Abstract
Locally advanced or metastatic pancreatic cancer has been a long-term challenge to clinicians, due to the poor overall survival rate compared with that of other gastrointestinal malignancies. Recently, with the emerging applications of therapeutic endoscopic ultrasonography (EUS), EUS- guided fine needle injection with antitumor agents is considered a promising modality. In this review, we summarize recently published data on the efficacy of endoscope guided interventional procedures with radioactive seeds. Firstly, EUS-guided iodine-125 seed implantation was reported to have a short-term efficacy on pancreatic cancer, with a three-month partial remission rate of 13.6% to 27%. Thereafter, feasibility of celiac ganglion radiation was tested in animal models to achieve pain relief. Recently, a seed-based stent has been introduced in the endoscopic retrograde cholangio-pancreatography (ERCP) drainage of biliary obstruction caused by pancreatic cancer, with a response rate of 72.7%. In addition, we discuss the potential of radioactive seed-based, endoscope-assisted interventional treatment of patients with locally advanced pancreatic cancer. Further studies should focus on the precise amount and distribution of seeds with the aim to improve the survival rate as well as the quality of life.
Key words: pancreatic cancer, endoscopic ultrasonography, brachytherapy, celiac ganglion radiation, radioactive stent, tumor response
Introduction
Pancreatic cancer has been accepted as one of the most difficult-to-treat cancers. There are few effective therapies available for the control of locally advanced or metastatic pancreatic cancer. Gemcitabine-based chemotherapy is an option that can improve survival rate and quality of life in these patients.1 Adding radiotherapy to chemotherapy may modestly improve the median survival rate over chemotherapy alone for those with locally advanced pancreatic cancer. External irradiation therapy is usually regarded as less effective to treat pancreatic cancer, although it can relieve pain in 50% to 85% of patients.2 Moreover, side effects on the gastrointestinal tract and other organs by external beam radiation therapy (EBRT) limit its further application. Early in the 1990s, intraoperative brachytherapy was introduced for its improvement on the survival time in patients who received palliative surgery. 3,4 Other ways to input radioactive seeds in the solid tumor include computerized tomography (CT) 5,6 or ultrasound-guided methods.7 Advances in image-guided placement of the seeds have enabled physicians to use CT or ultrasound to deliver conformal radiation doses to the tumor tissue, resulting in improved success rates for the local control and significant sparing of the surrounding normal tissues.
With the development of interventional endoscopic ultrasonography (EUS), multiple agents can be delivered by EUS via fine needle injection (FNI) for the treatment of pancreatic cancer.8–10 In addition, interventional EUS can also deliver recombinant adenovirus, cytoimplants, radiofrequency probes, photodynamic drugs and radioactive seeds into the solid pancreatic tumor. Compared with transdermal implantation via CT or abdominal ultrasound, EUS provides a more limpid real-time image, with a shorter puncture pathway. In the current review, the progress on EUS or encoscopic retrograde cholangio-pancreatography (ERCP)-guided brachytherapy and other up-to-date modalities with radioactive seeds in the treatment of unresectable pancreatic cancer is summarized.
EUS-guided iodine-125 implantation in pancreatic cancer
By searching Pubmed with the keywords “brachytherapy”, “seeds”, “endoscopy” and “pancreatic cancer” from January 2000 to July 2010, a list of 9 reports were summarized in Table 1. Before EUS-guided radioactive seed implantation could be applied in human tumor treatment, animal studies were necessary to confirm the safety and to simulate the protocol. Due to the resemblance of anatomy and physiology to humans, pigs became the most suitable animal for the model. Sun et al.11 pioneered the EUS-mediated implantation, with 4 iodine-125 (125I) seeds in each pig. All the tested six pigs tolerated the trial and the median diameter of the lesion around the seed was 3.8 cm after sacrifice. The surrounding pancreas was sonographically normal, and no seed migration occurred. Most importantly, localized tissue necrosis and fibrosis were only achieved in seed-contained pancreas, without significant complications. The usage of 18 or 19 gauge needles was proved to be safe during the puncture of the gastric wall. The study firstly confirmed that EUS-guided implantation of radioactive seeds was a safe and minimally invasive technique for interstitial brachytherapy.
Table 1.
Endoscope-assisted brachytherapy for pancreatic cancer
| Authors, year | Therapy | Trial | n | Tumor response (%) | Adverse events |
| Sun et al, 200511 | Iodine seeds implantation | Porcine | 6 | N/A | Hyperlipasemia |
| Sun et al, 200615 | Iodine seeds implantation | Patient | 15 | Partial (27) | Pancreatitis |
| Minimal (20) | Pseudocyst | ||||
| Stable (33) | Neutropenia | ||||
| Progression (20) | Anemia | ||||
| Jin et al, 200816 | Iodine seeds implantation plus chemotherapy | Patient | 22 | Partial (13) | Hyperamylasemia |
| Stable (46) | Mild fever | ||||
| Progression (41) | Seeds translocation | ||||
| Guo et al, 200936 | Iodine seeds implantation* | Patient | 1 | Complete remission | None |
| Wang et al, 200923 | Celiac ganglion irradiation | Porcine | 12 | N/A | None |
| Guo et al, 200726 | Radioactive stents** | Rabbit | 27 | N/A | N/A |
| Liu et al, 200724 | Radioactive stents | Porcine | 18 | N/A | Pancreatic duct perforation |
| Liu et al, 200925 | Radioactive stents*** | Porcine | 11 | N/A | Mild hyperplasia |
| Liu et al, 200930 | Radioactive stents | Patient | 11 | Stable (73) | None |
| Progression (27) |
*Retroperitoneal metastatic adenocarcinoma; **Esophageal cancer; ***Bile duct carcinoma; N/A: Not applicable.
The radioactive seeds recommended in brachytherapy are iodine-125, iridium-192 or palladium-103. Compared with the latter two sources, iodine-125 has a longer half-life of 59.7 days, which is appropriate in targeting the rapidly growing tumor such as pancreatic cancer. Iridium-192 is always introduced in brachytherapy for gynecological malignancies such as endometrial cancer, with a similar survival rate as the external beam radiotherapy.12 Palladium-103 has been widely accepted as a standard particle in brachytherapy for prostate and breast cancers.13,14 The source of iodine-125 is Na125I, and the package is a titanium alloy tube sealed by laser. Each seed source is 4.5 mm in length and 0.8 mm in diameter, with a mean photon energy of 27–35 KeV gamma ray, an initial dose rate of 7 cGy/h, and a mean radioactivity of 0.694 ± 0.021 mCi (25.6 MBq). As mostly concerned, the penetration distance in the human tissue for each seed is only 1.7 cm, which allows for localizing the energy inside the tumor instead of irradiating the surrounding organs. For the same reason, the implanted seeds are harmless to the patient's relatives. The potential harm to the operators can be minimized by adequate shielding.
Under EUS, the maximal diameter of the tumor is measured by real-time sector ultrasound and the relationship between the surrounding vasculature and the tumor is then identified. The puncture points should be determined by color Doppler technology to prevent the injury to the pancreatic duct or the vessels. Although the exact seed number needed in the therapy can be calculated by the three-dimensional computer system, the excise model for EUS-guided implantation has not been set up yet. The seeds should be well-distributed as shown in Figure 1.
Figure 1.
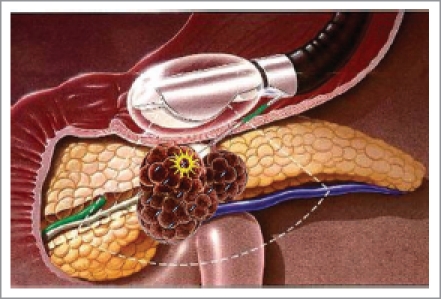
EUS-guided iodine-125 seed implantation in pancreatic cancer
By now, the only available two clinical trials on EUS-guided brachytherapy came from China.15,16 The number of patients enrolled in these two studies was 15 and 22, respectively, with stage III or IV pancreatic cancer in a majority of cases. The study conducted by Sun et al.15 reported an estimated median survival time of 10.6 months and 27% patients reached a partial tumor response toward a mean 22 seeds load per patient. Procedure-related pancreatitis or pseudocyst was only found in three patients, which was considered mild and easily managed. With the combination of gemcitabine, Jin et al.16 further evaluated the clinical efficacy and safety of EUS-guided interstitial implantation of radioactive iodine-125 seeds in advanced pancreatic cancer. Although this novel technique did not significantly improve the overall survival rate, it showed an estimated median survival time of 9.0 months, with a partial remission rate of 13.6% and an estimated one-year survival rate of 27.3%. Moreover, the visual analogue scale pain score significantly dropped from 5.07 to 1.73 one week after brachytherapy and maintained this score for one month. Therefore, these two reports show promising preliminary data that pancreatic cancer can be treated safely with EUS-brachytherapy. Additional larger studies are needed to establish this as an acceptable option for inoperable pancreatic cancer. Compared with single brachytherapy as reported by Sun et al., the seeddrug combined treatment did not show a better tumor response and long-term effect.16 Single gemcitabine chemotherapy provides a median one-year survival rate of 21% (11.0%–37.2%).17 Following the proved safety and feasibility in humans, upcoming randomized controlled trials with long-term follow-up are expected in order to evaluate the efficacy between single EUS-guided implantation and single standardized chemotherapy. It would also be of interest to compare the efficacy and tolerability between EUS-guided brachytherapy and conventional external beam radiation.
EUS-guided celiac ganglion radiation for pain relief
Partial pain relief can be achieved simultaneously when the radioactive seeds release the tumor-killed ray inside the tumor. It is the physician's duty to retrieve the patient from the unbearable pain which leads to the poor living conditions associated with the remaining lifetime of the patient. Celiac plexus neurolysis (CPN) and celiac plexus block (CPB) have been considered the first-line adjuvant therapies for the treatment of pain in pancreatic cancer patients.18 Gunaratnam et al.19 reported that EUS-guided CPN reduced pain in 78% of pancreatic cancer patients. However, CPN can only relieve the pain to a limited degree, and lasts a short period, and the analgesic effect is inversely correlated to the extent of invasion of celiac ganglia.20–22 EUS-guided brachytherapy with the implantation of iodine-125 seeds beside the celiac ganglion seems to be another choice for the pain relief. Recently, Wang et al.23 performed a pilot trial in a porcine model. Four pigs in each group were implanted with one 0.4 mCi or 0.8 mCi iodine-125 seed in either side of the celiac ganglion area with the help of EUS (Fig. 2). Pigs implanted with non-radioactive seeds were used as controls. All animals were sacrificed and the celiac ganglions were checked on days 14 and 60. Compared with the control group, neuronal apoptosis in the ganglion was seen in both brachytherapy groups, and the intensity of necrosis increased with the radiation dose increasing. More apoptotic cells (index as 0.53 and 0.94, respectively) were seen on day 60 of irradiation than on day 14 (0.27 and 0.76, respectively) in both 0.4 mCi and 0.8 mCi groups. There were no significant complications during the experiment. This is the first preliminary evidence for the feasibility and safety of EUS-guided celiac neuron brachytherapy. The new technique may introduce an alternative treatment for pain accompanied pancreatic diseases in humans. However iodine-125 seeds, which have a long period of decay and thus may lead to the maintenance of analgesia, can not reach as rapid effect as CPN or CPB. More animal and clinical trials are needed to determine whether celiac ganglion radiation is superior to classic CPN, and whether the technique can be applied eventually in clinical practice.
Figure 2.
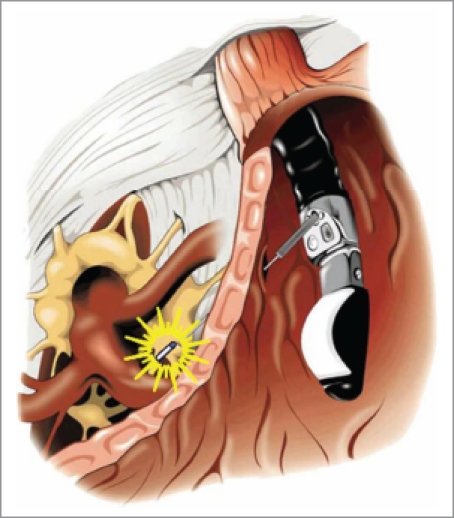
EUS-guided celiac ganglion brachytherapy
Radioactive stents in drainage of pancreatic cancer-induced biliary obstruction
Besides the pain, jaundice is always another symptom that occurs in pancreatic cancer patients, and also another reason for the poor quality of life. The traditional method to remove the obstruction at the lower common bile duct is to insert a plastic or metal stent by ERCP. Liu et al.24,25 reported two separate animal experiments for the usage of radioactive stents in pancreatic cancer and bile duct cancer. For pancreatic cancer, the authors invented a novel plastic cholangio-stent loaded with iodine-125 seeds. The stents were made of polyurethane, which had a negligible influence in shielding the gamma-rays. Brachytherapy was delivered by iodine-125 seeds inserted into the plastic stents. They observed the feasibility and safety by inserting different doses of 50 Gy, 100 Gy, 150 Gy and 200 Gy stents in the pancreatic duct (Fig. 3). The procedures were successfully done on 78% pigs, with four cases of pancreatic duct perforation. Histopathologic examination revealed that the stents were surrounded with necrotic tissues and lateral fibrous tissues after one month. The results indicate that the radioactive stents are safe in all above doses selected, and it is feasible to design a special radioactive stent for each patient according to the size, shape, and position of the pancreatic tumor. In addition to pancreatic cancer, radioactive stents could also be designed to treat esophageal cancer26 or cholangiocarcinoma.27,28 Actually, radiation inside the pancreatic duct is not new. Mutignani et al.29 tried intraluminal brachytherapy by endoscopy in 9 patients with pancreatic head cancer in 2002. Compared with the recently used radioactive stent, they used nasopancreatic drain as a guide-wire for the input of Iridium-192 wire into the pancreatic duct. In 2009, Liu et al.30 firstly reported a clinical trial of iodine-125 seed-coated stents in pateitns with peripancreatic-head cancer. Patients with inoperable extrahepatic bile-duct (n=2), pancreatic-head (n=6), or ampullary (n=3) carcinomas were treated by intraluminal implantation of radioactive stents designed according to a computerized treatment-planning system. Both radioactive stents and commonly used self-expanding metallic or plastic stents were placed in the common bile duct of the patients. A total of 16 radioactive stents were successively placed in all 11 patients and there were no life-threatening complications. The median survival rate was five months, and 72.7% patients had stable disease whereas the remaining patients showed progressive disease. The trial proved that the combination of radioactive stents and metallic and/or plastic stents was technically feasible and tolerable in patients with advanced tumors around the pancreatic-head area. According to the reviews on endoscopic stenting,31,32 newer stents will improve management of these difficult-to-treat diseases and provide prognostic as well as symptomatic benefit in the setting of malignant obstruction.
Figure 3.
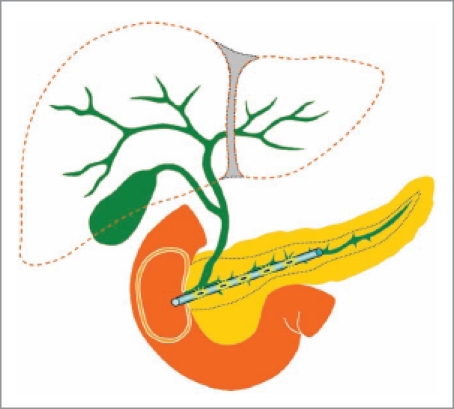
Insertion of a radioactive stent in the pancreatic duct
The therapeutic mechanism of endoscope-assisted brachytherapy
The biologic effects of radioactive seeds in pancreatic cancer cells remain to be determined. Wang et al.33 found that a continuous low-dose-rate (CLDR) irradiation of iodine-125 seeds was more effective in inducing cell apoptosis of Panc-1 cells than an acute high-dose-rate 60Co irradiation. Interestingly, CLDR irradiation by iodine-125 seeds can cause Panc-1 cell-cycle arrest in the G2/M phase and induce apoptosis, which may be an important mechanism underlying iodine-125 seed-induced Panc-1 cell inhibition. Cron et al.34 suggested that the best time for chemotherapy is within 3±4 days after implantation of iodine-125 seeds, because the permeability of the surrounding vasculature is promoted by the radiation effects of the seeds at that time.
Conclusion
Sufficient animal experiments have been done to prove the safety and feasibility of EUS-guided brachytherapy in patients with pancreatic cancer, but its clinical efficacy is still preliminary. In addition to brachytherapy, EUS may be useful in the management of gastro-intestinal cancers through the endoscopic placement of fiducial markers for image-guided radiation therapy.35 EUS-guided brachytherapy may also be used in the treatment of abdominal malignancies other than pancreatic cancer, as reported by Guo et al.36 However, the majority of associated studies were from China, and thus the technique needs to be further validated worldwide. Nevertheless, it is exciting to note that EUS-guided brachytherapy has recently become the topic of live demonstration in many international conferences. Patients with some kinds of malignancies such as prostate cancer have already taken the benefit of brachytherapy over the past decade, and the limited data available so far is encouraging for brachytherapy as a potential treatment for locally advanced pancreatic cancer. Although this technique appears to be feasible, safe and may improve pain control temporarily, larger studies are still needed to further confirm the long-term effect of EUS or ERCP-assisted brachytherapy. These studies must be done with the cooperation among endoscopists, radiologists and oncologists, and not only by the endoscopists alone.
Acknowledgements
The authors would thank Dr. Harry Hua-Xiang Xia, of Novartis Pharmaceuticals Corporation, for the constructive comments on and revision of the manuscript.
Abbreviations
- EUS
endoscopic ultrasonography
- ERCP
endoscopic retrograde cholangio-pancreatography
- EBRT
external beam radiation therapy
- CT
computerized tomography
- FNI
fine needle injection
- CPN
celiac plexus neurolysis
- CPB
celiac plexus block
- CLDR
continuous low-dose-rate
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
References
- 1.Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;3:CD002093. doi: 10.1002/14651858.CD002093.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Minsky BD, Hilaris B, Fuks Z. The role of radiation therapy in the control of pain from pancreatic carcinoma. J Pain Symptom Manage. 1988;3:199–205. doi: 10.1016/0885-3924(88)90031-0. [DOI] [PubMed] [Google Scholar]
- 3.Mohiuddin M, Rosato F, Barbot D, Schuricht A, Biermann W, Cantor R. Long-term results of combined modality treatment with I-125 implantation for carcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1992;23:305–311. doi: 10.1016/0360-3016(92)90746-5. [DOI] [PubMed] [Google Scholar]
- 4.Goertz SR, Ali MM, Parker GA. Local management of pancreatic carcinoma: iodine-125 implantation. Clin Oncol. 1990;2:22–26. doi: 10.1016/s0936-6555(05)80214-6. [DOI] [PubMed] [Google Scholar]
- 5.Wieners G, Pech M, Rudzinska M, Lehmkuhl L, Wlodarczyk W, Miersch A, et al. CT-guided interstitial brachytherapy in the local treatment of extrahepatic, extrapulmonary secondary malignancies. Eur Radiol. 2006;16:2586–2593. doi: 10.1007/s00330-006-0241-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhongmin W, Yu L, Fenju L, Kemin C, Gang H. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol. 2010;20:1786–1791. doi: 10.1007/s00330-009-1703-0. [DOI] [PubMed] [Google Scholar]
- 7.Joyce F, Burcharth F, Holm HH, Strøyer I. Ultrasonically guided percutaneous implantation of iodine-125 seeds in pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1049–1052. doi: 10.1016/0360-3016(90)90032-f. [DOI] [PubMed] [Google Scholar]
- 8.Al-Haddad M, Eloubeidi MA. Interventional EUS for the diagnosis and treatment of locally advanced pancreatic cancer. JOP. 2010;11:1–7. [PubMed] [Google Scholar]
- 9.Ashida R, Chang KJ. Interventional EUS for the treatment of pancreatic cancer. J Hepatobiliary Pancreat Surg. 2009;16:592–597. doi: 10.1007/s00534-009-0129-z. [DOI] [PubMed] [Google Scholar]
- 10.Yan BM, Van Dam J. Endoscopic ultrasound-guided intratumoural therapy for pancreatic cancer. Can J Gastroenterol. 2008;22:405–410. doi: 10.1155/2008/104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S, Qingjie L, Qiyong G, Mengchun W, Bo Q, Hong X. EUS-guided interstitial brachytherapy of the pancreas: a feasibility study. Gastrointest Endosc. 2005;62:775–779. doi: 10.1016/j.gie.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 12.Hampton T. Clinical trials probe new therapies for some difficult-to-treat cancers. JAMA. 2008;300:384–385. doi: 10.1001/jama.300.4.384. [DOI] [PubMed] [Google Scholar]
- 13.Herstein A, Wallner K, Merrick G, Orio P, Thornton K, Butler W, et al. There is a wide range of predictive dosimetric factors for I-125 and pd-103 prostate brachytherapy. Am J Clin Oncol. 2008;31:6–10. doi: 10.1097/COC.0b013e318068415b. [DOI] [PubMed] [Google Scholar]
- 14.Ravi A, Caldwell CB, Keller BM, Reznik A, Pignol JP. Online gamma-camera imaging of 103Pd seeds (OGIPS) for permanent breast seed implantation. Phys Med Biol. 2007;52:5921–5932. doi: 10.1088/0031-9155/52/19/013. [DOI] [PubMed] [Google Scholar]
- 15.Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399–403. doi: 10.1055/s-2006-925253. [DOI] [PubMed] [Google Scholar]
- 16.Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. EUS-guided interstitial implantation of iodine 125 seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314–320. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 17.Xie DR, Liang HL, Wang Y, Guo SS, Yang Q. Meta-analysis on inoperable pancreatic cancer: a comparison between gemcitabine-based combination therapy and gemcitabine alone. World J Gastroenterol. 2006;12:6973–6981. doi: 10.3748/wjg.v12.i43.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaels AJ, Draganov PV. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World J Gastroenterol. 2007;13:3575–3580. doi: 10.3748/wjg.v13.i26.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–324. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 20.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430–438. doi: 10.1111/j.1572-0241.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 21.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–662. doi: 10.1016/s0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 22.Akhan O, Ozmen MN, Basgun N, Akinci D, Oguz O, Koroglu M, et al. Long-term results of celiac Ganglia block: correlation of grade of tumoral invasion and pain relief. AJR Am J Roentgenol. 2004;182:891–896. doi: 10.2214/ajr.182.4.1820891. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Jin Z, Du Y, Chen J, Zhan X, Wang L, et al. Evaluation of endoscopic ultrasound guided celiac ganglion radiation with iodine 125 seeds: a pilot study in porcine model. Endoscopy. 2009;41:346–351. doi: 10.1055/s-0028-1119588. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Liu JL, Cai ZZ, Lu Z, Dong YH, Li ZS, et al. A novel approach for treatment of unresectable pancreatic cancer: design of radioactive stents and trial studies on normal pigs. Clin Cancer Res. 2007;13:3326–3332. doi: 10.1158/1078-0432.CCR-07-0154. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Liu JL, Cai ZZ, Lu Z, Gong YF, Wu HY, et al. A novel approach for treatment of unresectable extrahepatic bile duct carcinoma: design of radioactive stents and an experimental trial in healthy pigs. Gastrointest Endosc. 2009;69:517–524. doi: 10.1016/j.gie.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 26.Guo JH, Teng GJ, Zhu GY, He SC, Deng G, He J. Self-expandable stent loaded with 125I seeds: feasibility and safety in a rabbit model. Eur J Radiol. 2007;61:356–361. doi: 10.1016/j.ejrad.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 27.He GJ, Yu FQ, Wu R, Gao QY, Xu SH, Gao H, et al. 103Pd-induced apoptosis of proliferative smooth muscle cells in bile ducts of dogs: significance and effects on related genes. Hepatobiliary Pancreat Dis Int. 2007;6:521–526. [PubMed] [Google Scholar]
- 28.He GJ, Sun DD, Ji DW, Sui DM, Yu FQ, Gao QY, et al. Induction of biliary cholangiocarcinoma cell apoptosis by 103Pd cholangial radioactive stent gamma-rays. Chin Med J (Engl) 2008;121:1020–1024. [PubMed] [Google Scholar]
- 29.Mutignani M, Shah SK, Morganti AG, Perri V, Macchia G, Costamagna G. Treatment of unresectable pancreatic carcinoma by intraluminal brachytherapy in the duct of Wirsung. Endoscopy. 2002;34:555–559. doi: 10.1055/s-2002-33214. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Lu Z, Zou DW, Jin ZD, Liu F, Li SD, et al. Intraluminal implantation of radioactive stents for treatment of primary carcinomas of the peripancreatic-head region: a pilot study. Gastrointest Endosc. 2009;69:1067–1073. doi: 10.1016/j.gie.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 31.McLoughlin MT, Byrne MF. Endoscopic stenting: where are we now and where can we go? World J Gastroenterol. 2008;14:3798–3803. doi: 10.3748/wjg.14.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito S, Nagata H, Kosugi M, Toya K, Yorozu A. Brachytherapy with permanent seed implantation. Int J Clin Oncol. 2007;12:395–407. doi: 10.1007/s10147-007-0710-x. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Wang J, Liao A, Zhuang H, Zhao Y. The direct biologic effects of radioactive 125I seeds on pancreatic cancer cells PANC-1, at continuous low-dose rates. Cancer Biother Radiopharm. 2009;24:409–416. doi: 10.1089/cbr.2008.0563. [DOI] [PubMed] [Google Scholar]
- 34.Cron GO, Beghein N, Crokart N, Chavée E, Bernard S, Vynckier S, et al. Changes in the tumor microenvironment during low-dose-rate permanent seed implantation iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2005;63:1245–1251. doi: 10.1016/j.ijrobp.2005.07.971. [DOI] [PubMed] [Google Scholar]
- 35.Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, et al. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513–518. doi: 10.1016/j.gie.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Liu Y, Li Z, Wang D, Du Y, Chen J, et al. EUS-guided implantation of radioactive iodine-125 seeds in retroperitoneal metastatic adenocarcinoma. Endoscopy. 2009;41(Suppl 2):E301. doi: 10.1055/s-0029-1214499. [DOI] [PubMed] [Google Scholar]


