exposure. All of the radiographic images were randomized and independently assessed by two radiologists who were unaware of the treatment. The fracture healing was scored using a modified 5-point radiographic scoring system based on an earlier study23 (Table 1). The fracture callus was scored using a modified 5-point radiographic scoring system according to a previous protocol24 (Table 2).
Table 1.
The fracture healing stages (modified Warden's stages), using a 5-point radiograph-based scoring system (23).
| Fracture healing stage | Score |
| No evidence of healingCallus formation evident, but fracture gap not bridgedCallus formation evident, with possible bridging of the fracture gapCallus formation evident, with good bridging of the fracture gapFracture union | 01234 |
Table 2.
The callus stages using a 5-point radiograph-based scoring system (24).
| Calls stage | Score |
| No callusVery minimal callusMinimal callusModerate callusExuberant callus | 01234 |
Computerized tomographic (CT) scan
The frozen femora were thawed at room temperature for the radiological assessment. After the x-rays were performed, the dissected right femora were scanned using a computer tomography system (Somatom Sensation 64, Germany) that produced a narrow fan beam by means of an X-ray tube (120 kV and 40 mAs). The CT scans were performed using a thickness of 0.6 mm, an in-plane voxel size of 0.234 mm, and a matrix size of 512×512 pixels. The scanner was calibrated using a water phantom with 0 Hounsfield Units (HU) and a density of 1.0 g/cm3. The axial callous volumes of the fractured bones were measured at 1.0 cm above and below the fracture site using the CT scan (Software Version Syngo CT 2006A). The images (1 mm image) for each bone group were imported from the compact disc into the Osyrinx program (using an Apple Macintosh computer) and selected for volume measurement. The callus was measured from an axial image for each 1.5-mm slice using the selected caliper, and the program calculated the total volume of the callus for each bone (Fig. 2). All of the femora were measured. All of the CT scans were assessed by two radiologists who were unaware of the treatment the rat had received.
Figure 2.
A CT scan of an unfractured area of a femur (A) and a fractured callused area of the femur (B).
Statistical analysis
The statistical analyses were performed using the SPSS statistical package version 17. Normally distributed data were presented as mean plus minus SEM. Non-normally distributed data were presented as median with range in parenthesis. The normality of all of the variables was examined using the Shapiro-Wilk test. A p>0.05 was considered significant. The normally distributed variables were analyzed using an ANOVA model followed by Tukey's post-hoc test. The non-normally distributed variables were analyzed using the Kruskal-Wallis test followed by the Mann-Whitney T test.
RESULTS
CT scan
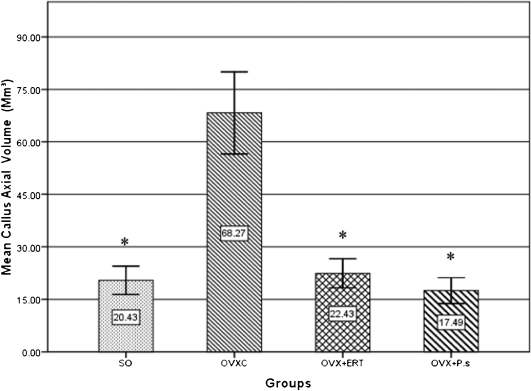
In the OVXC group, there was an abundance of callus, which was mainly composed of cartilage. In the SO, OVX+ERT and OVX+P.s. groups, the calluses were smaller, and the soft calluses had been replaced by woven bone, which was being remodeled into lamellar bone. The mean axial volume of the callus in the OVXC group was significantly greater than those of the SO, OVX+ERT and OVX+P.s. groups. The mean total callus volume was significantly lower in the OVX+P.s. group (17.5±3.68 mm3) than in the OVXC group (68.2±11.74 mm3). A higher callus volume indicates a delay in fracture healing. The callus volumes were similar in the SO and OVX+P.s. groups, indicating an improvement in the osteoporotic fracture healing (Fig 3).
Figure 3.
The mean callus axial volume after six weeks of treatment following the fracture of the right femora. *Significantly different compared to the OVXC group (p<0.05). Values are expressed as the mean ± SEM.
Radiographic findings
After the rats were sacrificed, the fractured right femora were almost healed, and the bony calluses exhibited greater remodeling in the SO, OVX+ERT and OVX+P.s. groups. In the OVXC group, the calluses were abundant, and soft tissues were still present within the calluses (Fig. 4). In the OVXC group at 6 weeks post-fracture, plain X-rays showed that the fracture line still existed and that the callus was abundant, suggesting a delay in fracture healing. In the OVX+ERT and OVX+P.s. groups, the fracture line was nearly undetectable, with good fracture bridging (Fig. 5). The fracture healing was identical in the SO and OVX+P.s. groups, suggesting an improvement in fracture healing. The median callus score of the OVXC group was significantly higher than those of the SO, OVX+ERT and OVX+P.s. groups (p<0.05) (Table 3). The median fracture-healing score of the OVXC group was significantly lower than the median scores of the SO, OVX+ERT and OVX+P.s. groups (p<0.05) (Table 4).
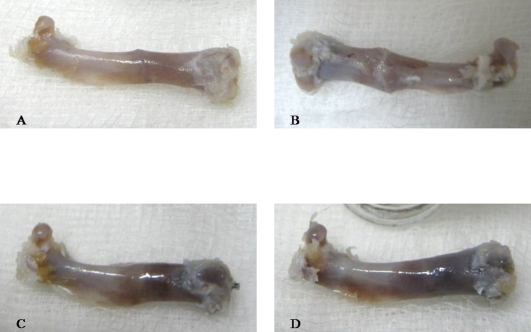
Figure 4.
Right femur samples harvested after sacrifice. Morphologically, the callus the OVXC sample (B) is large and mainly composed of soft callus. In the SO (A), OVX+ERT (C) and OVX+ P.s. (D) femora, the callus sizes were similar and mainly composed of mature bone.
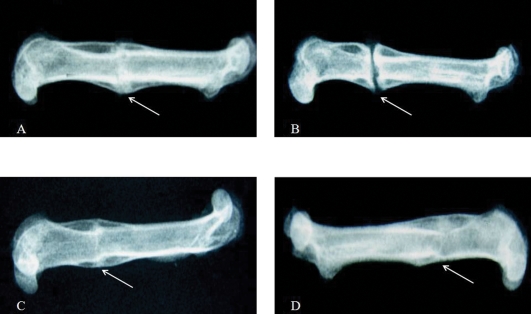
Figure 5.
Plain radiographic images after sacrifice. The OVXC (B) femur shows an existing fracture line with delayed fracture healing. In the SO (A), OVX+ERT (C), and OVX+P.s. (D) femora, the fracture lines were nearly undetectable, with good fracture bridging. The arrows indicate the fracture area.
Table 3.
The callus stages based on the radiographic assessment. The values are expressed as the median with the range in parentheses.
*Significant difference compared to the OVXC group (p<0.05).
Table 4.
The fracture healing stages based on the radiographic assessment. The values are expressed as the median with the range in parentheses.
| Group | Fracture Healing Stage |
| Sham (SO)OVXCOVX + ERTOVX + P.s. | 3.0 (3, 4)*2.0 (1, 2)3.0 (2, 3)*3.0 (2, 3)* |
*Significant difference compared to the OVXC group (p<0.05).
DISCUSSION
In normal rats, a closed fracture is thought to heal within 6 weeks.25 Fracture healing can be divided into four stages: the first and the second weeks, which involve the formation of granulation tissue; the third and fourth weeks, which involve the formation of the soft callus that will then be replaced by woven bone (hard callus) through endochondral ossification; and the fifth and sixth weeks, which involve the remodeling stage.26
The mean callus axial volume and the median callus score of the SO, OVX+ERT and OVX+P.s. groups were significantly lower than those of the OVXC group (p<0.05). The ovariectomized rats treated with the P.s. extract had a significantly lower mean callus axial volume and median callus score than the OVXC group (p<0.05). These results were in agreement with an earlier study that observed, through radiographic examinations, larger callus sizes in their ovariectomized-control group than in their sham group.5
The reduced callus volume and callus score after treatment with P.s. indicates improved callus maturity and restoration of the pre-fracture properties in an estrogen-deficient state. Treatment with P.s. reduces the level of ROS at the fracture site, which may prevent oxidative stress. The lower callus axial volume and callus score of the OVX+P.s. group indicate that the calluses were nearly mature and that the bone was at the advanced stage of healing in which the callus was undergoing reshaping to restore its pre-fracture properties. An earlier study observed that normal rats treated with saline had a lower mature-callus volume compared to normal rats treated with parathyroid hormone (PTH) at 5 weeks post-fracture.23 However, there were no significant differences in the total callus axial volume between the two groups. P.s. extract contains a natural antioxidant (naringenin), which belongs to the flavonoid family and may have a role in accelerating fracture healing.13 Research reports have suggested that flavonoids react with free radicals to produce stable or non-reactive compounds.17 It has been observed that flavonoids inhibit the activity of the enzymes involved in the oxidation of unsaturated fatty acids, thereby preventing oxidative stress at the fracture callus.27
The callus axial volume and callus score in the OVX+ERT group were significantly lower than those of the OVXC group (p<0.05). ERT was as effective as P.s. treatment in inducing the formation of a mature callus in the estrogen-deficient state. ERT improves the quality of the callus by inducing the mineralization of the soft callus and increasing the BMD of neoformed woven bone (the hard callus), leading to the formation of a mature callus. It has been reported that estrogen administration improves the early stages of fracture healing in ovariectomized rats.28 Postmenopausal ERT prevents bone loss and reduces fracture risk.29,30 Estrogen acts mainly by inhibiting osteoclast bone resorption and by preventing the overproduction of osteoclastogenesis-associated cytokines following the postmenopausal estrogen drop.31
The callus axial volume and callus score in the OVXC group were higher than those of the SO, OVX+ERT and OVX+P.s. groups (p<0.05). A larger total callus volume indicates that the callus is extensively composed of cartilage. An estrogen-deficient state results in decreased callus maturity, which suggests that the repairing phase was still on-going in the OVXC group. Estrogen loss delays the process of mineralization or endochondral ossification of the soft callus in the osteoporotic fractured femur. In osteoporosis following estrogen deficiency, the activity of osteoclasts increases as a result of the overproduction of ROS.20 This finding is consistent with a previous study that reported that the total callus volume in an ovariectomized-control group was higher than that of the control and treated groups at 8 weeks post-fracture.24 The callus quality was lower in the OVXC group due to the decreased BMD and reduced mineralization of the callus. The amount of cartilage in the calluses of the OVXC group was much greater than that of formed woven bone, which results in an immature callus. An earlier study has observed that the callus axial volume was lower in an ovariectomized-control group than in an ovariectomized + vitamin D3 group at 6 weeks post-fracture.32 This finding suggests that callus formation in the ovariectomized-control group was delayed and still in the early phase of repair, while the calluses of the ovariectomized + vitamin D3 group were in the early phases of remodeling. The findings of our study are inconsistent with those of that study due to a number of factors, such as the different species of rats used and the different fracture method.32 The increase in the callus axial volumes and callus scores in the OVXC group indicates that the fracture-healing process was in the middle of the repairing phase and that the callus was still immature.
The median fracture-healing score (Warden's grading) of the OVXC group was significantly lower than those of the SO, OVX+ERT and OVX+P.s. groups (p<0.05). Treatment with the P.s. extract resulted in higher fracture-healing scores compared to the OVXC group. The higher fracture-healing scores indicated better bridging of the fracture because the fracture line in the P.s. group was not detectable. The invisible fracture line in the P.s. group suggested that the hard callus (woven bone) was already formed and had totally replaced the cartilage (soft callus) through endochondral ossification. It has been reported that antioxidant supplementation may prevent bone loss and accelerate fracture healing in osteoporotic patients.20 The antioxidative activity of the P.s. extract, which occurs through its free radical scavenging activity, may have prevented oxidative stress at the fracture site, thereby accelerating the fracture bridging.
The ovariectomized rats treated with ERT showed an increase in their fracture-healing scores compared to those of the OVXC group (p<0.05). ERT accelerates fracture gap bridging and decreases fracture healing time. The treatment enhances the replacement of the soft callus with the hard callus by improving the mineralization and BMD of the neoformed woven bone. A few studies have observed that estrogen loss leads to decreased BMD in the callus and to the appearance of osteoporotic changes in the neoformed bony callus.5,33 A study performed on rabbits showed the appearance of osteoporotic changes in the callus of an ovariectomized-control group compared to a sham group at 4 weeks post-fracture.34 Hence, estrogen replacement is essential for improving the quality of the healed bone and for shortening healing time.
The untreated ovariectomized rats had a lower fracture healing score than those in the control and ovariectomized-treated groups (p<0.05). In contrast to the other groups, the fracture line was still detectable in the OVXC group. This finding suggests that estrogen loss delays the bridging and healing of an osteoporotic fractured femur. Estrogen deprivation leads to a delay in mineralization and a decrease in BMD during fracture healing. Cartilage is still predominant within the callus, and the replacement of the soft callus by the neoformed woven bone occurs slowly. The estrogen-deficient state is associated with the over-production of ROS, which in turn induce the production of cytokines involved in osteoclastogenesis.8 Osteoclastogenesis results in increased bone loss and the deterioration of fracture healing. These findings are similar to the findings of an earlier study that observed that the fracture gap was still clearly visible in an ovariectomized-control group compared to an ovariectomized + vitamin D3 group at 6 weeks post-fracture.32 These findings were also consistent with an earlier study that reported that fracture healing was improved in the sham and ovariectomized + calcium groups compared to the ovariectomized-control group at 8 weeks post-fracture.24 A previous study involving rabbits reported that callus maturity decreased with the appearance of osteoporotic changes in the ovariectomized-control group compared to the sham group.34 We believe that the osteoporotic fractured femur had a longer healing time because mineralization and replacement of the soft callus by the hard callus were delayed.
Bone fractures may form a union even in an estrogen-deficient state, but the quality of the healed bone is poor. The estrogen-deficient state affects fracture healing by delaying callus mineralization, inducing osteoporotic changes and increasing healing time and by decreasing callus maturity. In this study, treatment with the P.s. extract was beneficial to osteoporotic fracture healing, as assessed by quantitative and qualitative radiological analyses. The P.s. treatment decreased the callus axial volume and the callus score and increased the fracture healing score in the estrogen-deficient rats. Treatment with P.s. may improve fracture healing by preventing oxidative stress at the fracture site and by increasing mineralization and BMD of the callus, which results in the formation of a mature callus.
CONCLUSION
In conclusion, treatment with a P.s. extract has a beneficial effect on osteoporotic fracture healing, as shown by the quantitative and qualitative radiological findings. In estrogen-deficient rats, treatment with P.s. enhances callus maturity by decreasing the callus axial volume and the callus score and by increasing the fracture healing score. Further studies should be performed to explore the details of the healing process.
ACKNOWLEDGMENT
The authors acknowledge the Universiti Kebangsaan Malaysia for providing the financial assistance needed to conduct this study. The authors also acknowledge the kind help received from Prof. Dr. Baharudin Omar and Assoc. Prof. Zahiah Muhamed.
REFERENCES
- 1.Schuit SCE, van der Klift M, Weel AEAM, de Laet CEDH, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202. doi: 10.1016/j.bone.2003.10.001. 10.1016/j.bone.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Melton Iii LJ. Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res. 2003;18:1139–41. doi: 10.1359/jbmr.2003.18.6.1139. 10.1359/jbmr.2003.18.6.1139 [DOI] [PubMed] [Google Scholar]
- 3.Frost HM, Jee WSS. On the rat model of human osteopenias and osteoporoses. Bone Miner. 1992;18:227–36. doi: 10.1016/0169-6009(92)90809-r. 10.1016/0169-6009(92)90809-R [DOI] [PubMed] [Google Scholar]
- 4.Sartori AR, Moreira JA, Santos AMM, Cintra DEC, Sartori LR, Barauna MA, et al. Bone repair process in normal and osteopenic female rats' tibiae: a comparative study. Acta Ortop Bras. 2008;16:37–40. 10.1590/S1413-78522008000100007 [Google Scholar]
- 5.Kubo T, Shiga T, Hashimoto J, Yoshioka M, Honjo H, Urabe M, et al. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol Biol. 1999;68:197–202. doi: 10.1016/s0960-0760(99)00032-1. 10.1016/S0960-0760(99)00032-1 [DOI] [PubMed] [Google Scholar]
- 6.Riggs BL, Khosla S, Melton Iii LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. 10.1210/er.23.3.279 [DOI] [PubMed] [Google Scholar]
- 7.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. 10.1080/10715769900300851 [DOI] [PubMed] [Google Scholar]
- 8.Parhami F. Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor. Prostaglandins Leukot Essent Fatty Acids. 2003;68:373–8. doi: 10.1016/s0952-3278(03)00061-9. 10.1016/S0952-3278(03)00061-9 [DOI] [PubMed] [Google Scholar]
- 9.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Investig. 2003;112:915–23. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, et al. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360:81–6. doi: 10.1016/j.cccn.2005.04.014. 10.1016/j.cccn.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 11.Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005;146:728–35. doi: 10.1210/en.2004-1021. 10.1210/en.2004-1021 [DOI] [PubMed] [Google Scholar]
- 12.Aziz Z, Tey NP. Herbal medicines: Prevalence and predictors of use among Malaysian adults. Compl Ther Med. 2009;17:44–50. doi: 10.1016/j.ctim.2008.04.008. 10.1016/j.ctim.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 13.Subramaniam V, Adenan MI, Ahmad AR, Sahdan R. Natural antioxidants: Piper sarmentosum (Kadok) and Morinda elliptica (Mengkudu) Mal J Nutr. 2003;9:41–51. [PubMed] [Google Scholar]
- 14.Rukachaisirikul T, Siriwattanakit P, Sukcharoenphol K, Wongvein C, Ruttanaweang P, Wongwattanavuch P, et al. Chemical constituents and bioactivity of Piper sarmentosum. J Ethnopharmacol. 2004;93:173–6. doi: 10.1016/j.jep.2004.01.022. 10.1016/j.jep.2004.01.022 [DOI] [PubMed] [Google Scholar]
- 15.Seyoum A, Asres K, El-Fiky FK. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry. 2006;67:2058–70. doi: 10.1016/j.phytochem.2006.07.002. 10.1016/j.phytochem.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Horcajada MN, Habauzit V, Trzeciakiewicz A, Morand C, Gil-Izquierdo A, Mardon J, et al. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J Appl Physiol. 2008;104:648–54. doi: 10.1152/japplphysiol.00441.2007. 10.1152/japplphysiol.00441.2007 [DOI] [PubMed] [Google Scholar]
- 17.Van Acker FA, Schouten O, Haenen GR, Van der Vijgh WJ, Bast A. Flavonoids can replace alpha-tocopherol as an antioxidant. FEBS letters. 2000;473:145–8. doi: 10.1016/s0014-5793(00)01517-9. 10.1016/S0014-5793(00)01517-9 [DOI] [PubMed] [Google Scholar]
- 18.Peungvicha P. Hypoglycemic effect of the water extract of Piper sarmentosum in rats. J Ethnopharmacol. 1998;60:27–32. doi: 10.1016/s0378-8741(97)00127-x. 10.1016/S0378-8741(97)00127-X [DOI] [PubMed] [Google Scholar]
- 19.Ima-Nirwana S, Elvy-Suhana MR, Faizah O, Farihah S. Effects of Piper sarmentosum on bone resorption and its relationship to plasma cortisol in rats. Bone. 2009;44:S79–S80. 10.1016/j.bone.2009.01.177 [Google Scholar]
- 20.Sheweita SA, Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Current Drug Metabol. 2007;8:519–25. doi: 10.2174/138920007780866852. 10.2174/138920007780866852 [DOI] [PubMed] [Google Scholar]
- 21.Vialle E, Vialle LR, Boechat R, Bley JP, Scussiato R, Busato T, et al. Producao de fratura padronizada de femur em ratos. Rev Bras Ortop. 2004;39:323–9. [Google Scholar]
- 22.Hayward MA, Kharode YP, Becci MM, Kowal D. The effect of conjugated equine estrogens on ovariectomy-induced osteopenia in the rat. J Inflamm Res. 1990;31:152–6. doi: 10.1007/BF02003236. [DOI] [PubMed] [Google Scholar]
- 23.Warden SJ, Komatsu DE, Rydberg J, Bond JL, Hassett SM. Recombinant human parathyroid hormone (PTH 1-34) and low-intensity pulsed ultrasound have contrasting additive effects during fracture healing. Bone. 2009;44:485–94. doi: 10.1016/j.bone.2008.11.007. 10.1016/j.bone.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 24.Shuid AN, Mohamad S, Mohamed N, Fadzilah FM, Mokhtar SA, Abdullah S, et al. Effects of calcium supplements on fracture healing in a rat osteoporotic model. J Orthop Res. 2010;28:1651–6. doi: 10.1002/jor.21180. 10.1002/jor.21180 [DOI] [PubMed] [Google Scholar]
- 25.Tagil M, McDonald MM, Morse A, Peacock L, Mikulec K, Amanat N, et al. Intermittent PTH ((1-34)) does not increase union rates in open rat femoral fractures and exhibits attenuated anabolic effects compared to closed fractures. Bone. 2009;46:852–9. doi: 10.1016/j.bone.2009.11.009. 10.1016/j.bone.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 26.Udupa KN, Prasad GC. Chemical and histochemical studies on the organic constituents in fracture repair in rats. J Bone Joint Surg Br. 1963;45:770–9. [PubMed] [Google Scholar]
- 27.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–12. doi: 10.1021/jf000892m. 10.1021/jf000892m [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Xu K, Qiao L. Effects of estrogen on the expression of TGF-β in early fracture healing of ovariectomized rats. Bone. 2008;43:S54–S5. 10.1016/j.bone.2008.08.048 [Google Scholar]
- 29.Ribot C, Trémollières F. Hormone replacement therapy in the management of postmenopausal osteoporosis and prevention of fracture risk. Presse médicale (Paris, France: 1983) 2006;35:1557–63. doi: 10.1016/s0755-4982(06)74851-5. 10.1016/S0755-4982(06)74851-5 [DOI] [PubMed] [Google Scholar]
- 30.Compston J. How to manage osteoporosis after the menopause. Best Pract Res Clin Rheumatol. 2005;19:1007–19. doi: 10.1016/j.berh.2005.06.010. 10.1016/j.berh.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 31.Chen FP, Wang KC, Huang JD. Effect of estrogen on the activity and growth of human osteoclasts in vitro. Taiwan J Obstet Gynecol. 2009;48:350–5. doi: 10.1016/S1028-4559(09)60323-5. 10.1016/S1028-4559(09)60323-5 [DOI] [PubMed] [Google Scholar]
- 32.Fu L, Tang T, Miao Y, Hao Y, Dai K. Effect of 1, 25-dihydroxy vitamin D3 on fracture healing and bone remodeling in ovariectomized rat femora. Bone. 2009;44:893–8. doi: 10.1016/j.bone.2009.01.378. 10.1016/j.bone.2009.01.378 [DOI] [PubMed] [Google Scholar]
- 33.Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, et al. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–6. doi: 10.1016/s8756-3282(00)00414-2. 10.1016/S8756-3282(00)00414-2 [DOI] [PubMed] [Google Scholar]
- 34.Arslan H, Ketani A, Gezici A, Kapukaya A, Necmioglu S, Kesemenli C, et al. The effects of osteoporosis on distraction osteogenesis: an experimental study in an ovariectomised rabbit model. Acta Orthop Belg. 2003;69:67–73. [PubMed] [Google Scholar]