Abstract
Fracture healing and distraction osteogenesis have important applications in orthopedic, maxillofacial, and periodontal treatment. In this review, the cellular and molecular mechanisms that regulate fracture repair are contrasted with bone regeneration that occurs during distraction osteogenesis. While both processes have many common features, unique differences are observed in the temporal appearance and expression of specific molecular factors that regulate each. The relative importance of inflammatory cytokines in normal and diabetic healing, the transforming growth factor beta superfamily of bone morphogenetic mediators, and the process of angiogenesis are discussed as they relate to bone repair. A complete summary of biological activities and functions of various bioactive factors may be found at COPE (Cytokines & Cells Online Pathfinder Encyclopedia), http://www.copewithcytokines.de/cope.cgi.
Keywords: Fracture healing, distraction osteogenesis, morphogens, cytokines
Introduction
Bone has a substantial capacity for repair and regeneration in response to injury or surgical treatment. Both processes involve a complex integration of cells, growth factors, and the extracellular matrix. Repair simply restores the continuity of the injured tissues, without necessarily increasing bone volume. Regeneration, in contrast, involves the differentiation of new cells and the formation of new bone tissue that results in an overall increase in volume of new skeletal tissues.
Fracture healing is a multistage repair process that involves complex yet well-orchestrated steps that are initiated in response to injury, resulting eventually in the repair and restoration of function. Distraction osteogenesis, in contrast, is a controlled surgical procedure that initiates a regenerative process and uses mechanical strain to enhance the biological responses of the injured tissues to create new bone. This surgical model is used to bridge gap defects such as non-healing fractures, to treat disease conditions such as osteomyelitis, where there has been a net destruction of the bone tissues, to augment the alveolar bone around lost teeth, and to correct congenital skeletal deformities where there is a deficiency in the original structure of the skeleton (Tay et al., 1998). Both biological processes are controlled by complex molecular mechanisms that involve local and systemic factors that interact with many cell types that are recruited to the injury or surgical site from the surrounding tissues and circulation. Ongoing research has improved our understanding of the molecular mechanisms involved with fracture healing and distraction osteogenesis. The aim of this review is to characterize the cellular events that contribute to the healing process and to describe the complexity of signaling pathways and molecules involved. Fracture healing and distraction osteogenesis are presented to contrast two different mechanisms of bone repair and regeneration.
Fracture Healing
Fracture repair recapitulates the pathway of normal embryonic development with the coordinated participation of several cell types originating from the cortex, periosteum, surrounding soft tissue, and bone marrow space (Ferguson et al., 1999; Gerstenfeld et al., 2003b). The cellular and molecular processes of fracture healing are summarized in Tables 1 and 2.
Table 1. Summary of the Multiple Stages of Fracture Healing and the Accompanying Expression of Signaling Molecules (based on published results from Kon et al., 2001; Cho et al., 2002; Gerstenfeld et al., 2003b; Dimitriou et al., 2005).
| Stage of Fracture Repair | Biological Processes | Expression of Signaling Molecules and their Proposed Functions |
|---|---|---|
| Inflammation | Hematoma | IL-1, IL-6, and TNF-α play a role in initiating the repair cascade. |
| Inflammation | TGF-β, PDGF, and BMP-2 expression increases to initiate callus formation. | |
| Recruitment of mesenchymal stem cells | GDF-8 is restricted to day 1, suggesting its role in controlling cellular proliferation. | |
|
| ||
| Cartilage Formation and Periosteal Response | Chondrogenesis and endochondral ossification begins | TGF-β2, -β3, and GDF-5 peak due to their involvement in chondrogenesis and endochondral bone formation. |
| Cell proliferation in intramembranous ossification | BMP-5 and -6 rise. | |
| Vascular in-growth | Angiopoietins and VEGFs are induced to stimulate vascular in growth from vessels in the periosteum. | |
| Neo-angiogenesis | ||
|
| ||
| Cartilage Resorption and Primary Bone Formation | Phase of most active osteogenesis | TNF-α rises in association with mineralized cartilage resorption. This promotes the recruitment of mesenchymal stem cells and induces apoptosis of hypertrophic chondrocytes. |
| Bone cell recruitment and woven bone formation | RANKL and MCSF rise in association with mineralized cartilage resorption. | |
| Chondrocyte apoptosis and matrix proteolysis | ||
| Osteoclast recruitment and cartilage resorption | BMP-3, -4, -7, and -8 rise in association with the resorption of calcified cartilage. They promote recruitment of cells in the osteoblastic lineage. | |
| Neo-angiogenesis | BMP-5 and -6 remain high during this stage, suggesting a regulatory effect on both intramembranous and endochondral ossification. | |
| VEGFs are up-regulated to stimulate neo-angiogenesis. | ||
|
| ||
| Secondary Bone Formation and Remodeling | Bone remodeling coupled with osteoblast activity | IL-1 and IL-6 rise again in association with bone remodeling, whereas RANKL and MCSF display diminished levels. |
| Establishment of marrow | Diminished expression of members of the TGF-β superfamily. | |
Table 2. Summary of the Stages* of Fracture Repair and Their Associated Molecular Regulators.
| Signaling Molecules | Inflammation | Cartilage Formation and Periosteal Response | Cartilage Resorption and Primary Bone Formation | Secondary Bone Formation and Remodeling |
|---|---|---|---|---|
| Cytokines | ||||
| IL-1 | ↑↑↑ | ↑↑ | ||
| IL-6 | ↑↑↑ | |||
| TNF-α | ↑↑↑ | ↑↑ | ↑↑ | |
| RANKL | ↑↑↑ | ↑↑↑ | ↑↑ | |
| OPG | ↑↑↑ | ↑↑↑ | ||
| MCSF | ↑↑↑ | ↑↑↑ | ||
|
| ||||
| TGF-β Superfamily | ||||
| BMP-2 | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| BMP-3 | ↑ | ↑↑↑ | ↑ | |
| BMP-4 | ↑ | ↑↑↑ | ↑↑↑ | ↑ |
| BMP-5 | ↑ | ↑↑↑ | ↑↑↑ | ↑ |
| BMP-6 | ↑ | ↑↑↑ | ↑↑↑ | ↑ |
| BMP-7 | ↑↑↑ | |||
| BMP-8 | ↑↑↑ | |||
| TGF- 2 | ↑↑↑ | |||
| TGF- 3 | ↑↑↑ | ↑↑ | ||
| GDF-5 | ↑↑↑ | |||
| GDF-8 | ↑↑↑ | |||
| GDF-10 | ↑↑↑ | ↑↑↑ | ↑↑↑ | |
|
| ||||
| Angiogenic Factors | ||||
| VEGF A | ↑ | ↑↑ | ||
| VEGF B | ↑↑ | |||
| VEGF C | ↑↑ | |||
| VEGF D | ↑↑ | ↑ | ||
| Angiopoietin 1 | ↑↑ | ↑↑ | ↑ | ↑ |
| Angiopoietin 2 | ↑ | ↑ | ↑ | ↑ |
The different stages of the fracture-healing process are separated by dashed lines, since they overlap. The relative expressions of the different signaling molecules are denoted by arrows, indicating intensity of expression. Based on published results from Kon et al., 2001; Cho et al., 2002; Gerstenfeld et al., 2003b; Lehmann et al., 2005; Dimitriou et al., 2005.
The majority of fractures heal by the combination of both intramembranous and endochondral ossification. Endochondral bone formation usually occurs external to the periosteum in regions that are mechanically less stable and immediately adjacent to the fracture site, whereas intramembranous ossification occurs internal to the periosteum at the proximal and distal edges of the callus and forms hard callus (Dimitriou et al., 2005). It is the eventual bridging of hard callus areas across the central fracture gap that provides the initial stabilization and regaining of biomechanical function (Gerstenfeld et al., 2006). The repair process itself is comprised of four overlapping phases, initiated by an immediate inflammatory response that leads to the recruitment of mesenchymal stem cells and subsequent differentiation into chondrocytes that produce cartilage and osteoblasts, which form bone. After cartilage matrix is produced, it mineralizes, and a transition from mineralized cartilage to bone occurs, initiated by the resorption of mineralized cartilage. Primary bone formation is followed by remodeling, in which the initial bony callus is reshaped by secondary bone formation and resorption to restore the anatomical structure that supports mechanical loads (Gerstenfeld et al., 2003b). The biological processes taking place during each of these stages are tightly regulated by signaling molecules that can be categorized into three groups: (1) pro-inflammatory cytokines, (2) transforming growth factor-beta superfamily (TGF-β) members, and (3) angiogenic factors. Each of these groups of cytokines and morphogens has biological activities that promote overlapping biological processes and orchestrate interactions between differing cell populations. As an example, mesenchymal stem cells differentiate into both more specialized cells that make up the effects of each other's activities (Peng et al., 2005).
The Role of Pro-inflammatory Cytokines in Fracture Repair
Inflammatory cytokines involved in fracture repair are believed to play a role in initiating the repair cascade following injury. These cytokines are produced and function immediately after injury for a limited time period. At a mid-stage in healing, some of the inflammatory cytokines are up-regulated, so osteoclastogenesis is stimulated to remove mineralized cartilage, and others are induced at a later stage during bone remodeling.
Interleukins-1 and -6 (IL-1 and IL-6) and TNF-α have been shown to play a role in initiating the repair cascade. They induce a downstream response to injury by recruiting other inflammatory cells, enhancing extracellular matrix synthesis, and stimulating angiogenesis (Kon et al., 2001). They are secreted at the injury site by macrophages, inflammatory cells, and cells of mesenchymal origin. Their expression peaks within the first 24 hrs, then declines rapidly to nearly undetectable levels by day 3 (Kon et al., 2001; Cho et al., 2002). The expression of IL-1 and IL-6 rises again in association with bone remodeling during secondary bone formation, whereas the expression of TNF-α rises in association with mineralized cartilage resorption by the end of the endochondral phase of fracture repair (Gerstenfeld et al., 2003a). In addition to stimulating osteoclast function, TNF-α promotes the recruitment of mesenchymal stem cells and induces apoptosis of hypertrophic chondrocytes during endochondral bone formation. Its absence delays the resorption of mineralized cartilage and, consequently, prevents the formation of new bone. In situations where TNF-α is over-expressed, such as diabetic healing, there is premature cartilage removal that is associated with deficient bone formation and healing (Kayal et al., 2007).
The expression of RANKL and OPG (two members of the TNF-α superfamily), as well as macrophage colony-stimulating factor (MCSF), which are key regulatory factors in osteoclastogenesis, increases after initial injury as well as during the period of mineralized cartilage resorption. During the phase of secondary bone formation and bone remodeling, RANKL, OPG, and MCSF showed expression levels diminished from those seen during cartilage resorption. In contrast, IL-1 and IL-6 expression rose during late remodeling (Gerstenfeld et al., 2003b).
Pro-inflammatory Cytokines and Impaired Diabetic Fracture Healing
Diabetes causes diminished bone formation and increases the risk of fracture (Verhaeghe et al., 1990; Vestergaard, 2007). Moreover, fracture healing is impaired in diabetic humans and in animal models (Loder, 1988; Macey et al., 1989). There are likely to be multiple mechanisms through which diabetes may affect bone, including the expression of genes that regulate osteoblast differentiation and the expression of growth factors that promote bone formation (Kawaguchi et al., 1994; Lu et al., 2003). To gain insight into how diabetes affects fracture healing, investigators have carried out experiments in a type 1 diabetic model, focusing on the impact of diabetes on the transition from cartilage to bone (Kayal et al., 2007). There was relatively little difference in the initial amount of callus formed. However, diabetes caused an increase in mRNA levels of TNF-α, MCSF, and RANKL. The increase in these cytokines was accompanied by a similar increase in osteoclast numbers and a more rapid degradation of cartilage. The greater loss of cartilage also coincided with increased mRNA levels of ADAMTS 4 and 5 and aggrecanases that degrade cartilage (Kayal et al., 2007). The accelerated loss of cartilage may be physiologically significant, since it may leave a reduced template for endochondral bone formation. This may explain a decrease in callus size and a decrease in the strength of healing bone that is typically found in diabetic fracture healing (Funk et al., 2000; Beam et al., 2002). It is striking that the more rapid removal of cartilage, greater osteoclast formation, and enhanced expression of pro-inflammatory cytokines are the opposite of that observed in TNF receptor-deficient mice (Gerstenfeld et al., 2003a). Thus, in diabetes, high levels of TNF-α and other pro-inflammatory cytokines may increase osteoclastogenesis, which leads to excessive removal of cartilage, which in turn may interfere with the transition from cartilage to bone and impair fracture healing. In contrast, TNF receptor-deficient mice exhibit delayed osteoclast formation, failure to remove cartilage in timely fashion, and a longer time required for healing of fractured bone.
The Role of the Transforming Growth Factor Beta Superfamily in Fracture Repair
The transforming growth factor-beta (TGF-β) superfamily consists of a large number of growth and differentiation factors that include bone morphogenetic proteins (BMPs), transforming growth factor beta (TGF-β), growth differentiation factors (GDFs), activins, inhibins, and Müllerian inhibiting substance. Specific members of this family—such as BMPs (2-8), GDF (1, 5, 8, and 10), and TGF-β1-3—promote various stages of intramembranous and endochondral bone ossification during fracture healing (Cho et al., 2002).
Bone Morphogenetic Proteins
During fracture repair, BMPs are produced by mesenchymal cells, osteoblasts, and chondrocytes. Different BMPs function independently of or in collaboration with each other, as well as with other members of the TGF-β superfamily, to trigger a cascade of events that promote the formation of cartilage and bone. Cellular processes stimulated include chemotaxis, mesenchymal cell proliferation and differentiation, angiogenesis, and synthesis of extracellular matrix (Sakou, 1998; Reddi, 2001). Although different BMPs are closely related structurally and functionally, they exhibit different temporal patterns of expression at different stages of fracture healing, based on several animal experiments. In studies of murine fracture healing, BMP-2 mRNA expression showed maximal levels within 24 hrs of injury, suggesting that this BMP plays a role in initiating the repair cascade. Consistent with this finding are recent studies showing that BMP-2 is necessary for post-natal bone repair and is genetically associated with the maintenance of normal bone mass (Tsuji et al., 2006; Xiong et al., 2006). In contrast, BMP-2 is apparently not needed for embryological bone formation (Tsuji et al., 2006; Xiong et al., 2006). Other in vitro studies examining marrow stromal stem cell differentiation have shown that BMP-2 controls the expression of several other BMPs, and when its activity is blocked, marrow stromal stem cells fail to differentiate into osteoblasts (Edgar et al., 2007).
BMP- 3, -4, -7, and -8 show a restricted period of expression during fracture healing (days 14 through 21), when the resorption of calcified cartilage and osteoblastic recruitment are most active, and coupled bone formation takes place. BMP-5 and -6 and other members of the TGF-β superfamily are constitutively expressed from days 3-21 during fracture in mice, suggesting that they have a regulatory effect on both intramembranous and endochondral ossification (Cho et al., 2002).
It has been proposed that BMP-2, -6, and -9 may be the most potent inducers of mesenchymal cell differentiation to osteoblasts, while the remaining BMPs promote the maturation of committed osteoblasts (Cheng et al., 2003). BMP antagonists also play an important role in fracture repair. It has been reported that the expression of noggin, which blocks BMP-2, -4, and-7, is modulated during fracture healing (Yoshimura et al., 2001). The pattern of noggin expression is similar to that of BMP-4, suggesting that the noggin/BMP-4 balance could be an important factor in the regulation of callus formation during fracture healing. This is supported by findings that, in the absence of noggin, there is excess bone and cartilage formation during development, indicating that noggin plays an important role in limiting the formation of these tissues (Brunet et al., 1998).
Transforming Growth Factor Beta
All three isoforms of this group of morphogens are involved in fracture repair (TGF-β1-3). They are produced by degranulated platelets after initial injury, which suggests their involvement in the initiation of callus formation (Bolander, 1992; Bostrom, 1998). They are also produced by osteoblasts and chondrocytes at later stages, which enhances the proliferation of these cells as well as that of mesenchymal cells and pre-osteoblasts (Lieberman et al., 2002). TGF-β is thought to play an important role in chondrogenesis and endochondral bone formation (Barnes et al., 1999). It induces the expression of extracellular matrix proteins (Sandberg et al., 1993). The expression of TGF-β2 and TGF-β3 peaks on day 7 post-fracture in the mouse, when type II collagen expression rises, and appears to be associated with cartilage formation. The expression of TGF-β1 remains constant throughout the fracture-healing process. This suggests that TGF-β2 and TGF-β3 may play a more important role during fracture healing, since their expression peaks during the critical phase of chondrogenesis (Cho et al., 2002).
The Role of Angiogenic Factors in Fracture Repair
Optimal bone healing is dependent on adequate vascularization and therefore requires the development of new blood vessels. During endochondral fracture healing, the transition from a cartilaginous callus to new bone formation represents a crucial stage in the repair process. This stage includes four coordinated biological events: chondrocyte apoptosis; cartilaginous matrix degradation and removal; vascularization of the repair site; and osteogenic cell recruitment, differentiation, and bone matrix production. Disruption of any one of these can lead to delayed or impaired healing (Vu et al., 1998; Gerber et al., 1999; Aizawa et al., 2001; Colnot et al., 2003). In other biological processes, such as growth plate development, disruption of any of these events has been shown to interfere with the formation of skeletal bone. For example, disruption of cartilaginous matrix degradation through the loss of MMP-9 expression leads to a massive expansion of the hypertrophic zone (Vu et al., 1998), a consequence of abnormal regulation of the apoptotic process. Moreover, in mmp-9-/- mice, a delay is observed in the progression of fracture repair, and this effect can be rescued by the addition of exogenous VEGF (Colnot et al., 2003), suggesting that angiogenesis is linked to the apoptosis of chondrocytes.
The above observations have led to the suggestion that the molecular regulation of angiogenesis is linked with the removal of cartilage during endochondral bone formation. The interdependence of the various biologic processes in fracture healing was clearly demonstrated in data from our laboratory, which showed that lack of TNF-α signaling delays chondrocyte apoptosis, which then leads to delays in the resorption of mineralized cartilage and, ultimately, a delay in fracture healing (Gerstenfeld et al., 2003a; Lehmann et al., 2005).
Angiogenesis is regulated by 2 pathways, a vascular endothelial growth factor (VEGF)-dependent pathway and an angiopoietin-dependent pathway (Suri et al., 1996). Both pathways are speculated to be functional during fracture repair. The VEGF-related family of proteins includes endothelial cell mitogens and essential mediators of neo-angiogenesis (Ferrara and Davis-Smyth, 1997). It has been demonstrated that VEGF signaling plays a central role in neo-angiogenesis and in endochondral bone formation (Gerber et al., 1999; Street et al., 2002). Furthermore, fracture repair is enhanced by exogenous VEGF (Gerber et al., 1999; Street et al., 2002). Osteoblasts are known to express elevated amounts of VEGF, and therefore have been implicated as primary regulators of angiogenesis in fracture healing. Moreover, several studies have shown that BMPs stimulate the expression of VEGF and their receptors, suggesting an intimate relationship between these two families that promotes the formation of new bone (Yeh and Lee, 1999; Deckers et al., 2002).
A second pathway that regulates vascular growth includes angiopoietin-1 and -2 and their receptors. Angiopoietins are vascular morphogenetic proteins that are associated with the formation of larger vessels and the development of collateral branches from existing vessels. The role of angiopoietin in fracture repair is not as well-understood as the VEGF pathway. The expression of angiopoietin-1 is induced during the initial stages of fracture repair, suggesting that initial vascular in-growth from vessels in the periosteum plays an important role in the repair process (Lehmann et al., 2005).
In our studies of fracture healing, comparison of the expression profiles of angiogenic regulators demonstrated that the most prevalent factors expressed over the time-course of repair are angiopoietin-2, pigment epithelial-derived factor, pleiotrophin, Tie1, and vascular endothelial growth inhibitor (Gerstenfeld et al., 2003b). The VEGF gene family members detectable during fracture healing are VEGF-D, VEGF-A, and VEGF-C. They are expressed throughout the chondrogenic phase of healing, reaching maximal levels of expression during the late phases of calcification of the cartilage tissues, at the time when resorption is initiated. A relationship between the expression of some angiogenic factors and pro-inflammatory cytokines has been shown in mice lacking TNF receptors. The absence of TNF receptor signaling diminishes the expression of angiopoietins, metalloproteinases, and vascular endothelial growth inhibitor during fracture healing. However, the expression of VEGF family members that directly promote new vessel formation is not inhibited. Taken together, the results from this study suggest that, after injury, existent vessels are first dissociated into a pool of non-dividing endothelial cells through the actions of angiopoietin-2 and vascular endothelial growth inhibitor, the latter limiting proliferation. At the time when cartilage resorption and primary bone remodeling are initiated, VEGF levels rise, stimulating cell division of this pool of progenitors and promoting participation of these endothelial cells in neo-angiogenesis. These results suggest that TNF-α signaling in chondrocytes controls vascularization of cartilage through the regulation of angiopoietin and vascular endothelial growth inhibitor factor, which play counterbalancing roles in the induction of growth arrest and apoptosis of endothelial cells.
A third, more distantly related, member of the angiogenic signaling system is the platelet-derived growth factor (PDGF) family. PDGF consists of a group of factors that structurally belong to the larger family of angiogenic factors that include VEGF and placental growth factor (Heldin and Westermark, 1999). The PDGFs are secreted from the alpha granules of platelets as well as endothelial cells, vascular smooth-muscle cells, and macrophages (Meyer-Ingold and Eichner, 1995). There are multiple forms of PDGF (PDGF-A, -B, -C, and −D), which form hetero- and homodimers that are biologically active. The forms of PDGF found in human platelets—PDGF-AA, PDGF-AB, and PDGF-BB—bind to PDGF receptors alpha and beta. The target cells for PDGF are primarily mesenchymal cells and include dermal fibroblasts and smooth-muscle cells. These cell types express higher levels of PDGF ß-receptors (Heldin and Westermark, 1999).
The actions of PDGF depend on the target cell and stimulate cellular proliferation, chemotaxis, survival, and calcium mobilization from intracellular stores (Diliberto et al., 1992). The PDGFs also have a role in the remodeling of connective tissues through their stimulation of collagenase (Bauer et al., 1985). Consistent with these findings, PDGF-BB has been effectively used as a therapeutic agent to enhance dermal wound healing (Pierce et al., 1988, 1989).
Because PDGF has been shown to enhance osteoblast migration and proliferation, and to be secreted from osteoclasts, it has been suggested as a key factor in bone remodeling (Kubota et al., 2002). Systemic administration of PDGF in ovariectomized rats results in increased bone density and strength (Mitlak et al., 1996). PDGF enhances formation of a mineralizing matrix in vitro (Hsieh and Graves, 1998) and enhances bone formation in periodontal regeneration in vivo (Sarment et al., 2006). Exogenous PDGF enhances callus density and bone formation associated with healing osteotomies (Nash et al., 1994). PDGF can be detected in the callus tissue obtained from healing fractures during bone formation (Andrew et al., 1995). It has been suggested that PDGF is an essential component of normal fracture healing in experimental animal models (Fujii et al., 1999). In contrast, PDGF—along with TGF-β, fibroblast growth factor-β, BMP-2, and BMP-14 expression—is lacking in fractures that do not heal properly (Brownlow et al., 2001).
Distraction Osteogenesis
Distraction osteogenesis (DO) is a bone-regenerative process in which osteotomy followed by gradual distraction yields two vascularized bone surfaces, from which new bone is formed. First described by Codivilla (1905) for the treatment of limb length discrepancies, it was not until the work of Ilizarov (1989), more than 50 years later, that the technique of DO gained widespread clinical use as a method for enhancing bone regeneration in clinical orthopedics and oral/maxillofacial surgery (Aronson, 1994b). The cellular and molecular processes of distraction osteogenesis are summarized in Tables 3 and 4.
Table 3. Summary of the Biological Processes Taking Place during Multiple Stages of Distraction Osteogenesis and the Associated Expression of Signaling Molecules (based on published results from Sato et al., 1999; Choi et al., 2002; Pacicca et al., 2003; Cho et al., 2007).
| Stage of Distraction Osteogenesis | Biological Processes | Expression of Signaling Molecules and their Proposed Functions |
|---|---|---|
| Latency | Hematoma Inflammation Recruitment of mesenchymal stem cells Periosteal callus and cartilage formation |
IL-1 and IL-6 are up-regulated after osteotomy, then return to baseline. BMP-2 and BMP-4 rise during the early latency phase to accelerate differentiation of precursor cells into chondrogenic/osteogenic cells. RANKL/OPG ratio increases by the late latency phase in association with cartilage resorption. BMP-6 and TGF-β expression rise during the late latency phase, due to the role they play in endochondral ossification. |
| Active Distraction | The callus is stretched. Cartilage resorption and endochondral bone formation. Formation of a central fibrous interzone comprised of fibroblast cells and collagen fibers aligned parallel to the vector of elongation. Neo-angiogenesis between collagen fiber bundles. Osteoblast recruitment and arrangement along the new vessels, followed by intramembranous ossification and bone column formation. |
IL-6 rises again during this phase to contribute to intramembranous ossification by enhancing the differentiation of cells committed to the osteoblastic lineage. RANKL/OPG ratio remains high during the early distraction phase to promote resorption of the remaining mineralized cartilage formed during the latency phase. BMP-6 expression remains high during the early distraction phase. BMP-2, BMP-4, and TGF-β expression peak during this phase to stimulate uninterrupted bone formation in response to strain caused by distraction. IGF-1 and bFGF are induced during this phase. VEGF and angiopoietin-1 and -2 are up-regulated to stimulate new vessel formation and enhance the plasticity of existent larger vessels. |
| Consolidation | Bone columns interconnect. Osteoclast recruitment. Remodeling. |
BMP-2, BMP-4, and bFGF expression gradually disappears. TNF-α markedly increases toward the end of the consolidation phase, suggesting that it regulates bone remodeling. |
Table 4. Summary of the Stages* of Distraction Osteogenesis (DO) and Their Associated Molecular Regulators.
| Signaling Molecules | Latency | Active Distraction | Consolidation | |||
|---|---|---|---|---|---|---|
| Early | Late | Early | Late | Early | Late | |
| Cytokines | ||||||
| IL-1 | ↑↑↑ | |||||
| IL-6 | ↑↑↑ | ↑↑↑ | ↑↑↑ | |||
| TNF-α | ↑↑↑ | |||||
| RANKL | ↑↑↑ | ↑↑↑ | ||||
| OPG | ↑↑ | ↑↑↑ | ||||
|
| ||||||
| TGF-β Superfamily | ||||||
| BMP-2 | ↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | ↑ | |
| BMP-4 | ↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | ↑ | |
| BMP-6 | ↑↑↑ | ↑↑↑ | ↑ | |||
| TGF-β | ↑↑↑ | ↑↑↑ | ↑↑↑ | |||
| bFGF | ↑↑ | ↑↑ | ↑ | ↑ | ||
| IGF | ↑↑ | ↑↑ | ||||
|
| ||||||
| Angiogenic Factors | ||||||
| VEGF A | ↑ | ↑ | ↑ | |||
| VEGF B | ↑ | ↑ | ↑ | |||
| VEGF C | ↑↑ | ↑ | ↑ | ↑ | ||
| VEGF D | ↑↑ | ↑ | ↑ | |||
| Angiopoietin 1 | ↑ | ↑ | ||||
| Angiopoietin 2 | ↑ | ↑ | ||||
The different stages of DO are denoted in the top cells and subdivided into early and late stages. The number of arrows indicates their intensity of expression. Based on published results (Sato et al., 1999; Choi et al., 2002; Pacicca et al., 2003; Cho et al., 2007).
Three modes of ossification take place during DO. Although endochondral ossification occurs during early stages of DO, intramembranous bone formation is the predominant mechanism of ossification, particularly in later stages. A third form of ossification, called ‘transchondroid bone formation’, has been suggested to occur. During transchondroid ossification, chondroid bone is formed directly by chondrocyte-like cells, with transition from fibrous tissue to bone occurring gradually (Yasui et al., 1997; Choi et al., 2002). Cartilage that forms during DO is usually observed at the level of the periosteum, but not between the ends of the cortices within the distraction gap.
Distraction osteogenesis can be divided into three temporal and dynamic phases: latency, distraction, and consolidation. The latency phase allows for the initial trauma response to take place. It starts immediately following creation of the osteotomy and extends until the onset of active distraction. Events taking place during this phase are basically the same as those in the early stages of fracture repair. However, by the time the active distraction phase has been initiated, the primary inflammatory processes have been completed. During the distraction phase, tensile forces are applied to the callus with a specific rate and rhythm. As the callus is stretched, a central fibrous zone, called the fibrous interzone (FIZ), forms. It is rich in chondrocyte-like cells, fibroblasts, and oval cells, which are morphologically intermediate between fibroblasts and chondrocytes (Vauhkonen et al., 1990; Aronson, 1994b; Sato et al., 1998). The differentiating osteoblasts at the fibrous interzone deposit osteoid along collagen bundles. They subsequently undergo mineral crystallization parallel to the collagen bundles, forming a zone called the ‘zone of microcolumn formation’ (MCF). In between the fibrous interzone and microcolumn formation, a zone of highly proliferating cells, called the ‘primary matrix’ or ‘mineralization front’ (PMF), is observed (Aronson et al., 1990). Once the desired bone length is achieved, distraction ceases, marking the beginning of the consolidation phase, where bone and extensive amounts of osteoid undergo mineralization and eventual remodeling.
Bone regeneration during distraction osteogenesis is believed to occur in response to the longitudinal mechanical strain applied to the callus during healing. The exact mechanism by which strain stimulates bone formation remains unclear. It has been suggested that living tissues become metabolically activated by slow, steady traction, a phenomenon called ‘mechano-transduction’, characterized by the stimulation of proliferative and biosynthetic cellular functions (Ilizarov, 1989). Also, recent molecular investigations have indicated that the molecular signaling cascade plays an important role in the relationship between induced strain and bone regeneration. Although distraction regenerates the bone tissues by a process very different from that of fracture repair, the molecular signals that drive the regenerative process are similar and include the pro-inflammatory cytokines, the transforming growth factor beta superfamily, and angiogenic factors.
The Role of Pro-inflammatory Cytokines in Distraction Osteogenesis
Just like fracture, the expression of pro-inflammatory cytokines IL-1 and IL-6 is up-regulated after osteotomy and then returns to baseline levels rapidly during the latency period. However, the expression of IL-6 is elevated a second time, once distraction is started and mechanical strain is applied to the callus. During the distraction phase, IL-6 is expressed by the oval cells residing in the fibrous interzone, the zone at which tensile strains are the highest, as well as by osteoblasts and chondrocytes. Therefore, IL-6 released in response to stress has been hypothesized to contribute to intramembranous ossification, by enhancing the differentiation of cells committed to the osteoblastic linage (Cho et al., 2007).
It has been reported that the expression of TNF-α remains silent throughout the distraction osteogenesis process, suggesting that its expression is induced only by a more substantial trauma (Cho et al., 2007). However, studies conducted in our laboratories reached a different conclusion when the temporal patterns of expression of TNF-α superfamily members were examined. During distraction osteogenesis in mouse tibiae, TNF-α mRNA levels markedly increased toward the end of consolidation (unpublished data). In addition, the RANKL/OPG expression ratio increased at the beginning of the distraction phase, and decreased by the end of consolidation. These results are similar to those from another study conducted on mandibular distraction osteogenesis (Wang et al., 2005). A comparison of these results suggests that the resorption of mineralized cartilage in the external callus areas that form adjacent to the ends of the bone tissues and in the gap during the latency phase of distraction osteogenesis is more dependent on the levels of RANKL and OPG and less affected by other cytokines (Gerstenfeld et al., 2003a). In contrast, cartilage removal and bone remodeling in fracture healing are more dependent on the activity of TNF-α (Gerstenfeld et al., 2003a).
The Role of the Transforming Growth Factor Beta Superfamily in Distraction Osteogenesis
Bone Morphogenetic Proteins
Different BMPs exhibit different temporal patterns of expression during distraction osteogenesis. The expression of BMP-2 and BMP-4 rises in the early latency phase, probably to accelerate the differentiation of precursor cells into chondrogenic/osteogenic cells. The expression of BMP-2 and -4 is strongly enhanced by the application of mechanical strain during the distraction phase. They are produced by chondrogenic cells involved in cartilage formation and osteogenic cells at the primary mineralizing front. Also, they are produced by the oval cells residing in the fibrous interzone, which may form bone in response to strain (Lammens et al., 1998; Li et al., 1998; Liu et al., 1999; Sato et al., 1999; Rauch et al., 2000; Farhadieh et al., 2004). Once distraction has stopped, the expression of BMP-2 and BMP-4 gradually disappears (Sato et al., 1999; Rauch et al., 2000; Marukawa et al., 2006). It has been reported that the expression of these BMPs could last for up to two weeks after the cessation of distraction, implying that they play a role in the proliferation of cells required for the completion of bone healing (Yazawa et al., 2003; Marukawa et al., 2006). Since BMP-2 has osteo-inductive properties, the administration of exogenous BMP-2 has been used successfully to shorten the treatment time during distraction osteogenesis by accelerating bone formation during the consolidation stage (Yonezawa et al., 2006). BMP-6, in contrast, peaks during the late phase of latency and the early phase of distraction. It then declines toward the late distraction phase, as the mode of ossification transforms from endochondral to intramembranous, reflecting its role in the endochondral phase (Li et al., 1998; Sato et al., 1999; Rauch et al., 2000). It has been reported that BMP-7 plays a role similar to that of BMP-2 and BMP-4 in distraction osteogenesis (Rauch et al., 2000); however, most experiments have detected only weak levels or no expression of BMP-7 during distraction osteogenesis (Sato et al., 1999; Campisi et al., 2003; Yazawa et al., 2003).
Transforming Growth Factor Beta
Toward the end of latency, TGF-β displays an increased level of expression that lasts into the distraction phase. It displays diffuse expression throughout the distraction gap (Liu et al., 1999). An inverse relationship between TGF-β and osteocalcin has been observed in a canine distraction model, where elevated TGF-β levels were accompanied by lower levels of osteocalcin after the initiation of distraction osteogenesis (Lammens et al., 1998). These observations suggest that TGF-β suppresses osteoblast maturation by delaying differentiation of osteoblasts during the mineralization stage of distraction osteogenesis.
Other Morphogens and Growth Factors
Insulin-like growth factor 1 (IGF-1) and basic fibroblast growth factor (bFGF) are also induced during distraction. Basic fibroblast growth factor is mainly expressed by cells of osteoblastic lineage and mesenchymal cells on the newly formed trabecular bone (Farhadieh et al., 1999). Insulin-like growth factor-1, in contrast, is diffusely expressed throughout the distraction gap (Liu et al., 1999). Once distraction has ceased, the expression of insulin-like growth factor-1 returns to basal levels, whereas basic fibroblast growth factor drops to levels lower than those observed during distraction, although some osteoblasts continue to express it during consolidation (Liu et al., 1999; Yeung et al., 2001).
The Role of Angiogenic Factors in Distraction Osteogenesis
Similar to fracture healing, distraction osteogenesis increases the demand on the surrounding tissues to increase blood flow, so that successful induction of new bony regeneration can occur (Aronson, 1994a; Carvalho et al., 2004). Neo-angiogenesis during distraction osteogenesis may be induced by VEGF-A and neuropilin, an alternative receptor for VEGF. During distraction osteogenesis, the expression of other VEGF ligands and receptors is many-fold less than that of VEGF-A and neuropilin1 and is difficult to quantify. The one exception is VEGF-D, which peaks at the end of the latency period and in early periods of active distraction, but shows diminished expression at later stages (Carvalho et al., 2004). The expression of VEGF-A is primarily localized to maturing osteoblasts at the primary mineralizing front and to osteoclasts located at the zone of microcolumn formation (Choi et al., 2002). The localization of VEGF-A to the primary mineralizing front suggests that there is a tight spatial coordination between areas of neovascularization and new bone formation (Pacicca et al., 2003).
Another family of angiogenic factors, the angiopoietins, is also expressed during distraction. The temporal appearance of angiopoietin-1 is followed by angiopoietin-2, which in turn is followed by a maximal expression of VEGF-A in the distraction model. Angiopoietin-2 by itself is antagonistic to angiopoietin-1. However, it has been proposed that the combination of angiopoieten-2 and VEGF-A stimulates new vessel formation, enhances the plasticity of existent larger vessels, and contributes to new vessel formation (Pacicca et al., 2003). It has also been reported that the increase in VEGF-A and angiopoietin-1 expression is associated with an up-regulation in the expression of hypoxia-induced factor 1 alpha (Hif1α), which is one of the key transcription factors regulating genes associated with an angiogenic response, such as VEGF-A and angiopoietin-1 (Pacicca et al., 2003; Carvalho et al., 2004).
Differences between Fracture Repair and Distraction Osteogenesis
Although bone regeneration during distraction osteogenesis uses many of the same basic processes involved in healing fractures, there are also unique cellular and molecular aspects to this form of tissue repair. The most striking differences are illustrated when one contrasts the histological and computerized tomographic sections of fracture calluses and distraction osteogenesis (Figs. 1, 2). These comparisons show both differences in temporal processes of healing and bone formation and variations in the spatial localization of the bone tissue formed.
Figure 1.
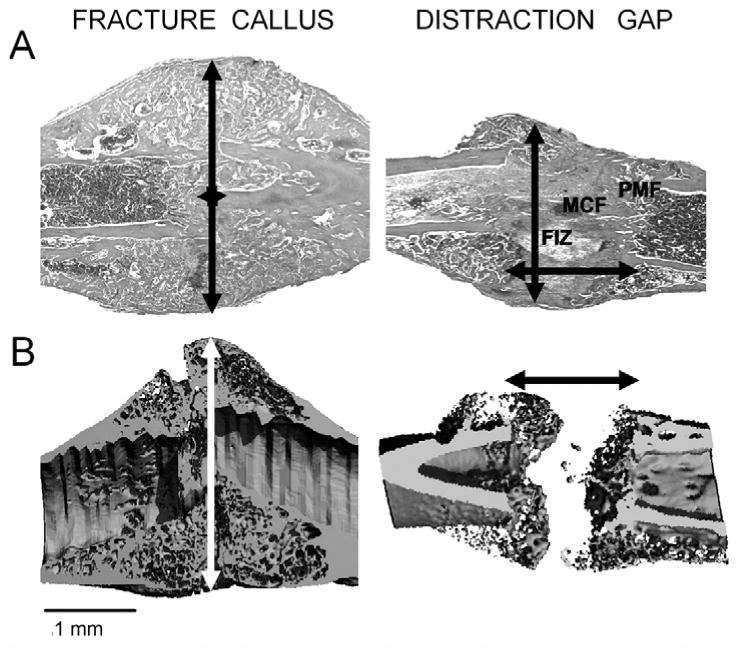
Comparisons of the tissue histology and mineralized tissue structure of fracture callus and distraction gap tissues. Murine femur fracture calluses and tibia distraction gap tissue were prepared from specimens obtained 21 days post-fracture or at 21 days post-surgery. (Panel A) Representative longitudinal sections of fracture and distraction osteogenesis were stained with Safranin-O/fast green. Original magnification 25×. (Panel B) Representative longitudinal microCT images at a resolution of 12 microns. Arrows indicate the extent of new bone formation. Both sets of images are presented with the distal and proximal orientations, left to right. The various zones in distraction osteogenesis are indicated. The central fibrous zone, histologically called the fibrous interzone (FIZ), is rich in chondrocyte-like cells, fibroblasts, and oval cells that are morphologically intermediate between fibroblasts and chondrocytes. The fibrous interzone contains differentiating osteoblasts that deposit osteoid along collagen bundles. When these collagen bundles mineralize, they form a zone called the zone of microcolumn formation (MCF). In between the fibrous interzone and the zone of microcolumn formation is a zone of high cell density called the primary matrix or mineralization front (PMF). Separate scale bars for both the histological and microCT images are presented below each image (1 mm).
Figure 2.
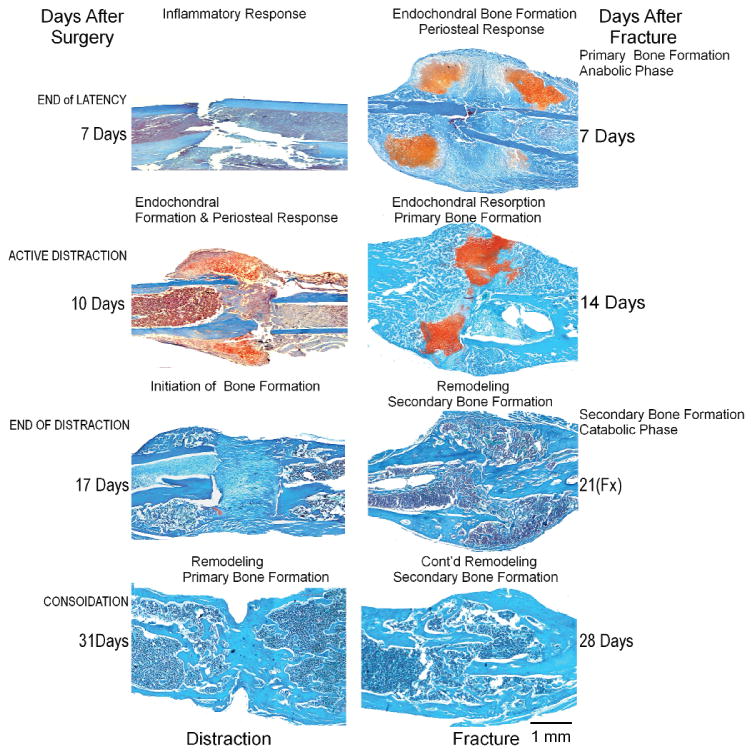
Comparison of the progression of healing in fractures and distraction osteogenesis. Murine femur fracture calluses and tibia distraction gap tissues were prepared at the indicated time-points. The different stages of healing and bone formation are given for each. Representative histologic specimens are stained with Safranin-O/fast green, which stains cartilage bright red. The scale bar in the lower right indicates 1 mm for all panels.
The regenerative processes of distraction osteogenesis and fracture healing are both characterized by intramembranous and endochondral bone formation. However, endochondral bone formation is considered the predominant form of ossification during fracture repair, whereas intramembranous ossification dominates over endochondral bone formation during distraction osteogenesis (Yasui et al., 1997; Einhorn, 1998). Differences in the timing of these two types of bone formation processes and spatial differences in where these processes occur can be noted in Figs. 1 and 2. In the case of fracture healing, endochondral bone formation robustly occurs in the first week after fracture and leads to a substantial volume of tissue formed outside the bone in the periosteal space. In contrast, much less cartilage is formed and is restricted to the early periods after distraction is initiated, after which it is rapidly resorbed. Other features worth noting are the large amounts of unmineralized osteoid in the central region of the distraction gap, whereas, at comparable times after injury, endochondral bone in the fracture callus has calcified and is undergoing the first round of primary bone formation (compare days 17 and 20 DO with day 21 fracture; Figs. 1, 2). One last feature to note is the primary difference in timing of the initiation of angiogenesis. In fracture healing, these processes are initiated in the period between days 7 and 14, as the chondrogenic tissues undergo apoptosis and resorption. In contrast, during distraction, these processes are initiated only after active distraction has begun, and are believed to be driven by the actual distraction processes, and not by signals elaborated by the development of chondrogenesis. This conclusion is supported by the observation that development of the majority of the new blood vessels occurs in the medullary space (Pacicca et al., 2003; Jacobsen et al., 2005) of the distraction regenerate. In contrast, the majority of vessels in fracture healing form in the external callus and are associated with the transition from cartilage to bone (Duvall et al., 2007).
Differences between fracture healing and distraction osteogenesis are seen at the level of molecular signaling. During fracture repair, the levels of IL-1, IL-6, and TNF-α are elevated within the first 24 hrs after injury, then decline below their basal levels during the endochondral phase. They then return to their basal levels during the remodeling stage. In contrast, during distraction osteogenesis, only IL-1 and IL-6 display elevated expression in response to initial injury, whereas TNF-α is increased significantly less. This may be due to the fact that injury associated with osteotomy in distraction osteogenesis is not as profound as the trauma associated with a fracture, and there is less chondroid tissue resorption. In addition, IL-6 levels are elevated in response to mechanical strain, suggesting that this cytokine has an anabolic effect during distraction osteogenesis, rather than a catabolic effect, as is observed during fracture repair (Cho et al., 2007). The enhanced bone formation observed during distraction osteogenesis may be mediated by the collaborative induction of BMP-2 and -4 expression during the distraction phase, since BMP-2 expression is restricted to the inflammatory phase of fracture repair, whereas BMP-4 is induced during the phases of both endochondral and primary bone formation (Sato et al., 1999; Cho et al., 2002).
An optimal angiogenic response has been shown to be directly related to the rate of distraction. Numerous investigators have speculated that it is this characteristic that drives bone formation, through an intramembranous pathway (Lewinson et al., 2001; Meyer et al., 2001). It should be noted that while both fracture repair and bone formation during distraction osteogenesis require increased blood flow, greater vascularization is observed during distraction osteogenesis compared with fracture healing (Aronson et al., 1990). During both fracture repair and distraction osteogenesis, the same angiogenic factors directly regulate vessel formation. While all four forms of VEGF expression are seen in distraction osteogenesis and fracture healing, the expression of VEGFs, compared with that of the angiopoietins and Tie receptors, is lower in distraction osteogenesis. In contrast, during fracture healing, the expression patterns are reversed, so that the expression of VEGFs is higher than that of angiopoietins and Tie receptors. It is also interesting to note that while angiopoietin-1 is expressed at fairly high levels in distraction osteogenesis, it is expressed to a lesser degree than angiopoietin-2 in the fracture calluses. These observations suggest that while the stabilization and growth of existing vessels in the endosteal space provide initial vascularization of the distraction gap, the vascularization during fracture healing is driven by new vessel formation within the periosteum (Jazrawi et al., 1998; Claes et al., 2002). Other interesting differences to note include the very high levels of pleiotrophin (Ptn), TGF-β2, fibroblast growth factor receptor-4, and the higher levels of VEGF-D, in comparison with levels of other VEGFs, in distraction osteogenesis relative to those levels seen in fractures. Although not as extensively examined in distraction as in fracture healing, platelet-derived growth factor expression has also been shown in distraction tissues and in cells found within distraction regenerates. Platelet-derived growth factor and basic fibroblast growth factor are observed adjacent to areas of new vessel formation (Knabe et al., 2005).
Our own studies have shown that the regulation of angiogenesis in distraction tissues is associated with much higher levels of hypoxia-inducible factor 1α, all of the thrombospondins, and tenascin, compared with fracture calluses (Pacicca et al., 2003). In this regard, the transient up-regulation of hypoxia-inducible factor 1α in response to each round of distraction would suggest that many of the downstream genes that are targets of transcriptional activation of hypoxia-inducible factor 1α, such as VEGF-A, may play a major role in promoting new bone formation during distraction osteogenesis. Studies examining the role of VEGF activity in distraction osteogenesis by inhibiting both receptors VEGF receptor-1 (Flt-1) and VEGF receptor-2 (Flk-1), or only VEGF receptor-2, with selective antibody blockade showed that both angiogenesis and osteogenesis in distraction osteogenesis were dependent on the activity of both VEGF receptors 1 and 2 (Jacobsen et al., 2005). In contrast, studies of fracture healing in which VEGF activity was inhibited led to delayed healing and a failure to progress from a cartilaginous to a bony callus (Street et al., 2002). The basic differences were that while bone healing almost totally fails in the absence of angiogenesis in distraction osteogenesis, the formation of cartilage occurs but fails to progress to bone formation when angiogenesis is inhibited. This further differentiates the role of angiogenesis in intramembranous and endochondral bone formation.
In summary, fracture healing and distraction osteogenesis are driven by the activities of molecular mediators of inflammation, the TGF-β superfamily of morphogens, and mediators of angiogenesis. The primary differences between the two processes of new bone formation are in the relative levels of expression of individual mediators and their timing of expression.
Acknowledgments
The authors are supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (PO1AR049920). Institutional support was provided by the Department of Orthopaedic Surgery, Boston University School of Medicine, and by the Boston University School of Medicine.
Abbreviations
- TGF
tumor-derived growth factor
- BMP
bone morphogenetic protein
- TNF
tumor necrosis factor
- IL
interleukin
- RANKL
receptor activator of nuclear factor-kappaB ligand
- Rank
receptor activator of nuclear factor-kappaB receptor
- OPG
osteoprotegerin
- M-CSF
macrophage colony-stimulating factor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- VEGI
vascular endothelial growth inhibitor
- Ang
angiopoietin
- PDGF
platelet-derived growth factor
- IGF
insulin-derived growth factor
- FGF
fibroblast-derived growth factor
- PEDF
pigment epithelium-derived factor
- Nrp
neuropilin
All other abbreviations and acronyms are denoted in the text.
Footnotes
The review of this paper was overseen by the Editor-in-Chief, and, to avoid any potential conflict of interest, the Associate Editor for Critical Reviews was not involved in the review process.
References
- Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res. 2001;19:785–796. doi: 10.1016/S0736-0266(00)00078-4. [DOI] [PubMed] [Google Scholar]
- Andrew JG, Hoyland JA, Freemont AJ, Marsh DR. Platelet-derived growth factor expression in normally healing human fractures. Bone. 1995;16:455–460. doi: 10.1016/8756-3282(95)90191-4. [DOI] [PubMed] [Google Scholar]
- Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994a;301:124–131. [PubMed] [Google Scholar]
- Aronson J. Experimental and clinical experience with distraction osteogenesis. Cleft Palate Craniofac J. 1994b;31:473–482. doi: 10.1597/1545-1569_1994_031_0473_eacewd_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Aronson J, Good B, Stewart C, Harrison B, Harp J. Preliminary studies of mineralization during distraction osteogenesis. Clin Orthop Relat Res. 1990;250:43–49. [PubMed] [Google Scholar]
- Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805–1815. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- Bauer EA, Cooper TW, Huang JS, Altman J, Deuel TF. Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc Natl Acad Sci USA. 1985;82:4132–4136. doi: 10.1073/pnas.82.12.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam H, Parsons J, Lin S. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res. 2002;20:1210–1216. doi: 10.1016/S0736-0266(02)00066-9. [DOI] [PubMed] [Google Scholar]
- Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200:165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- Bostrom MP. Expression of bone morphogenetic proteins in fracture healing. Clin Orthop Relat Res. 1998;355(Suppl):S116–S123. doi: 10.1097/00003086-199810001-00013. [DOI] [PubMed] [Google Scholar]
- Brownlow HC, Reed A, Simpson AH. Growth factor expression during the development of atrophic non-union. Injury. 2001;32:519–524. doi: 10.1016/s0020-1383(00)00249-7. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Campisi P, Hamdy RC, Lauzier D, Amako M, Rauch F, Lessard ML. Expression of bone morphogenetic proteins during mandibular distraction osteogenesis. Plast Reconstr Surg. 2003;111:201–208. doi: 10.1097/01.PRS.0000034932.99249.34. discussion 209-210. [DOI] [PubMed] [Google Scholar]
- Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, et al. The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone. 2004;34:849–861. doi: 10.1016/j.bone.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85(A)(8):1544–1552. doi: 10.2106/00004623-200308000-00017. published erratum, J Bone Joint Surg Am 86(A): 141. [DOI] [PubMed] [Google Scholar]
- Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- Cho TJ, Kim JA, Chung CY, Yoo WJ, Gerstenfeld LC, Einhorn TA, et al. Expression and role of interleukin-6 in distraction osteogenesis. Calcif Tissue Int. 2007;80:192–200. doi: 10.1007/s00223-006-0240-y. [DOI] [PubMed] [Google Scholar]
- Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. J Korean Med Sci. 2002;17:435–447. doi: 10.3346/jkms.2002.17.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L, Eckert-Hubner K, Augat P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J Orthop Res. 2002;20:1099–1105. doi: 10.1016/S0736-0266(02)00044-X. [DOI] [PubMed] [Google Scholar]
- Codivilla A. On the means of lengthening in the lower limbs. Am J Orthop Surg. 1905;2:353–369. [Google Scholar]
- Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- Diliberto PA, Gordon GW, Yu CL, Earp HS, Herman B. Platelet-derived growth factor (PDGF) alpha receptor activation modulates the calcium mobilizing activity of the PDGF beta receptor in Balb/c3T3 fibroblasts. J Biol Chem. 1992;267:11888–11897. [PubMed] [Google Scholar]
- Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22:286–297. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007;40:1389–1398. doi: 10.1016/j.bone.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;355(Suppl):S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- Farhadieh RD, Dickinson R, Yu Y, Gianoutsos MP, Walsh WR. The role of transforming growth factor-beta, insulin-like growth factor I, and basic fibroblast growth factor in distraction osteogenesis of the mandible. J Craniofac Surg. 1999;10:80–86. doi: 10.1097/00001665-199901000-00016. [DOI] [PubMed] [Google Scholar]
- Farhadieh RD, Gianoutsos MP, Yu Y, Walsh WR. The role of bone morphogenetic proteins BMP-2 and BMP-4 and their related postreceptor signaling system (Smads) in distraction osteogenesis of the mandible. J Craniofac Surg. 2004;15:714–718. doi: 10.1097/00001665-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Fujii H, Kitazawa R, Maeda S, Mizuno K, Kitazawa S. Expression of platelet-derived growth factor proteins and their receptor alpha and beta mRNAs during fracture healing in the normal mouse. Histochem Cell Biol. 1999;112:131–138. doi: 10.1007/s004180050399. [DOI] [PubMed] [Google Scholar]
- Funk JR, Hale JE, Carmines D, Gooch HL, Hurwitz SR. Biomechanical evaluation of early fracture healing in normal and diabetic rats. J Orthop Res. 2000;18:126–132. doi: 10.1002/jor.1100180118. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003a;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003b;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Alkhiary YM, Krall EA, Nicholls FH, Stapleton SN, Fitch JL, et al. Three-dimensional reconstruction of fracture callus morphogenesis. J Histochem Cytochem. 2006;54:1215–1228. doi: 10.1369/jhc.6A6959.2006. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Hsieh SC, Graves DT. Pulse application of platelet-derived growth factor enhances formation of a mineralizing matrix while continuous application is inhibitory. J Cell Biochem. 1998;69:169–180. doi: 10.1002/(sici)1097-4644(19980501)69:2<169::aid-jcb7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- Jacobsen KA, Tsiridis E, Kaker S, Einhorn TA, Gerstenfeld LC. Bone formation during distraction osteogenesis requires VEGF receptor signalling. In: Landis WJ, Sodek J, editors. Proceedings of the Eighth International Conference on the Chemistry and Biology of Mineralized Tissues; Toronto, Canada: University of Toronto Press; 2005. [Google Scholar]
- Jazrawi LM, Majeska RJ, Klein ML, Kagel E, Stromberg L, Einhorn TA. Bone and cartilage formation in an experimental model of distraction osteogenesis. J Orthop Trauma. 1998;12:111–116. doi: 10.1097/00005131-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Kurokawa T, Hanada K, Hiyama Y, Tamura M, Ogata E, et al. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994;135:774–781. doi: 10.1210/endo.135.2.8033826. [DOI] [PubMed] [Google Scholar]
- Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22:560–568. doi: 10.1359/jbmr.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabe C, Nicklin S, Yu Y, Walsh WR, Radlanski RJ, Marks C, et al. Growth factor expression following clinical mandibular distraction osteogenesis in humans and its comparison with existing animal studies. J Craniomaxillofac Surg. 2005;33:361–369. doi: 10.1016/j.jcms.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- Kubota K, Sakikawa C, Katsumata M, Nakamura T, Wakabayashi K. Platelet-derived growth factor BB secreted from osteoclasts acts as an osteoblastogenesis inhibitory factor. J Bone Miner Res. 2002;17:257–265. doi: 10.1359/jbmr.2002.17.2.257. [DOI] [PubMed] [Google Scholar]
- Lammens J, Liu Z, Aerssens J, Dequeker J, Fabry G. Distraction bone healing versus osteotomy healing: a comparative biochemical analysis. J Bone Miner Res. 1998;13:279–286. doi: 10.1359/jbmr.1998.13.2.279. [DOI] [PubMed] [Google Scholar]
- Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, et al. Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36:300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lewinson D, Maor G, Rozen N, Rabinovich I, Stahl S, Rachmiel A. Expression of vascular antigens by bone cells during bone regeneration in a membranous bone distraction system. Histochem Cell Biol. 2001;116:381–388. doi: 10.1007/s004180100331. [DOI] [PubMed] [Google Scholar]
- Li G, Berven S, Simpson H, Triffitt JT. Expression of BMP-4 mRNA during distraction osteogenesis in rabbits. Acta Orthop Scand. 1998;69:420–425. doi: 10.3109/17453679808999060. [DOI] [PubMed] [Google Scholar]
- Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84(A):1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- Liu Z, Luyten FP, Lammens J, Dequeker J. Molecular signaling in bone fracture healing and distraction osteogenesis. Histol Histopathol. 1999;14:587–595. doi: 10.14670/HH-14.587. [DOI] [PubMed] [Google Scholar]
- Loder R. The influence of diabetes mellitus on the healing of closed fractures (review) Clin Orthop Relat Res. 1988;232:210–216. [PubMed] [Google Scholar]
- Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003;144:346–352. doi: 10.1210/en.2002-220072. [DOI] [PubMed] [Google Scholar]
- Macey LR, Kana SM, Jingushi S, Terek RM, Borretos J, Bolander ME. Defects of early fracture-healing in experimental diabetes. J Bone Joint Surg Am. 1989;71:722–733. [PubMed] [Google Scholar]
- Marukawa K, Ueki K, Alam S, Shimada M, Nakagawa K, Yamamoto E. Expression of bone morphogenetic protein-2 and proliferating cell nuclear antigen during distraction osteogenesis in the mandible in rabbits. Br J Oral Maxillofac Surg. 2006;44:141–145. doi: 10.1016/j.bjoms.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Meyer U, Meyer T, Wiesmann HP, Kruse-Losler B, Vollmer D, Stratmann U, et al. Mechanical tension in distraction osteogenesis regulates chondrocytic differentiation. Int J Oral Maxillofac Surg. 2001;30:522–530. doi: 10.1054/ijom.2001.0159. [DOI] [PubMed] [Google Scholar]
- Meyer-Ingold W, Eichner W. Platelet-derived growth factor. Cell Biol Int. 1995;19:389–398. doi: 10.1006/cbir.1995.1084. [DOI] [PubMed] [Google Scholar]
- Mitlak BH, Finkelman RD, Hill EL, Li J, Martin B, Smith T, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res. 1996;11:238–247. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203–208. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, et al. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33:889–898. doi: 10.1016/j.bone.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- Pierce GF, Mustoe TA, Senior RM, Reed J, Griffin GL, Thomason A, et al. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988;167:974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989;109:429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch F, Lauzier D, Croteau S, Travers R, Glorieux FH, Hamdy R. Temporal and spatial expression of bone morphogenetic protein-2, -4, and -7 during distraction osteogenesis in rabbits. Bone. 2000;27:453–459. doi: 10.1016/s8756-3282(00)00337-9. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg Am. 2001;83(A)((Suppl 1)(Pt 1)):S1–S6. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- Sakou T. Bone morphogenetic proteins: from basic studies to clinical approaches. Bone. 1998;22:591–603. doi: 10.1016/s8756-3282(98)00053-2. [DOI] [PubMed] [Google Scholar]
- Sandberg MM, Aro HT, Vuorio EI. Gene expression during bone repair. Clin Orthop Relat Res. 1993;289:292–312. [PubMed] [Google Scholar]
- Sarment DP, Cooke JW, Miller SE, Jin Q, McGuire MK, Kao RT, et al. Effect of rhPDGF-BB on bone turnover during periodontal repair. J Clin Periodontol. 2006;33:135–140. doi: 10.1111/j.1600-051X.2005.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Yasui N, Nakase T, Kawahata H, Sugimoto M, Hirota S, et al. Expression of bone matrix proteins mRNA during distraction osteogenesis. J Bone Miner Res. 1998;13:1221–1231. doi: 10.1359/jbmr.1998.13.8.1221. [DOI] [PubMed] [Google Scholar]
- Sato M, Ochi T, Nakase T, Hirota S, Kitamura Y, Nomura S, et al. Mechanical tension-stress induces expression of bone morphogenetic protein (BMP)-2 and BMP-4, but not BMP-6, BMP-7, and GDF-5 mRNA, during distraction osteogenesis. J Bone Miner Res. 1999;14:1084–1095. doi: 10.1359/jbmr.1999.14.7.1084. [DOI] [PubMed] [Google Scholar]
- Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tay BK, Le AX, Gould SE, Helms JA. Histochemical and molecular analyses of distraction osteogenesis in a mouse model. J Orthop Res. 1998;16:636–642. doi: 10.1002/jor.1100160518. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Vauhkonen M, Peltonen J, Karaharju E, Aalto K, Alitalo I. Collagen synthesis and mineralization in the early phase of distraction bone healing. Bone Miner. 1990;10:171–181. doi: 10.1016/0169-6009(90)90260-m. [DOI] [PubMed] [Google Scholar]
- Verhaeghe J, van Herck E, Visser WJ, Suiker AM, Thomasset M, Einhorn TA, et al. Bone and mineral metabolism in BB rats with long-term diabetes. Decreased bone turnover and osteoporosis. Diabetes. 1990;39:477–482. doi: 10.2337/diab.39.4.477. [DOI] [PubMed] [Google Scholar]
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Takahashi I, Sasano Y, Sugawara J, Mitani H. Osteoclastogenic activity during mandibular distraction osteogenesis. J Dent Res. 2005;84:1010–1015. doi: 10.1177/154405910508401108. [DOI] [PubMed] [Google Scholar]
- Xiong DH, Shen H, Zhao LJ, Xiao P, Yang TL, Guo Y, et al. Robust and comprehensive analysis of 20 osteoporosis candidate genes by very high-density single-nucleotide polymorphism screen among 405 white nuclear families identified significant association and gene-gene interaction. J Bone Miner Res. 2006;21:1678–1695. doi: 10.1359/JBMR.060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui N, Sato M, Ochi T, Kimura T, Kawahata H, Kitamura Y, et al. Three modes of ossification during distraction osteogenesis in the rat. J Bone Joint Surg Br. 1997;79:824–830. doi: 10.1302/0301-620x.79b5.7423. [DOI] [PubMed] [Google Scholar]
- Yazawa M, Kishi K, Nakajima H, Nakajima T. Expression of bone morphogenetic proteins during mandibular distraction osteogenesis in rabbits. J Oral Maxillofac Surg. 2003;61:587–592. doi: 10.1053/joms.2003.50116. [DOI] [PubMed] [Google Scholar]
- Yeh LC, Lee JC. Osteogenic protein-1 increases gene expression of vascular endothelial growth factor in primary cultures of fetal rat calvaria cells. Mol Cell Endocrinol. 1999;153:113–124. doi: 10.1016/s0303-7207(99)00076-3. [DOI] [PubMed] [Google Scholar]
- Yeung HY, Lee SK, Fung KP, Leung KS. Expression of basic fibroblast growth factor during distraction osteogenesis. Clin Orthop Relat Res. 2001;385:219–229. doi: 10.1097/00003086-200104000-00033. [DOI] [PubMed] [Google Scholar]
- Yonezawa H, Harada K, Ikebe T, Shinohara M, Enomoto S. Effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on bone consolidation on distraction osteogenesis: a preliminary study in rabbit mandibles. J Craniomaxillofac Surg. 2006;34:270–276. doi: 10.1016/j.jcms.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Nomura S, Kawasaki S, Tsutsumimoto T, Shimizu T, Takaoka K. Colocalization of noggin and bone morphogenetic protein-4 during fracture healing. J Bone Miner Res. 2001;16:876–884. doi: 10.1359/jbmr.2001.16.5.876. [DOI] [PubMed] [Google Scholar]


