Abstract
The molecular clock controls 24-hour cycles of behavioral and physiological processes across the day-night cycle. Disruption of circadian rhythmicity has been implicated in the pathogenesis of several diseases, including the metabolic syndrome, although the role of clock genes in these disorders is still not well understood. Studies of the etiology of diabetes in circadian mutant mice have revealed a novel role for the clock in pancreatic β-cell insulin secretion, suggesting that a major cellular function of the circadian network involves control of protein exocytosis.
Key words: circadian clock, glucose homeostasis, islet, insulin secretion, metabolism, exocytosis
Central vs. Peripheral Clocks
The circadian clock drives alternating cycles of energy harvesting and utilization in organisms ranging from plants to animals in order to maintain synchrony with the rotation of the Earth on its axis. While the existence of an endogenous clock was proposed in the 18th century, genetic studies over the past twenty years have provided details on the molecular components of the clock, which is encoded by a transcription-translation feedback loop comprised of members of the basic helix-loop-helix (bHLH)-Per-Arnt-Sim (PAS) domain transcription factor superfamily (Fig. 1).1 In vertebrates, the circadian system is organized hierarchically, with the ‘master’ clock located within pacemaker neurons of the suprachiasmatic nucleus (SCN) (Fig. 2). The SCN in turn maintains circadian organization through both direct projections to extra-SCN neurons and through indirect release of signaling neuropeptides that synchronize downstream neurons.
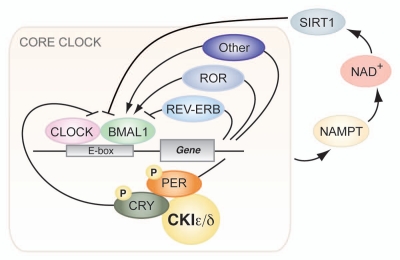
Figure 1.
Core circadian clock network. The core molecular clock machinery is encoded by a transcription-translation feedback loop that oscillates with a ∼24 hour periodicity. The bHLH-PAS transcription factors CLOCK and BMAL1 heterodimerize to drive expression of downstream target genes containing E-box sequences. Among these, the period (PER) and cryptochrome (CRY) proteins become phosphorylated by CKIepsilon/delta, multimerize and inhibit the action of the CLOCK:BMAL1 complex. The NAD+-dependent deacetylase SIRT1 comprises a recently discovered regulatory clock feedback loop.
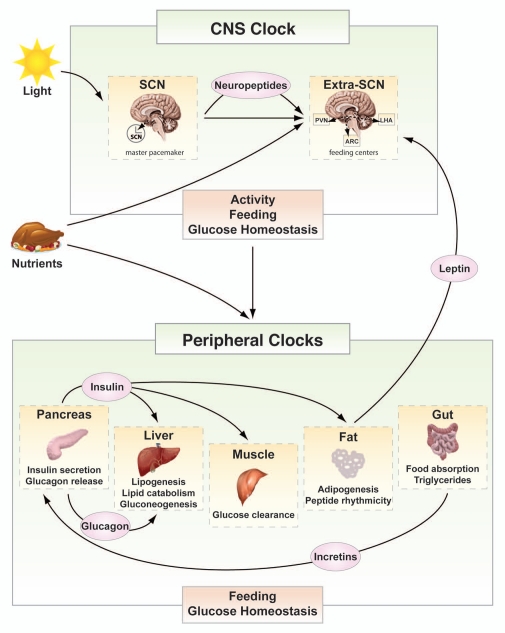
Figure 2.
Circadian regulation of glucose metabolism. Both brain and peripheral tissue clocks participate in the regulation of metabolic homeostasis. The CNS clock, entrained by environmental cues such as light and food availability, affects behavior and rhythmicity of caloric intake and impacts metabolic function of peripheral tissues through rhythmic hormonal and autonomic nervous system signaling. Peripheral tissue oscillators, entrained by signals from the master pacemaker and by rhythmic nutrient availability, impart rhythmicity in the expression of tissue-specific genes to optimize glucose metabolism and storage during times of feeding and to boost glucose production in periods of fasting. Rhythmic signaling of insulin, glucagon and incretin release between peripheral tissues is essential for the maintenance of normal glucose levels. Peripheral clocks also secrete satiety signals, such as leptin and ghrelin, to the brain, thereby modulating feeding behavior. Circadian misalignment in the integration of feeding, glucose metabolism and energetics contributes to hyperglycemia and metabolic dysregulation. (Extra-SCN: hypothalamic tissue excluding the SCN; PVN: paraventricular nucleus; LHA: lateral hypothalamus; ARC: arcuate nucleus).
The discovery in 1998 that mouse fibroblasts possess self-sustained 24-hr clocks demonstrated that circadian clocks are present in nearly all tissues of the body.2 Microarray analyses in liver, heart, brain and other tissues revealed rhythmic variation in the expression of nearly 10% of the transcriptome, including genes encoding rate-limiting enzymes in lipogenesis, sterol biosynthesis, oxidative-phosphorylation, gluconeogenesis, xenobiotic metabolism, and proliferation, suggesting that local tissue clocks may impact physiology.3–5 Many nuclear hormone receptors central to control of reproduction, energy balance and metabolism also exhibit highly rhythmic patterns of gene expression.6 A remaining question is whether these 24-hr cycles across the transcriptome are driven by local (autonomous) circadian clocks, or are instead controlled (non-autonomously) by rhythmic changes in metabolites, hormones or temperature that are programmed by the SCN clock. Notwithstanding questions on direct vs. indirect clock gene effects on metabolic cycles, additional evidence suggests interdependence of metabolic and circadian gene regulatory networks. For instance, McKnight and colleagues showed that levels of NAD+ and the cellular redox status affect the transcriptional activity of CLOCK and its homolog Neuronal PAS Domain Protein 2 (NPAS2).7 The McKnight studies point towards parallels between circadian and metabolic systems; both are responsive to nutrient flux and synchronized by oscillation of metabolites such as NAD+.
Clocks and Glucose Metabolism
Clues from clinical studies also indicate that the circadian system may play a role in feeding and glucose metabolism. For example, individuals subjected to shift work or restricted sleep have higher risk of metabolic disorders.8–10 In addition, while glucose tolerance and insulin levels in humans vary according to the time of day, these circadian patterns are disordered in individuals at risk for diabetes (see ref. 11). Studies using isolated islets of Langerhans from rats revealed that insulin secretion is also rhythmic, indicating that circadian variation in insulin secretion may be a property of the β-cell itself.12,13 Finally, polymorphisms in Clock and Bmal1 have been correlated with susceptibility to obesity and type 2 diabetes in humans, and polymorphisms in the Clock homolog Npas2 and the circadian gene Per2 have been linked to high fasting glucose levels.14–17
Experimental genetic models have provided an entry point to dissect the interconnections between clock genes and metabolic physiology.18,19 Mice with global clock gene mutations develop increased diet-induced obesity with high lipid and glucose levels. Surprisingly, rather than displaying hyperinsulinemia in an attempt to maintain normoglycemia, these mice have inappropriately low levels of insulin. The combination of hyperglycemia and hypoinsulinemia suggested a primary role of the clock transcription factor(s) in insulin production or secretion. Because these early analyses were in multi-tissue mutants, however, it was not possible to separate central versus peripheral effects of the mutation on glucose homeostasis, nor was it clear whether the hyperglycemia might have arisen merely as a consequence of the altered activity behavior in these animals. In an additional twist, mice with selective ablation of the clock within liver had low glucose levels.20 While the biochemical pathways involved in liver clock glucose metabolism are still incompletely known, it became increasingly clear that the clock displays tissue-specific functions.
Clock in the Pancreas
The most convincing evidence that clock function within endocrine pancreas impacts glucose homeostasis has emerged from our recent studies in mice with tissue-specific ablation of Bmal1 using the Cre-LoxP system to eliminate function in Pdx1-expressing cells.21 Loss of Bmal1 in PdxCre;Bmal1flx/flx mice is restricted to the pancreas, and Bmal1 expression in liver, skeletal muscle and adipose is intact, thereby preserving function in these “insulin-responsive tissues.” Despite normal locomotor activity rhythms, pancreas-specific Bmal1 knockout mice display much more severe hyperglycemia earlier in life than the multi-tissue mutant. This observation is consistent with opposing effects of the mutation in pancreas versus liver (and possibly skeletal muscle and fat). Thus the severe diabetes of the PdxCre;Bmal1flx/flx mouse demonstrates that β-cell failure is masked by loss of clock gene function in insulin-sensitive tissues in the whole body knockout (recent independent studies of pancreatic clock ablation have also observed hypoinsulinemia).22
Clocks and Islet Size
While the overall islet architecture in circadian mutant islets was normal, we observed decreased islet size and survival, as mutations in either Clock or Bmal1 decrease proliferation (via downregulation of expression of cell cycle genes) and increase cell death (via upregulation of apoptotic genes) in islets.21 These observations are consistent with previous reports of circadian control of cell proliferation in liver and skeletal muscle.3 This raises the possibility that, similar to the impaired liver regeneration in Cry1-/-;Cry2-/- mice, islets from circadian mutant mice may have increased sensitivity to glucose-induced oxidative damage during aging.23 However, it is still unclear whether the decrease in islet size in the circadian mutants is due to developmental effects, or rather is secondary to insulin deficiency and lack of autocrine growth factor stimulation. Future studies using inducible islet-specific circadian gene knockout mice will be necessary to discriminate between developmental and post-developmental effects and will help elucidate the molecular underpinnings of the reduced islet mass.
Clocks and Exocytosis
How does loss of clock function lead to β-cell failure? Isolated islet experiments from both Clock and Bmal1 mutant mice reveal impaired insulin release in response to both glucose and pharmacological secretagogues. However, because glucose-stimulated calcium influx in circadian gene mutant islets is normal and because KCl-induced depolarization does not trigger exocytosis, we infer that the defect in insulin secretion likely lies downstream of β-cell membrane depolarization (Fig. 3).21 Consistent with these findings, Clock mutant islets exhibit significant alterations in the expression of genes involved in post-translational modification and protein packaging, such as Syntaxin6 (a SNARE protein implicated in vesicle transport and docking, as well as insulin granule maturation) and Vamp3 (an insulin granule membrane-bound protein involved in docking and fusion of secretory granules to the plasma membrane).21,24–26 While our studies have localized clock function to the late stage in insulin secretion, the precise molecular details remain to be elucidated. Future experiments examining insulin granule maturation, trafficking, vesicle membrane fusion and insulin release in circadian gene mutant islets are likely to shed light on this critical relationship. A related question is whether the clock gene network also affects protein packaging and exocytosis in other neuroendocrine and/or neuronal cells as well. Finally, it is interesting to speculate that disrupted NAD+ biosynthesis and NAD+-dependent deacetylase SIRT1 activity may be involved in clock islet dysfunction, as SIRT1 has been shown to both comprise part of a novel regulatory clock feedback loop and regulate insulin secretion, potentially at the level of insulin granule exocytosis.27–31 Such a finding would have potential implications for understanding how the clock network functions in different tissues throughout aging.
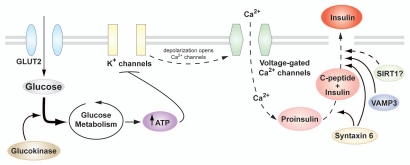
Figure 3.
Glucose metabolism and insulin secretion in the β-cell. Glucose enters the β-cell through the facilitative GLUT2 transporter and is metabolized during glycolysis and the Krebs cycle, resulting in rapid generation of ATP and closure of the ATP-sensitive K+ channels. Subsequent plasma membrane depolarization leads to opening of voltage-gated Ca2+ channels, and the consequent influx of Ca2+ triggers insulin granule exocytosis. Also shown are genes important for insulin granule maturation, transport and docking to the plasma membrane of the β-cell.
Conclusion
A critical remaining question is whether Clock and Bmal1 regulate glucose metabolism through their roles as components of the clock machinery or through a function independent of timing. We cannot exclude the possibility that, as trans-activators, both CLOCK and BMAL1 may have pleiotropic effects independent of the circadian clock that could impinge on metabolism and cause the phenotypes we observed. However, several considerations argue strongly for a defective pancreatic clock as the primary cause of dysregulation of glucose metabolism. First, the variety of genes exhibiting circadian profiles in the pancreas suggests a fundamental role of the clock network in islet physiology (Fig. 3). Second, the circadian phase of expression of oscillating genes in the islet is consistent with their function in glucose regulation (i.e., Glut2 and Gck peak at the beginning of the dark period in WT mice).21 Finally, the effects of mutations of Clock and Bmal1, the two components of the ‘forward limb’ of the clock, on glucose metabolism and insulin secretion are very similar, strongly implying an underlying circadian process.
The prevalence and increasing burden of diabetes and its complications have incited great interest in dissecting the mechanisms regulating glucose metabolism and insulin secretion. The interplay between the clock and metabolic systems is complex, involving both neural and peripheral tissues coordinating behavioral and metabolic systems (Fig. 2). Our findings point toward insulin secretion and exocytosis as downstream of the clock network, opening the possibility that circadian systems may provide new targets for pharmacological control of glucose metabolism.
Acknowledgement
This work was supported by NIH (PO1 AG011412 and R01HL097817), Chicago Biomedical Consortium Searle Finds, Islet Biology Core of the University of Chicago DRTC, ADA and JDRF to J.B.
Abbreviations
- bHLH-PAS
basic helix-loop-helix - period-arnt-singleminded
- BMAL1
brain and muscle arnt-like protein-1
- CLOCK
circadian locomotor output cycles kaput
- CRY
cryptochrome
- GCK
glucokinase
- GLUT2
glucose transporter type 2
- NAD+
nicotinamide adenine dinucleotide
- NPAS2
neuronal PAS domain protein 2
- SCN
supra-chiasmatic nucleus
- SIRT1
sirtuin 1
- SNARE
soluble N-ethylmaleimide sensitive factor attachment protein receptor
- VAMP3
vesicle-associated membrane protein 3
Conflict of Interest
J.B. is a member of the scientific advisory board of Reset Therapeutics. He also is an advisor and receives support from Amylin Pharmaceuticals.
References
- 1.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 3.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 5.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 7.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Lorenzo L, De Pergola G, Zocchetti C, L'Abbate N, Basso A, Pannacciulli N, Cignarelli M, Giorgino R, Soleo L. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord. 2003;27:1353–1358. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 11.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delattre E, Cipolla-Neto J, Boschero AC. Diurnal variations in insulin secretion and K+ permeability in isolated rat islets. Clin Exp Pharmacol Physiol. 1999;26:505–510. doi: 10.1046/j.1440-1681.1999.03073.x. [DOI] [PubMed] [Google Scholar]
- 13.Peschke E, Peschke D. Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia. 1998;41:1085–1092. doi: 10.1007/s001250051034. [DOI] [PubMed] [Google Scholar]
- 14.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 15.Sookoian S, Gemma C, Gianotti TF, Burgueno A, Castano G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 16.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Englund A, Kovanen L, Saarikoski ST, Haukka J, Reunanen A, Aromaa A, Lonnqvist J, Partonen T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadacca LA, Lamia KA, Delemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2010;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 24.Bock JB, Klumperman J, Davanger S, Scheller RH. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuliawat R, Kalinina E, Bock J, Fricker L, McGraw TE, Kim SR, Zhong J, Scheller R, Arvan P. Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic beta-cells. Mol Biol Cell. 2004;15:1690–1701. doi: 10.1091/mbc.E03-08-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omatsu-Kanbe M, Ding WG, Hashiramoto M, Kitasato H. Immunohistochemical localization of cellubrevin on secretory granules in pancreatic B-cells. Arch Histol Cytol. 1997;60:289–295. doi: 10.1679/aohc.60.289. [DOI] [PubMed] [Google Scholar]
- 27.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 28.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai SI, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]