Abstract
The mechanism of melanosome transfer from melanocytes to keratinocytes has not been fully clarified. We now show a route of melanosome transfer using co-cultures of normal human melanocytes and keratinocytes. Substantial levels of melanosome transfer were elicited in co-cultures of melanocytes and keratinocytes separated by a microporous membrane filter. The melanocyte dendrites penetrated into the keratinocyte layer through the filter and many pigment globules were observed in keratinocytes. Electron microscopic observations revealed that melanosomes incorporated in keratinocytes were packed in clusters enclosed by a double membrane. Numerous pigment globules budded off from melanocyte dendrites and were released into the culture medium. Those pigment globules were filled with multiple melanosomes and a few mitochondria but no nuclei. When those globules were added to the culture medium of keratinocytes, they were incorporated and showed double membrane-enclosed melano-phagolysosomes consistent with the structures obtained from the co-culture system. In contrast, when individual naked melanosomes isolated from melanocytes were added to keratinocytes, they were also phagocytosed by keratinocytes but were enclosed by a single membrane in a manner distinct from the co-culture system. These results suggest a novel mechanism of melanosome transfer, wherein melanosomes are packed in membrane globules that bud off from melanocyte dendrites, where they are released into the extracellular space and then phagocytosed by keratinocytes.
Key words: keratinocyte, melanocyte, melanosome, membrane, phagocytosis
Introduction
Solar ultraviolet radiation can elicit hyperpigmentation of the skin, which is caused by increased production of melanin pigment produced by melanocytes, specialized cells located in the basal layer of the epidermis. Melanocytes produce specific organelles, termed melanosomes, in which melanin pigment is ultimately synthesized and deposited. In the skin and hair, melanosomes are transferred from melanocytes to neighboring keratinocytes to form melanin caps above their nuclei.1 Those melanosomes play a role not only in protection against solar ultraviolet-induced nuclear DNA damage as an internal sunscreen but also in the production of visible color and cosmetic appearance that is a psychological concern to men and women.
A thorough understanding of melanosome transfer is crucial for designing treatments for hyper- and hypopigmentary disorders of the skin, such as melasma, age spots and vitiligo. Three possible mechanisms of melanosome transfer have been proposed, (i) direct inoculation of melanosomes into keratinocytes via keratinocyte-melanocyte membrane fusions through nanotubular filopodia, (ii) individual melanosome release from melanocytes and uptake by keratinocytes via phagocytosis, and/or (iii) partial cytophagocytosis of melanocyte dendrite tips containing melanosomes by adjacent keratinocytes.2–7 The finding that protease-activated receptor-2 (PAR-2), a seven-transmembrane G-protein-coupled receptor expressed by keratinocytes,8 regulates melanosome transfer via keratinocyte phagocytosis9–12 reinforces the notion that the release of melanosomes from melanocytes, at least in part, is involved in the machinery of melanosome transfer. However, the exact mechanism of the transfer process of melanosomes from melanocytes to keratinocytes has not been fully clarified.
In order to clarify the transfer mechanism, a variety of melanocyte-keratinocyte co-culture models have been developed.13–16 Further, evaluation assays for melanosomes transferred to keratinocytes in co-cultures have used many approaches, for example, fluorescently or radioactively labeled melanosomes detected by microscopy or by flow cytometric separation and quantification. However, those techniques are often insufficient with respect to the reproducibility and reliability of the assay systems.17 In this study, we developed a new melanocyte-keratinocyte co-culture system using a microporous membrane filter that allows frequent and reproducible melanosome transfer and can isolate keratinocytes reliably. Using that method, a new mechanism for the transfer of melanosomes was identified where pigment globules containing multiple melanosomes are released into the extracellular space by melanocytes and are then ingested by keratinocytes.
Results
Melanosomes are transferred to keratinocytes via melanocyte-dendrites that penetrate a microporous membrane filter.
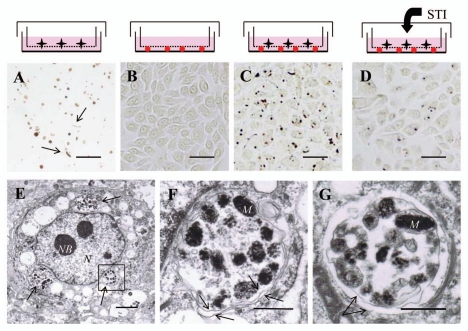
In order to observe melanosome transfer into keratinocytes under conditions independent of melanocyte contamination, a microporous membrane filter was utilized. Melanocytes and keratinocytes were separated by a 1 µm-microporous membrane filter through which only the dendrites of melanocytes can penetrate. After normal human melanocytes were seeded on the inner side of a Millicell® put in collagen I-coated culture dishes and incubated for 6 days, many melanocyte segments but no melanocytes were observed in the culture dishes. Those segments of melanocytes were mainly composed of globular structures and also some long and thin filaments (Fig. 1A). To establish the recipient cells, normal human keratinocytes were grown under the Millicell® for 6 days and we confirmed that no pigmentation was observed by Fontana-Masson staining (Fig. 1B). When normal human melanocytes and keratinocytes were co-cultured under conditions where those two kinds of cells can contact each other only through the 1 µm pores in the microporous membrane filter, a high frequency of melanosome uptake by keratinocytes was observed after removal of the Millicell®. Only individual or a few pigment globules were observed that were closely associated with keratinocyte nuclei (Fig. 1C). When soybean trypsin inhibitor (STI), a known inhibitor of PAR-2 that is involved in phagocytosis by keratinocytes,9 was added to the culture medium during the culture period, the number of melanosomes transferred into keratinocytes was decreased (Fig. 1D), as previously reported.18 Electron microscopic observations of keratinocytes co-cultured with microporous membrane filter-separated melanocytes for 6 days revealed that the incorporated melanosomes were frequently packed in globules which were located in the perinuclear area (Fig. 1E). Higher magnifications of globules containing multiple melanosomes showed that they were enclosed by a double membrane, that is, a structure categorized as a melanophagolysosome (Fig. 1F and G), as previously observed in a manner similar to the melanophore-derived melanosomes incorporated into frog fibroblasts that were also enclosed by a double membrane.19
Figure 1.
The microporous membrane filter-separated melanocyte and/or keratinocyte culture system. (A) Light microscopic image (bright field) of the bottom of a culture dish after growth of normal human melanocytes for 6 days. During the culture, there was a 1 µm-pore microporous membrane filter between the melanocytes and the bottom of the culture dish. After the membrane filter was removed, Fontana-Masson staining was performed. Many globules and a few segments (arrows) of melanocyte dendrites were dispersed. Scale bars in (A–D): 50 µm. (B) Light microscopic image (bright field) of the bottom of the culture dish after growth of normal human keratinocytes for 6 days. Fontana-Masson staining was performed to detect melanin but no staining was observed. (C) Light microscopic image (bright field) of the bottom of a culture dish after growth of normal human melanocytes and keratinocytes for 6 days. During the culture period, there was a 1 µm-pore microporous membrane filter between melanocytes and keratinocytes. Fontana-Masson staining was performed to detect melanin and single or clusters of pigment granules was/were observed closely associated with nuclei. (D) Light microscopic image (bright field) of the keratinocyte layer in the presence of 2 mg/mL STI, a known inhibitor of melanosome transfer, for 6 days. Fontana-Masson staining was performed and a decrease of melanosome transfer was observed. (E) Representative electron microscopic image of a normal human keratinocyte in C; the ingested melanosomes were packed and located in the perinuclear area (arrows). Scale bar: 2 µm. N: nuclei, NB: nuclear bodies. (F) Higher magnification image of the boxed region in E showing various stages of melanosomes or premelanosomes surrounded by a double membrane (arrows). Scale bar: 0.5 µm. M: representative melanosomes, N: nuclei. (G) Another electron microscopic image of ingested melanosomes in the co-culture system that shows an apparent double membrane (arrows) surrounding melanosomes. Scale bar: 0.5 µm. M: representative melanosomes.
Melanocytes release membrane-bound globules containing multiple melanosomes from their dendrite tips.
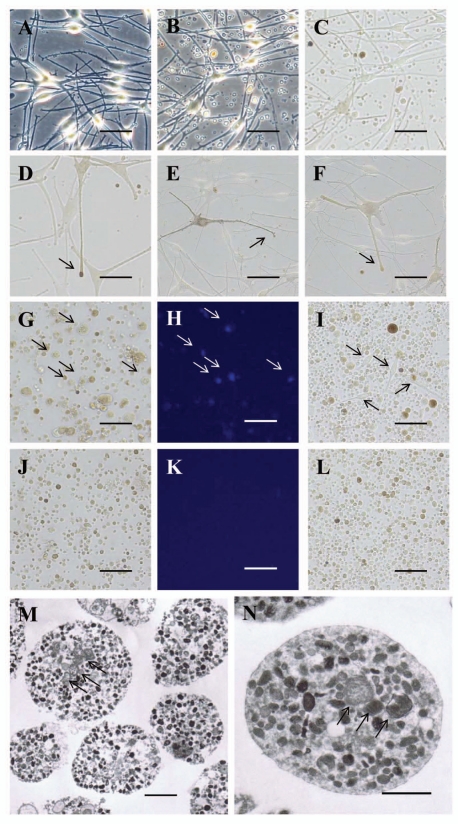
During the mono-culture of normal human melanocytes, many pigment globules that were smaller than melanocytes were observed in the culture medium. The number of pigment globules dramatically increased 24 hours after the culture medium was changed (Fig. 2A–C). Those globules were often seen to be generated by budding off from the extended tips of melanocyte dendrites (Fig. 2D–F). When the culture medium was centrifuged and the pellets were stained with 4',6-diamidino-2-phenylindole (DAPI) to detect nuclei, many floating melanocytes with DAPI fluorescence were observed (Fig. 2G and H). In fact, when the pellets were resuspended and re-seeded in medium 254 containing HMGS, pigment globules of various sizes and many melanocytes appeared after 24 hours of incubation (Fig. 2I). On the other hand, when the pellets were resuspended and filtered through an 8 µm-pore membrane to remove floating melanocytes and the relatively large globules, no fluorescence was observed after the nuclear DAPI stain (Fig. 2J and K). Further, when the filtered pellets were re-seeded, only globules smaller than 8 µm diameter but no melanocyte cell bodies were observed after 24 hours of incubation, i.e. bright field microscopy of the filtered globules revealed various degrees of pigmentation (Fig. 2L). Interestingly, electron microscopy revealed that almost all the filtered globules contained large numbers of melanosomes in circles of various diameters (Fig. 2M). At higher magnification, not only large numbers of melanosomes and various stages of premelanosomes but also a small number of mitochondria were observed and they were enclosed by a single membrane with no nuclei (Fig. 2N).
Figure 2.
Pigment globules containing multiple melanosomes released into the culture medium by normal human melanocytes. (A) Light microscopic image (phase contrast) of normal human melanocytes immediately after the culture medium was changed. Scale bars in (A–L): 50 µm. (B) Light microscopic image (phase contrast) of normal human melanocytes 24 hours after the culture medium was changed; note that numerous globules are present in the culture medium. (C) Bright field image of B; various degrees of pigmentation are observed in the globules. (D–F) Representative light microscopic images (bright field) of dendrites of normal human melanocytes. Pigment globules can be seen in the extended tip of a melanocyte dendrite (arrows). (G–I) Light microscopic images (G and I: bright field, H: fluorescence) of the culture medium of B after nuclear staining with DAPI (G and H) or incubated for 24 hours after re-seeding (I); many melanocytes (arrows in G and H indicate nuclei from the same positions, arrows in I indicate melanocyte cell bodies) and globules of various sizes are seen in the culture medium. (J–L) Light microscopic images (J and L: bright field, K: fluorescence) of the culture medium of B after filtration through an 8 µm-pore membrane filter; globules smaller than 8 µm diameter are seen but no melanocyte nuclei or melanocyte cell bodies are observed after DAPI staining (J and K) or after 24 hours of incubation (L). (M) Representative electron microscopic image of the purified pigment globules in (J–L); almost all globules contain multiple melanosomes and premelanosomes and a small number of mitochondria (arrows). Scale bar: 2 µm. (N) Higher magnification image of M; a large number of melanosomes and a small number of mitochondria (arrows) are surrounded by a single membrane. Scale bar: 1 µm.
Pigment globules containing multiple melanosomes are phagocytosed by keratinocytes.
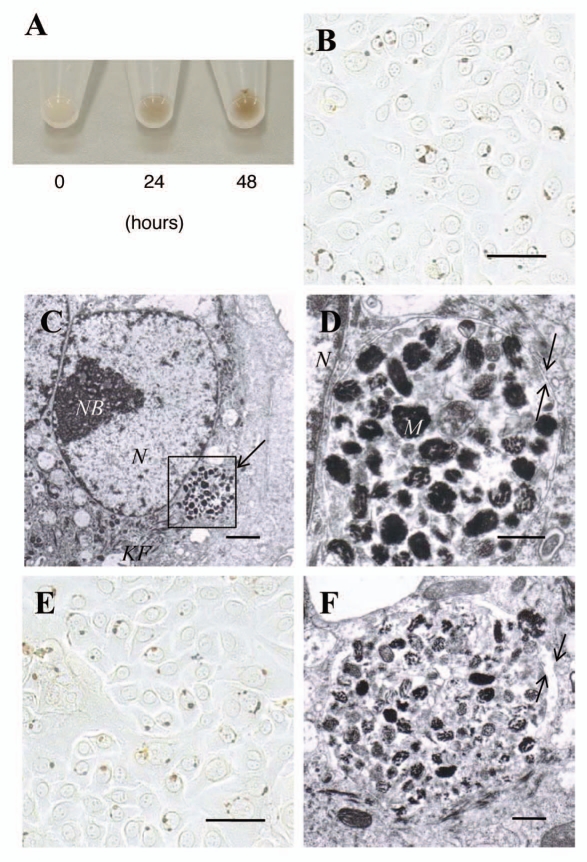
In order to investigate whether the membrane-coated pigment globules released into the culture medium are involved in the transport of melanosomes, pigment globules filtered through an 8 µm-pore membrane filter were added to the culture medium of normal human keratinocytes. After incubation of the filtered pigment globules with keratinocytes for 24 or 48 hours, the pigmentation of the keratinocyte pellets increased in a time-dependent manner (Fig. 3A). Fontana-Masson staining of keratinocytes incubated with the filtered pigment globules for 48 hours revealed that they were located in the perinuclear area similar to the microporous membrane filter-separated melanocyte-keratinocyte co-cultures (Fig. 3B). Electron microscopy of keratinocytes incubated with the filtered pigment globules for 48 hours showed that pigment globules containing multiple melanosomes and premelanosomes were incorporated and were localized in the perinuclear area (Fig. 3C). Higher magnifications of the incorporated pigment globules in keratinocytes revealed that the aggregated melanosomes and various stages of premelanosomes were enclosed by a double membrane consistent with the case observed in melanocyte- keratinocyte co-cultures (Fig. 3D). Furthermore, when keratinocytes were treated with pigment globules that had penetrated through the 1 µm membrane filter as shown in Figure 1A, it was also revealed that the incorporated melanosomes in clusters were located in the perinuclear area (Fig. 3E) and were enclosed by a double membrane (Fig. 3F), in a manner consistent with that of the coculture system.
Figure 3.
Pigment globules containing multiple melanosomes derived from normal human melanocytes retain the capacity to transfer melanosomes to normal human keratinocytes. (A) Cell pellets of normal human keratinocytes incubated with the filtered pigment globules for 0, 24 or 48 hours; the pigmentation in the pellets after 24 and 48 hours of incubation is due to the pigment globules incorporated into the keratinocytes. (B) Light microscopic image (bright field) of normal human keratinocytes after incubation with the filtered pigment globules for 48 hours; Fontana-Masson staining revealed single or clustered pigment globules closely associated with nuclei. Scale bar: 50 µm. (C) Representative electron microscopic image of a normal human keratinocyte in (B); melanosomes and premelanosomes are packed and located in the perinuclear area. Scale bar: 2 µm. N: nuclei, NB: nuclear bodies, KF: keratin fibers. (D) Higher magnification image of the boxed region in C showing that the ingested melanosomes and premelanosomes are surrounded by a double membrane (arrows). Scale bar: 0.5 µm. M: representative melanosomes, N: nuclei. (E) Light microscopic image (bright field) of normal human keratinocytes after incubation with the globules or segments generated from melanocyte dendrites that had penetrated through the 1 µm membrane filter for 48 hours; Fontana-Masson staining revealed single or clustered pigment globules closely associated with nuclei. Scale bar: 50 µm. (F) Representative electron microscopic image of a normal human keratinocyte in E at higher magnification; melanosomes and premelanosomes are enclosed by a double membrane (arrows). Scale bar: 0.5 µm. M: representative melanosomes.
The addition of individual naked melanosomes also generates melanophagolysosomes but in a manner distinct from the melanocyte-keratinocyte co-cultures.
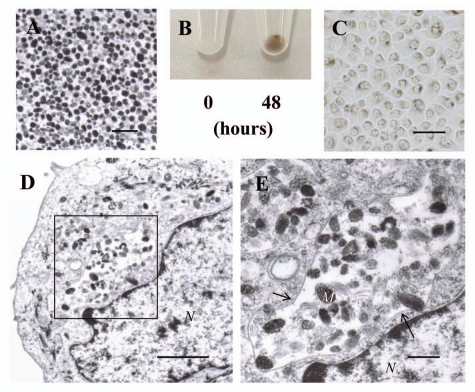
In order to investigate the manner of uptake of individual naked melanosomes compared to pigment globules containing multiple melanosomes, naked melanosomes isolated from normal human melanocytes were added to cultures of normal human keratinocytes. Many melanosomes and various stages of premelanosomes but few other organelles were observed in the purified individual naked melanosomes (Fig. 4A). When those individual naked melanosomes were added to cultures of normal human keratinocytes and were incubated for 48 hours, the pellets of incubated keratinocytes were pigmented (Fig. 4B, right) compared to keratinocyte pellets in which the pigment globules had been removed immediately after addition (Fig. 4B, left). Light microscopy of keratinocytes incubated with individual naked melanosomes for 48 hours showed that many pigment granules were dispersed around the nuclei as observed in the supranuclear melanin caps found in the epidermis and in cultured reconstructed epidermis containing melanocytes,20,21 although, the distribution patterns of incorporated melanosomes was quite different from those obtained from the co-cultures and the pigment globule-treated keratinocytes (Fig. 4C). Electron microscopy of keratinocytes incubated with individual naked melanosomes for 48 hours showed that melanosomes and premelanosomes were present in clusters and were enclosed by an amorphous membrane (Fig. 4D). Higher magnifications revealed that the ingested melanosomes and various stages of premelanosomes were surrounded by a distorted single membrane (Fig. 4E).
Figure 4.
Uptake of individual naked melanosomes isolated from normal human melanocytes by normal human keratinocytes. (A) Electron microscopic image of isolated naked melanosomes. Scale bar: 1 µm. (B) Cell pellets of normal human keratinocytes incubated with isolated naked melanosomes for 0 or 48 hours. The pigmentation in the pellet after 48 hours of incubation is elicited by the naked melanosomes incorporated by normal human keratinocytes. (C) Light microscopic image (bright field) of normal human keratinocytes after incubation with isolated naked melanosomes for 48 hours; Fontana-Masson staining revealed that the dispersed pigment granules had accumulated in the perinuclear area. Scale bar: 50 µm. (D) Representative electron microscopic image of a normal human keratinocyte in (C); the melanosomes and premelanosomes are present in clusters and are enclosed by an amorphous structure membrane. Scale bar: 2 µm. N: nuclei. (E) Higher magnification image of the boxed region in (D) showing that the ingested melanosomes and premelanosomes are surrounded by a distorted single membrane (arrows). Scale bar: 0.5 µm. M: representative melanosomes, N: nuclei.
Discussion
A high frequency of melanosome transfer occurs in our novel melanocyte-keratinocyte co-culture system that utilizes a microporous membrane filter. Since melanocytes are able to penetrate their dendrites through the 1 µm-pore membrane filter, this method enables the convenient detection of melanosomes transferred to keratinocytes without contamination by the co-cultured melanocytes. In order to separate keratinocytes from melanocyte- keratinocyte co-cultures, cell sorter systems have often been used, however, those methods are complicated to handle and are unable to get reliable and reproducible data.17 Our newly developed method allows convenient and stable observation of melanosomes ingested by keratinocytes using light and electron microscopy under conditions where the pigmentation observed is certainly derived from incorporated melanosomes and not from contaminating melanocytes. In addition, the treatment with STI, a known inhibitor of melanosome transfer,18 caused an apparent decrease of melanosome transfer, suggesting that the newly developed melanocyte-keratinocyte co-culture system actually reflects melanosome transfer as is observed physiologically.
One of the interesting findings in this study was that pigment globules containing multiple melanosomes are released into the extracellular space by melanocytes. To date, the evaluation of the possible first step of melanosome transfer, that is, the release of melanosomes from melanocytes, has been measured by the chemical analysis of melanin released into the culture medium.15 In addition, the appearance of membrane vesicles that contain small numbers of melanosomes from human melanoma cells has previously been observed when melanin synthesis was stimulated by 1-oleoyl-2-acetylglycerol.22 However, to our knowledge, there have been no reports of pigment globules containing large numbers of melanosomes being released into the culture medium from melanocyte dendrites, although the existence of floating globules in the culture medium might have been noticed previously during the culture of normal human melanocytes. Since the floating globules in the culture medium of normal human melanocytes contain many floating melanocytes, it might have been difficult to investigate the substance or nature of the floating globules other than melanocytes. In this study, filtration by capillary action through the 8 µm-microporous membrane filter allowed the removal of melanocytes from the mixed floating globules as determined by the lack of staining with DAPI to detect nuclei and thus pigment globules containing multiple melanosomes were found to be released spontaneously into the culture medium.
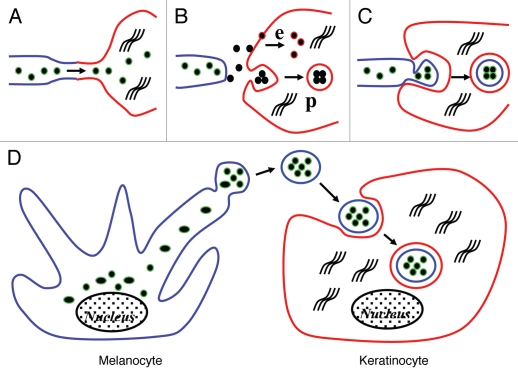
When the existence and structure of lipid membranes surrounding the clusters of incorporated melanosomes in keratinocytes are taken into consideration, one can speculate how melanosomes are transferred to keratinocytes. Among the traditional proposed mechanisms of melanosome transfer, melanosomes were expected to be transferred to keratinocytes via direct inoculation through nanotubular filopodia in cases where there were no surrounding lipid membranes around the incorporated melanosomes, shown schematically in Figure 5A, or via exocytosis and membrane fusion-associated endocytosis of individual melanosomes, shown in Figure 5B. On the other hand, the presence of lipid membranes surrounding the incorporated melanosomes supports the notion that melanosomes are released into the extracellular space and then are phagocytosed by keratinocytes. In cases where the surrounding membrane is single, melanosomes are expected to be exocytosed into the extracellular space individually from melanocytes and then to be phagocytosed by keratinocytes, which results in the single enclosure by keratinocyte-derived plasma membranes (Fig. 5B). Further, in cases where the membrane enclosure is double-layered, it is expected that the melanocyte dendrites containing melanosomes are pinched off and partially cytophagocytosed by keratinocytes (Fig. 5C). In this study, melanosomes transferred in the co-culture system had a double membrane-enclosure surrounding them, suggesting that the ingested melanosomes were cytophagocytosed by keratinocytes together with the plasma membrane of melanocyte dendrites. However, numerous pigment globules containing multiple melanosomes, which had been spontaneously released into the culture medium, were found. In addition, incubation of pigment globules (isolated from the culture medium by filtration through an 8 µm-pore membrane filter or the deposited debris/segments generated from melanocyte dendrites passed through a 1 µm-pore membrane filter) with cultured normal human keratinocytes revealed that melanosomes incorporated into keratinocytes had the same double membraneenclosure observed in the co-culture system. Furthermore, exogenously added individual naked melanosomes were taken up by normal human keratinocytes with a single membrane-enclosure (as depicted in Fig. 5B) in a manner distinct from the co-culture system. Taking these findings into consideration, it seems likely that a novel mechanism of melanosome transfer exists, that is, melanosomes in clusters enclosed by plasma membranes derived from melanocytes are released into the extracellular space and then are phagocytosed by keratinocytes, although partial cytophagocytosis of melanocyte dendrites by keratinocytes can not be ruled out. In the present study, no individual melanosomes without membrane enclosures were observed in keratinocytes in the melanocyte-keratinocyte co-cultures. Therefore, we speculate that the direct inoculation of melanosomes into keratinocytes may not occur nor does the transfer of melanosomes in the pigment globules into keratinocytes via membrane fusion-associated endocytosis, at least in this melanocyte-keratinocyte co-culture system. However, whether a single transfer mechanism or multiple mechanisms are involved in the melanosome transfer pathway remains to be determined.
Figure 5.
Scheme depicting traditional concepts and the new proposed melanosome transfer pathway. (A) Traditional concept showing direct inoculation of melanosomes into keratinocytes through nanotubular filopodia. (B) Traditional concept showing individual melanosome exocytosed from melanocytes and taken up by keratinocytes via membrane fusion-associated endocytosis (e) or phagocytosis (p). (C) Traditional concept showing cytophagocytosis of melanocyte dendrite tips by adjacent keratinocytes. (D) New concept showing numerous melanosomes packed in globules enclosed by the plasma membrane of the melanocyte, released into the extracellular space (into the culture medium) and finally being phagocytosed by normal human keratinocytes and then turned to melano-phagolysosomes enclosed by a double membrane. Color indications: melanocyte plasma membrane is blue, keratinocyte plasma membrane is red, and melanosomal membrane is green.
In conclusion, our results suggest a new mechanism of melanosome transfer wherein melanosomes are packed in globular bodies while budding off from melanocyte dendrites, are released into the extracellular space, and then are phagocytosed by keratinocytes. Although existing cell culture models may not perfectly represent in vivo processes, the novel membrane filter-separated co-culture system we developed will help to elucidate the mechanism of melanosome-containing globule generation from melanocytes and their incorporation by keratinocytes. It may also be useful to explore the destiny of incorporated melanosomes, for example, melanosome degradation spontaneously observed in the skin and hair.11,23
Materials and Methods
Microporous membrane filter-separated co-cultures of melanocytes and keratinocytes.
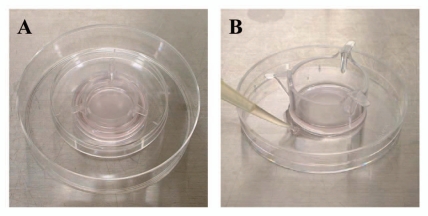
Normal human epidermal keratinocytes (derived from darkly pigmented newborn foreskins, passage 3) (Cascade Biologics) were cultured in medium 154 (Cascade Biologics, 0.2 mM Ca2+) supplemented with a commercial cocktail of growth factors (HKGS, consisting of 0.2 ng/mL human recombinant epidermal growth factor, 0.18 µg/mL hydrocortisone, 5 µg/mL insulin, 5 µg/mL transferrin, and 0.2% (v/v) bovine pituitary extract) and an antibiotic/antimycotic solution (Sigma-Aldrich Company, A5955) at 37°C in a humidified atmosphere with 5% CO2. Keratinocytes were seeded in collagen I-coated 35-mm tissue culture dishes (Becton Dickinson Labware, 35-4456) at 5 × 105 cells/2 mL/well. After 24 hours, the culture medium was changed to 2 mL fresh medium 154 containing HKGS and hanging cell culture inserts, 6-well Millicell®, 1 µm PET (Millipore Corporation, PIRP30R48) were placed on the keratinocytes (the bottom of the Millicell® was attached to the keratinocytes). Normal human melanocytes (derived from darkly pigmented newborn foreskins, passage up to 6) (Cascade Biologics) were then seeded into the Millicell® at 5 × 105 cells/2 mL/well in medium 254 (Cascade Biologics) supplemented with a commercial cocktail of growth factors (HMGS-2, consisting of 10 nM endothelin-1, 3 ng/mL human recombinant basic fibroblast growth factor, 3 µg/mL heparin, 500 nM hydrocortisone, 5 µg/mL insulin, 5 µg/mL transferrin, 0.2 % (v/v) bovine pituitary extract, and 0.5 % (v/v) fetal bovine serum) and an antibiotic/antimycotic solution. In order to place each Millicell® on the keratinocytes softly and to maintain sterility, the top cover of a 60-mm tissue culture dish (Becton Dickinson Labware, 35-3002) was put on each Millicell® (Fig. 6A). After three days, the culture medium was replaced with 4 mL (2.5 mL inside and 1.5 mL outside of the Millicell®) of an equal volume of medium 154 and medium 254 containing the relevant growth factors (HKGS and HMGS-2) and an antibiotic/antimycotic solution. In culture dishes treated with STI, the culture medium was replaced with the same medium containing 2 mg/mL STI 4 hours after the seeding of normal human melanocytes. After an additional three days, each Millicell® was removed slowly and Fontana-Masson staining was performed for some dishes, i.e., the keratinocytes were washed twice with Ca2+ and Mg2+ free Dulbecco's phosphate-buffered saline (D-PBS), and they were then fixed with cold methanol for 10 min at 4°C, washed twice with distilled water, incubated with the Fontana ammoniacal silver solution (Muto Pure Chemicals) for 1 hour at 37°C in a thermostatic oven, and finally washed twice with distilled water.24 Melanosome-incorporated keratinocytes were examined using light microscopy and/or electron microscopy. In order to observe melanocyte-derived segments that had penetrated through the 1 µm-pores of the microporous membrane filters, normal human melanocytes were seeded in the same manner as the co-culture system described above, except with no added keratinocytes, and they were incubated for 6 days with a medium change at three days. After removal of the Millicell®, the bottom of each collagen I-coated 35-mm tissue culture dish was stained with Fontana ammoniacal silver solution and observed by light microscopy as previously detailed.24
Figure 6.
Photos of the originally established materials and methods. (A) Material for the microporous (1 µm-pore) membrane filter separating the melanocyte-keratinocyte co-culture system. The microporous membrane filter is placed between the culture dish (lower) and a top cover (upper). The material was finally placed on the 10 mm dish in order to hold and handle easily. (B) Method of filtering the culture medium of normal human melanocytes. A microporous (8 µm-pore) membrane filter was put on the dish and the culture medium was poured into the inner side of the microporous membrane filter. The liquid containing the pigment globules (diameter less than 8 µm) that penetrated the filter to remove melanocytes was collected slowly by capillary action.
Isolation of pigment globules from the melanocyte culture medium.
Normal human melanocytes were incubated in medium 254 supplemented with a commercial cocktail of growth factors (HMGS, consisting of 10 ng/mL phorbol 12-myristate 13-acetate (PMA), 3 ng/mL human recombinant basic fibroblast growth factor, 3 µg/mL heparin, 500 nM hydrocortisone, 5 µg/mL insulin, 5 µg/mL transferrin, 0.2 % (v/v) bovine pituitary extract, and 0.5 % (v/v) fetal bovine serum) and an antibiotic/antimycotic solution. After two or three days in culture, the culture medium was collected. The collected culture medium from three of the 10 mm tissue culture dishes was centrifuged at 2 × 103 g for 10 min at room temperature (RT) to precipitate floating globules and melanocytes. After the supernatant was decanted, the pellet was suspended in 1 mL of medium 254 supplemented with HMGS-2 to remove PMA before the membrane-coated globules were added to keratinocytes, because PMA has the potency to differentiate keratinocytes.25 The suspension was then further centrifuged at 2 × 104 g for 5 min at R.T. and the supernatant was decanted. The pellet was sequentially suspended by pipetting 20 times in HMGS-2-containing medium 254 in order to dissect out floating globules and melanocytes. The suspension was then poured into a hanging cell culture insert, 6-well Millicell®, 8 µm PET (Millipore Corporation, PIEP30R48) touching the bottom of a 60-mm tissue culture dish and the culture medium penetrated the filter to remove floating melanocytes was collected slowly by capillary action (Fig. 6B). The collected medium was finally centrifuged at 2 × 104 g for 5 min at RT and the pellet was used as the isolated globules fraction. The pellets were stored at −20°C until used to treat the normal human keratinocytes, under the condition that a small amount of culture medium remained on each pellet to prevent desiccation. In order to evaluate whether the purified globules contained melanocytes, fluorescence staining of nuclei by DAPI was performed. The filtered or non-filtered pellets were fixed with 4% formaldehyde neutral buffer solution (Sigma-Aldrich Company, 11-0705-7) for 10 min at RT, washed with D-PBS, incubated with 0.05 µg/mL DAPI solution (Dojindo Laboratories, 340-07971) in D-PBS for 5 min, washed with D-PBS and then observed by fluorescence microscopy (λex = 390 nm, λem = 511 nm).
Treatment of normal human keratinocytes with pigment globules.
Pellets containing the isolated pigment globules were suspended in 100 µL of medium 254 supplemented with HMGS-2 using a pipette 20 times. In order to identify the uniformity between pigment globules isolated from the culture medium and those isolated from the melanocyte dendrites that had penetrated through the 1 µm membrane filter, those globules under the 1 µm membrane filter were also collected and resuspended. The entire suspension of each pellet was added to the culture medium of collagen I-coated six-well plates (Becton Dickinson Labware, 35-4400) in which keratinocytes had been seeded at 5 × 105 cells/2 mL/well 24 hours earlier. After 48 hours of incubation, keratinocytes adhering to the plates were gently washed three times with D-PBS to remove non-ingested pigment globules. Some dishes were used for Fontana-Masson staining. To the other dishes, 1 mL trypsin-EDTA solution (Sigma-Aldrich Company, 25300) was added to each well for 10 min at 37°C and the detached cells were harvested. After centrifugation at 2 × 104 g for 5 min, each cell pellet was washed with D-PBS. After further centrifugation, the cells were used for electron microscopic observations or to evaluate pigmentation.
Preparation and treatment of the individual naked melanosome-rich fraction.
Cultured normal human melanocytes were kept frozen at 2 × 106 cells per Eppendorf tube (1.5 mL). After thawing the frozen cells, 1 mL cold lysis buffer, consisting of 0.1 M Tris-HCl (Invitrogen, 15567-027), pH 7.5, 1% Igepal CA-630 (Sigma-Aldrich, I3021) and 0.01% SDS, was added to the cells and was stored at 4°C for 20 min with mixing every 10 min. After centrifugation (1 × 103 g for 5 min at 4°C), the supernatants were transferred to new Eppendorf tubes (1.5 mL) and centrifuged again in the same manner. The supernatants were then further centrifuged (2 × 104 g for 5 min at 4°C) and the precipitates were washed twice with D-PBS and brief and gentle mixing in order to avoid dispersion and were centrifuged again (2 × 104 g for 5 min at 4°C). The pellets were then used immediately as the individual melanosome-rich fraction. For the treatment of normal human keratinocytes with isolated individual naked melanosomes, 110 µL D-PBS was added to each melanosome pellet (obtained from 2 × 106 melanocytes) and was mixed by pipetting fifty times in order to disperse the individual naked melanosomes. When the melanosomal suspension was homogeneous, 100 µL was added to each well of six-well plates in which keratinocytes had been seeded at 5 × 105 cells/2 mL/well 24 hr earlier.24
Electron microscopic observations.
Cells were harvested with trypsin-EDTA, washed with D-PBS, and fixed with 2% glutaraldehyde in D-PBS at 4°C for at least two hours. After washing twice with D-PBS for 15 min each, the cells were post-fixed with 2% osmium tetroxide for 1.5 hours. After fixation, they were dehydrated in a graded series of ethanol and propylene oxide, and embedded in epoxy resin (EPON812) for 48 hours at 60°C. Ultrathin sections were cut and then stained with uranyl acetate and lead citrate and were examined with an electron microscope (JEM-1200EX, JEOL, Japan) at 80 kV.
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- D-PBS
Ca2+ and Mg2+ free Dulbecco's phosphate buffered saline
- PAR-2
protease-activated receptor-2
- PMA
phorbol 12-myristate 13-acetate
- RT
room temperature
- STI
soybean trypsin inhibitor
References
- 1.Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Mottaz JH, Zelickson AS. Melanin transfer: a possible phagocytic process. J Invest Dermatol. 1967;49:605–610. doi: 10.1038/jid.1967.187. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto O, Bhawan J. Three modes of melanosome transfers in Caucasian facial skin: hypothesis based on an ultrastructural study. Pigment Cell Res. 1994;7:158–169. doi: 10.1111/j.1600-0749.1994.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 4.Seiberg M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. 2001;14:236–242. doi: 10.1034/j.1600-0749.2001.140402.x. [DOI] [PubMed] [Google Scholar]
- 5.Scott G, Leopardi S, Printup S, Madden BC. Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci. 2002;115:1441–1451. doi: 10.1242/jcs.115.7.1441. [DOI] [PubMed] [Google Scholar]
- 6.Van Den Bossche K, Naeyaert JM, Lambert J. The quest for the mechanism of melanin transfer. Traffic. 2006;7:769–778. doi: 10.1111/j.1600-0854.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh SK, Nizard C, Kurfurst R, Bonte F, Schnebert S, Tobin DJ. The silver locus product (Silv/gp100/Pmel17) as a new tool for the analysis of melanosome transfer in human melanocyte-keratinocyte co-culture. Exp Dermatol. 2008;17:418–426. doi: 10.1111/j.1600-0625.2008.00702.x. [DOI] [PubMed] [Google Scholar]
- 8.Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, et al. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp Cell Res. 2000;254:25–32. doi: 10.1006/excr.1999.4692. [DOI] [PubMed] [Google Scholar]
- 9.Sharlow ER, Paine CS, Babiarz L, Eisinger M, Shapiro S, Seiberg M. The protease-activated receptor 2 upregulates keratinocyte phagocytosis. J Cell Sci. 2000;113:3093–3101. doi: 10.1242/jcs.113.17.3093. [DOI] [PubMed] [Google Scholar]
- 10.Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162–167. doi: 10.1046/j.1523-1747.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 11.Boissy RE. Melanosome transfer to and translocation in the keratinocyte. Exp Dermatol. 2003;12:5–12. doi: 10.1034/j.1600-0625.12.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 12.Cardinali G, Ceccarelli S, Kovacs D, Aspite N, Lotti LV, Torrisi MR, et al. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J Invest Dermatol. 2005;125:1190–1199. doi: 10.1111/j.0022-202X.2005.23929.x. [DOI] [PubMed] [Google Scholar]
- 13.Minwalla L, Zhao Y, Cornelius J, Babcock GF, Wickett RR, Le Poole IC, et al. Inhibition of melanosome transfer from melanocytes to keratinocytes by lectins and neoglycoproteins in an in vitro model system. Pigment Cell Res. 2001;14:185–194. doi: 10.1034/j.1600-0749.2001.140308.x. [DOI] [PubMed] [Google Scholar]
- 14.Minwalla L, Zhao Y, Le Poole IC, Wickett RR, Boissy RE. Keratinocytes play a role in regulating distribution patterns of recipient melanosomes in vitro. J Invest Dermatol. 2001;117:341–347. doi: 10.1046/j.0022-202x.2001.01411.x. [DOI] [PubMed] [Google Scholar]
- 15.Virador VM, Muller J, Wu X, Abdel-Malek ZA, Yu ZX, Ferrans VJ, et al. Influence of alpha-melanocyte-stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes. FASEB J. 2002;16:105–107. doi: 10.1096/fj.01-0518fje. [DOI] [PubMed] [Google Scholar]
- 16.Yoon TJ, Hearing VJ. Co-culture of mouse epidermal cells for studies of pigmentation. Pigment Cell Res. 2003;16:159–163. doi: 10.1034/j.1600-0749.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 17.Berens W, Van Den Bossche K, Yoon TJ, Westbroek W, Valencia JC, Out CJ, et al. Different approaches for assaying melanosome transfer. Pigment Cell Res. 2005;18:370–381. doi: 10.1111/j.1600-0749.2005.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paine C, Sharlow E, Liebel F, Eisinger M, Shapiro S, Seiberg M. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol. 2001;116:587–595. doi: 10.1046/j.1523-1747.2001.01291.x. [DOI] [PubMed] [Google Scholar]
- 19.Aspengren S, Hedberg D, Wallin M. Studies of pigment transfer between Xenopus laevis melanophores and fibroblasts in vitro and in vivo. Pigment Cell Res. 2006;19:136–145. doi: 10.1111/j.1600-0749.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 20.Byers HR, Maheshwary S, Amodeo DM, Dykstra SG. Role of cytoplasmic dynein in perinuclear aggregation of phagocytosed melanosomes and supranuclear melanin cap formation in human keratinocytes. J Invest Dermatol. 2003;121:813–820. doi: 10.1046/j.1523-1747.2003.12481.x. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs S, Murli S, De Boer G, Mulder A, Mommaas AM, Ponec M. Melanosome capping of keratinocytes in pigmented reconstructed epidermis - effect of ultraviolet radiation and 3-isobutyl-1-methyl-xanthine on melanogenesis. Pigment Cell Res. 2000;13:458–466. doi: 10.1034/j.1600-0749.2000.130608.x. [DOI] [PubMed] [Google Scholar]
- 22.Cerdan D, Redziniak G, Bourgeois CA, Monsigny M, Kieda C. C32 human melanoma cell endogenous lectins: characterization and implication in vesicle-mediated melanin transfer to keratinocytes. Exp Cell Res. 1992;203:164–173. doi: 10.1016/0014-4827(92)90052-a. [DOI] [PubMed] [Google Scholar]
- 23.Ito M, Hashimoto K, Organisciak DT. Ultrastructural, histochemical, and biochemical studies of the melanin metabolism in eye and skin of pallid mice. J Invest Dermatol. 1982;78:414–424. doi: 10.1111/1523-1747.ep12507677. [DOI] [PubMed] [Google Scholar]
- 24.Ando H, Niki Y, Yoshida M, Ito M, Akiyama K, Kim JH, et al. Keratinocytes in culture accumulate phagocytosed melanosomes in the perinuclear area. Pigment Cell Melanoma Res. 2010;23:129–133. doi: 10.1111/j.1755-148X.2009.00640.x. [DOI] [PubMed] [Google Scholar]
- 25.Dlugosz AA, Yuspa SH. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J Cell Biol. 1993;120:217–225. doi: 10.1083/jcb.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]