Summary
Crohn’s disease and ulcerative colitis are the two major forms of chronic relapsing inflammatory disorders of the human intestines collectively referred to as inflammatory bowel disease (IBD). Though a complex set of autoinflammatory disorders that can be precipitated by diverse genetic and environmental factors, a feature that appears common to IBD pathogenesis is a dysregulated effector T cell response to the commensal microbiota. Due to the heightened effector T cell activity in IBD, developmental and functional pathways that give rise to these cells are potential targets for therapeutic intervention. In this review, we highlight recent advances in our understanding of effector T cell biology in the context of intestinal immune regulation and speculate on their potential clinical significance.
Introduction
CD4+ T cells are selected in the thymus to generate a broad repertoire of clonal specificities that enable peripheral immune surveillance of antigens that are presented by the MHC II pathway. This allows for protective anti-pathogenic T cell responses to microbial threats that enter antigen-presenting cells (APCs) primarily through phagocytic and pinocytotic routes, but it also holds the potential for deleterious responses to host self-antigens and components of the commensal microbiota. Commensal bacteria comprise the largest number and diversity of extracellular, ‘non-self’ antigens that can be encountered by CD4+ T cells, and by far, the greatest number of these reside in the intestinal tract (Ley et al., 2006). Despite robust innate barrier and innate immune mechanisms that largely sequester the gut commensal microbiota from the CD4+ T cell compartment, on-going ‘sampling’ of the flora occurs, reflected in the representation of specificities to antigens of the commensal flora in the peripheral T cell repertoire (Lathrop et al., 2008).
In the setting of immune homeostasis, both effector and regulatory CD4+ T cells (Tregs) that recognize enteric microbial antigens are highly enriched in the gut-associated lymphoid tissues (GALT). Realization that the normal CD4+ T cell repertoire includes specificities that can mount — as well as contain — inflammatory responses to the microbiota came from groundbreaking studies demonstrating that the transfer into immunodeficient mice of normal CD4+ T cells depleted of endogenous regulatory cells resulted in IBD (Morrissey et al., 1993; Powrie et al., 1993), and opened the door to much of our current understanding of T cell regulation of innate and adaptive immune responses to the enteric microbiota. As the organ dedicated to nutrient uptake, however, the intestines also contain a diversity of dietary antigens that pass through the lumen on a daily basis. Remarkably, then, despite T cell receptor specificities directed against ingested food and commensal microbial antigens, immune interactions in the intestines are normally dominated by regulatory activities such that a perfectly mutualistic relationship is maintained with the intestinal microbiota and aberrant effector CD4+ T cell responses to commensal antigens are comparatively rare.
In spite of a dominant system of intestinal immune regulation, spontaneous inflammation still develops in the intestines of about 1 in 1000 individuals in the developed world. It is likely that multiple factors and events must intersect to disrupt the robust system of immune tolerance that has evolved to enable homeostatic coexistence with the intestinal microbiota. Whereas breakdowns in intrinsic barrier and innate immune system functions may initiate IBD, it is evident that effector T cells of the adaptive immune system figure prominently in the sustaining disease, and are probably essential to disease chronicity. The attributes of immune memory intrinsic to CD4+ T cells, coupled with the broad antigenic pool represented in a commensal microbiota that can not be effectively cleared, set the stage for chronic, organ-specific inflammation that is characteristic of many autoimmune disorders. Accordingly, a better understanding of the developmental and functional pathways of effector T cells that are involved in IBD pathogenesis is paramount to improved therapeutics aimed at interrupting the cycle of immune inflammation they propagate.
The two most prevalent forms of these diseases, Crohn’s disease (CD) and ulcerative colitis (UC), can have similar clinical presentations, marked by abdominal pain, bloody diarrhea, weight loss, fever, and fatigue, but differ with respect to histopathologic features, distribution of involvement along the gastrointestinal axis, risk of associated malignancy and, in some cases, treatment options. Crohn’s Disease is a chronic relapsing inflammatory disorder that has historically been considered a Th1-mediated disease. However, recent discovery of the Th17 pathway has prompted a reassessment of whether Th1 cells are pathogenic, as key molecules associated with the development, function, and maintenance of Th17 cells are up-regulated in patients with CD compared to healthy subjects. In contrast to the T cell populations associated with CD, UC is generally considered a “Th2-like” disease, due to the presence of elevated Th2-type cytokines in lesional tissue from UC patients. IL-13 appears to be the predominant T cell-derived cytokine in UC, along with IL-5; IL-4 is typically not prominent. Although classical αβ TCR-expressing effector T cells likely play a role at least early in the pathogenesis of UC, natural killer T (NKT) cells also contribute, a view supported by experimental evidence from animal models (Fuss and Strober, 2008).
Mechanisms limiting effector T cell development and function in the normal intestine
The bulk of effector CD4+ T cells represented in the normal immune repertoire are distributed in the gut-associated lymphoid tissues (GALT), particularly in the lamina propriae and lymphoid follicles of the small and large intestines. The number and activity of intestinal T cells are normally controlled by a carefully orchestrated regulatory system comprised of multiple cell types and regulatory molecules, which act to ensure that intestinal immune homeostasis is the default pathway and spontaneous inflammation an anomaly. The pathways utilized can be loosely grouped into mechanisms that, (1) sequester the commensal microbiota within the intestinal lumen, thereby limiting contact of microbes with the intestinal epithelium, as well as innate and adaptive immune cells (see Review by Duerkop et al. in this issue), (2) restrict activation of innate immune cells exposed to commensal microbial products to limit pro-inflammatory cytokine responses that favor induction of effector T cell responses (see review in this issue by Strober), (3) tolerize the adaptive immune system to the commensal microbiota by promoting the differentiation of regulatory rather than effector T cells (see review in this issue by Barnes and Powrie), and (4) directly inhibit effector T cell differentiation or function (Figure 1).
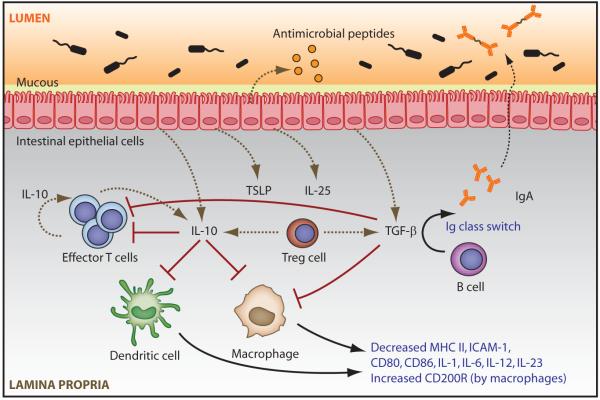
Figure 1. Mechanisms inhibiting the development and local function of intestinal effector CD4+ T cells.
The intestinal epithelium serves as a physical barrier between the intestinal lumen and the lamina propria. Intestinal epithelial cells (IECs) secrete antimicrobial peptides, and goblet cells (not shown) secrete mucous that forms a barrier on the epithelial surface. IECs sense microbederived substances and respond with production of anti-inflammatory cytokines such as TSLP, IL-25, TGF-β and IL-10. IL-10, which is produced by multiple cell types in the intestines, down-regulates APC expression of MHC II, adhesion molecules (eg, ICAM-1), co-stimulatory molecules (eg, CD80 and CD86), and pro-inflammatory cytokines (eg, IL6, IL-23 or IL-12), thereby inhibiting effector T cell (TE) activation. Both IL-10 and TGF-β can induce high amounts of the negative regulator CD200 receptor (CD200R) on tissue macrophages in the small intestine. As a product of effector T cell lineages, IL-10 can also act in an autocrine manner to down-regulate effector T cell responses. Locally activated TGF-β suppresses both the development and function of effector T cells via APC- and/or T cell-directed mechanisms, and promotes iTreg (TR) development (in the absence of pro-inflammatory factors). Active TGF-β also promotes B cell immunoglobulin (Ig) isotype class switching, resulting in the secretion of flora-reactive IgA which is translocated across IECs in association with J-chain into the intestinal lumen, thereby sequestering bacterial antigens from mucosal APCs.
Despite these robust regulatory mechanisms, the intestines of genetically-susceptible individuals still become spontaneously inflamed. It is now clear that there are multiple pathways to intestinal inflammation and increasingly, subsets of UC or CD are being identified. However, excessive or inadequately controlled CD4+ T cell responses feature prominently in the disease cascade, such that CD4+ T cells isolated from diseased patients produce much higher amounts of pro-inflammatory effector cytokines than cells isolated from healthy patients. The combination of T cell hyper-activation and an accompanying breakdown in T cell-mediated regulation set the stage for T cell–associated perpetuation of inflammation.
Overview of effector CD4+ T cell subsets
There are currently, three established subsets of effector CD4+ T cells — Th1, Th2, and Th17 — each of which can be detected in one or another variant of IBD. Cytokines derived from innate immune cells are dominant in initiating the transcriptional programs that specify effector cell differentiation, and key cytokine products of each lineage are crucial for lineage self-preservation, through positive feedback or ‘autoamplification’ loops — as well as through cross-regulatory inhibition of other lineages. Until recently it has been thought that mature CD4+ effector T cells are inherently stable, and do not develop via, and are resistant to, interconversion. However, several studies have now convincingly demonstrated Treg to Th17 and Th17 to Th1 transitions (Lee et al., 2009a; Zhou et al., 2009).
Classical Th1 cells develop in response to antigens of intracellular bacteria and viruses through a program that involves sequential activation of the transcription factors signal transducer of activated T cells (STAT)-1, and STAT4 by type I and II interferons (IFNα, β and IFNγ, respectively) and IL-12 (Murphy et al., 2000). This promotes induction of T-box transcription factor expressed in T cells (T-bet) and IFNγ, the signature transcription factor and cytokine, respectively, of the Th1 lineage. Th1 cells are distinguished from other effector lineages by surface expression of CCR1 and CCR5, as well as CXCR3, which allows trafficking of differentiated Th1 cells to inflammatory beds. IL-27, a member of the IL-12 cytokine family that can also induce expression of T-bet in a STAT1–dependent manner (Kamiya et al., 2004; Takeda et al., 2003), has emerged as a possible player in Th1 differentiation, particularly in the intestines (discussed below).
Th2 cells develop primarily in response to parasitic helminthes that promote innate immune cell production of IL-4, which activates STAT6 and induces up-regulation of the lineage-specifying transcription factor, GATA3 (Ansel et al., 2006). Th2 cells are characterized by their production of the cytokines IL-4, IL-5, and IL-13, and the chemokine receptors CCR3 and CCR4 (O’Garra et al., 1998). Although not considered classical Th2 cells, a distinct population of IL-13-producing NKT cells are detectable in the large intestine of mice and humans, in the context of some types of IBD (see below).
Th17 cells appear specialized for responses to extracellular bacteria and fungi, and their differentiation is induced by combined actions of TGF-β and IL-6, which induce sequential recruitment of STAT3 and retinoic acid receptor-related orphan nuclear receptor (ROR)γt (Weaver et al., 2007), albeit in cooperation with other transcription factors (Brustle et al., 2007; Quintana et al., 2008; Schraml et al., 2009; Veldhoen et al., 2008a; Yang et al., 2008b). IL-17A and IL-17F are characteristic of Th17 cells and promote neutrophil development recruitment and enhanced epithelial barrier function. IL-22, another Th17 cytokine, is important in intestinal epithelial barrier function (Zheng et al., 2007). IL-21 can act as an autocrine growth factor for Th17 cells, although it is not unique to this CD4+ lineage. Th17 cells co-express CCR6 and CCR4 (Acosta-Rodriguez et al., 2007), but despite their preferential accumulation in the intestines at steady state, no association has been made with homing molecules unique to the gut. In fact, retinoic acid, which induces gut tropism in activated T cells, inhibits Th17 development (Mucida et al., 2007), leaving open the question of what controls gut-specific Th17 homing. Developing Th17 cells acquire responsiveness to the IL-12 family cytokine, IL-23, through up-regulation of the inducible component of the IL-23 receptor (IL-23R) (Weaver et al., 2006). Although precise functions of IL-23 remain to be defined, IL-23 appears critical for late developmental functions of Th17 cells that are essential for their immune effector activity and pathogenicity (McGeachy et al., 2007; McGeachy et al., 2009).
Recent reports have proposed the existence of a fourth subset of effector CD4+ T cells, referred to as ‘Th9’ cells, which are characterized by the production of IL-9 and can be induced by treatment of Th2 cells with TGF-β or by antigenic stimulation of naïve T cells in the presence of TGF-β and IL-4 (Dardalhon et al., 2008; Veldhoen et al., 2008b). However, although these cells lack expression of transcription factors typical of other effector lineages, no lineage specification factors have yet been defined for this subset, and IL-9 can also be induced in Th17 and Treg cells (Elyaman et al., 2009; Lu et al., 2006; Nowak et al., 2009), and definitive designation of Th9 as a distinct lineage awaits further studies.
Development of effector T cells is favored in the intestines at steady state
The GALT of the normal intestines includes both effector and regulatory T cell subsets. Because at homeostasis T cell-mediated immune suppression appears to be the default pathway, the normal intestinal lamina propria and its associated lymphoid follicles are home to a large number and relatively high frequency of Foxp3- and/or IL-10-expressing CD4+ T cells (Kamanaka et al., 2006; Maynard et al., 2007; Uhlig et al., 2006). However, Th1 and Th17 cells are also detectable in the uninflamed intestinal lamina propria, in greater numbers than perhaps any other tissue site. The fact that IL-10-producing cells develop alongside all known effector lineages, coupled with increased frequencies of Th1 and Th17 cells in IL-10-deficient mice, suggest a dominant role for IL-10-expressing CD4+ T cells in suppressing intestinal effector T cell development and function, and is consistent with the spontaneous development of colitis under conditions of genetic deficiency of IL-10 production by CD4+ T cells (Roers et al., 2004).
In contrast to Th1 and Th17 cells, Th2 cells are rare or absent from the normal, uninfected intestines. In view of the role of Th2 cells in coordinating host responses to parasitic helminthes, and the relative scarcity of these organisms in the intestinal flora of specific pathogen-free mice and humans living in countries with good sanitation, this is perhaps not surprising. Although in early reports Th2 cells were found in abundance in the intestines (Taguchi et al., 1990), in retrospect, this likely reflected the presence of intestinal parasites in the mouse colonies studied. Thus, mice raised in pathogen-free environments are essentially devoid of intestinal Th2 cells until infected with helminths, in accordance the observed absence of IL-4 production by intestinal DCs that produce basal amounts of pro-inflammatory cytokines that drive Th1 and Th17 responses (Artis et al., 2005).
Development of, or divergence to, intestinal Th1 cells in the steady state
Th1 cells are normally present in both the small and large intestines. Early studies of IL-12 family cytokines found significant expression of mRNA encoding IL-12p40, particularly in the terminal ileum (Becker et al., 2003), suggesting that IL-12 might drive Th1 cell differentiation. It has also been demonstrated that IL-23 is more highly expressed by intestinal APCs, whereas IL-12 is predominant from APCs of peripheral lymphoid tissues, such as spleen (Hue et al., 2006). Although IL-23 can elicit production of IFNγ from memory CD4+ T cells (Oppmann et al., 2000), it does not drive classical Th1 differentiation. Collectively, these data suggest that ‘Th1’ cells detectable in the intestines at steady state might develop via an IL-23-dependent pathway, via an alternative, IL-12-independent pathway, or migrate there following induction in peripheral lymphoid tissues (Figure 2).
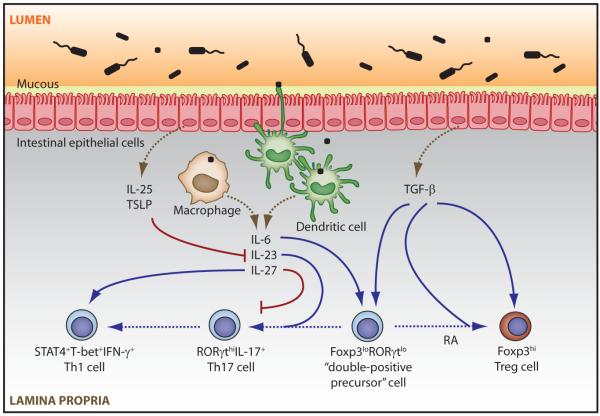
Figure 2. Possible mechanisms of induction and control of intestinal T cell lineage development.
Activation of intestinal APCs by commensal microbes acquired by direct sampling of the luminal contents or via ingestion of transcytosed or invading microbes results in secretion of pro-inflammatory cytokines, such as IL-6, IL-23, and IL-27. Along with TGF-β, IL-6 induces differentiation of a population of Foxp3 and RORγt ‘double-positive precursor’ cells, which express low amounts of both transcription factors. Sustained or increased TGF-β stimulation coupled with reduced IL-6 signaling in the presence of retinoic acid (RA) skews this population towards increased, stable expression of Foxp3 and down-regulation of RORγt and the Th17 program. Conversely, lower amounts of TGF-β signaling in the continued presence of IL-6 favor down-regulation of Foxp3 and divergence towards RORγt-expressing Th17 cells. IL-27 can inhibit Th17 development while directly promoting STAT1-driven differentiation of Th1 effectors. IL-23 produced by TLR and/or NLR-activated APCs promotes late stages of pro-inflammatory Th17 effector functions. Intestinal Th1 cells might emerge from Th17 precursors, or develop independently of the Th17 pathway. Commensal antigen stimulation of IECs induces expression of TSLP and IL-25, which can inhibit Th17 maintenance by suppressing local expression of IL-23.
Although the source of Th1-type cells in the normal GALT is currently unclear, it is now apparent that developing Th17 cells can retain responsiveness to IL-12 and be deviated to a Th1 phenotype late in their development, and at low concentrations of TGF-β, can also deviate to a Th1-like phenotype in the presence of IL-23 and absence of IL-12 (see below). Alternatively, IL-27R-deficient mice display substantially reduced frequencies of IFNγ-expressing CD4+ T cells in the intestinal lamina propria (Troy et al., 2009). Further, IL-27-deficiency is associated with elevated expression of IL-17 in the intestines, consistent with the reported ability of this IL-12 family member to inhibit Th17 differentiation (Batten et al., 2006; Stumhofer et al., 2006). Thus, IL-27 might favor intestinal Th1 development while suppressing Th17 development. However, IL-27 also promotes IL-10 expression in CD4+ T cells and could thus modulate intestinal effector cell homeostasis through indirect, IL-10-dependent effects that favor reciprocal Th1 and Th17 differentiation. None of these mechanisms is mutually exclusive, and a complete understanding of the developmental origins of Th1-type effectors in the intestinal tissues awaits further study.
Steady state intestinal Th17 cells – current concepts and controversies
The enrichment of Th17 cells in the intestinal lamina propria compared to other tissues suggests a role for the commensal flora in the differentiation of this subset, although studies examining this issue have produced conflicting results. In one recent study, the presence of Th17 cells in the SI lamina propria was dependent on commensal microbiota, as mice raised under germ-free conditions were essentially devoid of Th17 cells, and mice of the same genetic strain raised in distinct environments displayed different frequencies of Th17 cells (Ivanov et al., 2008). However, these findings were contradicted by an independent report showing that germ-free mice have normal levels of Th17 cells in the SI lamina propria (Zaph et al., 2008). There have also been reports of the intestinal microbiota both promoting (Atarashi et al., 2008) and suppressing (Zaph et al., 2008) colonic Th17 cell development. The basis for these conflicting results are currently unclear.
Normal frequencies of Th17 cells were present in the small intestine of mice lacking key adapter proteins critical for signaling in the toll-like receptor (TLR) system (Myd88 and TRIF), suggesting that although the commensal microbiota may be critical for intestinal Th17 development, the mechanism can be TLR-independent (Atarashi et al., 2008; Ivanov et al., 2008). Yet, mice deficient for TLR9, which requires MyD88 for signaling, have reduced frequencies of IL-17+ CD4+ T cells in the SI lamina propria (Hall et al., 2008). The reasons for these apparent discrepancies are not known, but it appears that the relationship between innate sensing of the intestinal microbiota and stimulation of Th17 development at steady state may be complex and is likely to multifactorial.
The transcription factors and cytokines important for Th17 development have begun to be studied in the context of the intestines. The transcription factor RORγt is required for optimal development Th17 cells in the small intestines (Ivanov et al., 2006), although small numbers of Th17 cells can also the ROR family member, RORα for intestinal Th17 development (Yang et al., 2008b). To date, there have been no reports examining Th17 development in the intestines of mice with deficiencies of other transcription factors important for Th17 development (eg, IRF4, AhR and Batf).
TGF-β is abundant in the intestines, where it is produced by epithelial cells, as well as innate and adaptive immune cells. Consistent with in vitro findings, TGF-β appears to be required for intestinal Th17 development since mice with TGFβ1 deficiency or defective TGF-β activation restricted to the extracellular matrix lacked intestinal Th17 cells (Ivanov et al., 2008; Mangan et al., 2006). It should be noted, however, that studies to date do not exclude the possibility that reduced frequencies of Th17 cells observed in the absence of active TGF-β are due in part to inhibition of Th17 development by the increased amounts of IFNγ present in mice with defective TGF-β function.
The majority of TGFβ in the intestines is associated with the extracellular matrix in latent form, and multiple cell types can release active TGF-β — and through multiple mechanisms. Notably, however, DCs that express the latent TGFβ-binding integrin, αvβ8 (Mu et al., 2002), can release active TGFβ , which appears to play an important role in TGFβ regulation in the intestines (Travis et al., 2007). The lamina propria of the small intestines harbors a population of CD11chi CD11b+ CD103lo CX3CR1hi DCs that preferentially induces IL-17 in CD4+ T cells via a mechanism that is at least partially dependent on TGF-β (Denning et al., 2007), and in the lamina propria of the large intestine, a population of CD11loCD70hi DCs that harnesses commensal-derived adenosine triphosphate (ATP) to drive Th17 development expresses αvβ8 (Atarashi et al., 2008). Thus, although specific cell populations and mechanisms by which active TGF-β is generated to drive induction of Th17 cells remain to be fully characterized, it is likely that DCs expressing αvβ8 integrin play an important role in the intestines.
To our knowledge, there have been no reports on the status of Th17 cells in intestinal tissues of mice deficient for IL-6, although given its central role in Th17 development, and the production of IL-6 by intestinal DCs (Hart et al., 2005), it is presumed that steady state Th17 development is dependent on IL-6. However, despite the reported requirement for autocrine IL-21 in optimal Th17 development ex vivo, Th17 frequencies in the SI lamina propria appeared to be enhanced in the absence of IL-21 (Ivanov et al., 2008). This is in contrast to peripheral lymphoid tissues where deficient IL-21 signaling was reported to reduce the number of IL-17+ memory CD4+ T cells (Korn et al., 2007). Notably, IL-21 also promotes induction of IL-10 (Maynard et al., 2009; Pot et al., 2009; Spolski et al., 2009), and it is possible that particularly in the steady state intestines, this function of IL-21 is dominant such that enhanced frequencies of Th17 cells associated with IL-21 deficiency might result from reduced suppression by IL-10.
A role, if any, for IL-23 in regulating the numbers of intestinal Th17 cells at steady state is largely unresolved. The frequency of Th17 cells in the small intestines, as well as the degree of IL-17 expression there appeared to be only slightly diminished in the absence of IL-23 (Ivanov et al., 2008). Similarly, Th17 cells were present in the large intestine of uninfected IL-23-deficient mice, which also mounted a robust Th17 response to infection with Citrobacter rodentium (Mangan et al., 2006), suggesting that IL-23 is dispensable for Th17 development in the large intestine in the steady state or in response to infection. In contrast, commensal-dependent production of IL-25 by intestinal epithelial cells results in reduced numbers of Th17 cells in the lamina propria of the large, but not the small intestine, through suppressed induction of IL-23 (Zaph et al., 2008). Indeed, compared to wild type mice, IL-25-deficient mice have increased Il23p19 transcripts and a corresponding increase in IL-17+ cells in the large intestine (Zaph et al., 2008).
Effector T cells in IBD; lessons from animal models and human correlates
Due to limitations inherent to immune studies of human populations, much of our current understanding of the pathogenetic basis for IBD has been derived from studies of mouse models, which have provided important insights into the development of distinct effector populations and how these pathways contribute to IBD. There is still no animal model that is a perfect phenocopy for human IBD, particularly with respect to one of the least understood aspects of human disease — the characteristic cycles of relapse and remission typical of the clinical course in many IBD patients. Instead, most mouse models involve either an acute disease that resolves spontaneously or a chronic, non-remitting disease that eventually results in death of the animal. Also, most models are heavily biased towards inflammation of the large intestine (Table 1). Nevertheless, animal models of IBD have been invaluable in defining pathogenic mechanisms, and offer the advantage of facilitating study of defined alterations in host barrier function, innate immunity and adaptive immunity or alterations of the intestinal microbiota that impact development of intestinal inflammation (Elson et al., 2005).
Table 1.
T Cell-Associated Animal Models of IBD
| Mouse Model | Tissue Affected | Histology Resembles | Effector T Cells Detected | References |
|---|---|---|---|---|
| T cell transfer | Colon | CD | Th1, Th17 | (Izcue et al., 2008; Morrissey et al., 1993; Powrie et al., 1993; Powrie et al., 1994) |
| IL-10-deficient | Colon | CD | Th1, Th17 | (Kuhn et al., 1993; Yen et al., 2006) |
| STAT-4 transgenic | Colon | CD | Th1 | (Wirtz et al., 1999) |
| C3H/HeJBir | Cecum | CD | Th1, Th17 | (Cong et al., 1998; Sundberg et al., 1994) |
| SAMP1/Yit | Distal SI | CD | Th1, Th2 | (Kosiewicz et al., 2001; Matsumoto et al., 1998) |
| TNBS Colitis | Colon | CD | Th1, Th17 | (Alex et al., 2009; Strober et al., 2002) |
| Chronic DSS Colitis | Colon | UC | Th1, Th2, Th17 | (Dieleman et al., 1998; Takedatsu et al., 2008) |
| Oxazolone Colitis | Colon | UC | Th2, NKT Cells | (Boirivant et al., 1998; Heller et al., 2002) |
| TCRα-deficient | Colon | UC | “Th1,” “Th2” | (Mizoguchi et al., 1996; Mombaerts et al., 1993; Nishiyori et al., 2009) |
Whereas a number of mouse models with germline deficiencies of genes linked to immune regulation result in intestinal inflammation, many of these models also induce multi-organ inflammation. These include mice with targeted deficiencies of the genes encoding IL-2, IL-2 receptor, TGF-β, TGF-β receptor II, SMAD3, and A20, as well as CD40 ligand transgenic and TNF-α gene-targeted (TnfαΔARE/ΔARE) mice. Here we highlight those models in which disease is primarily restricted to the intestinal tissues and is dependent on effector T cell responses for induction, perpetuation, or both (Table 1), and discuss correlates with human IBD.
Crohn’s disease: Th1, Th17, or both?
Although Crohn’s disease and mouse models that share features with CD were originally attributed to Th1-mediated pathogenesis, the recent emergence of the Th17 lineage has precipitated a reassessment of its immune-based etiology. The discovery of IL-23 and its subsequent link to induction of IL-17 cytokines and the Th17 pathway resulted in a re-examination of the relative roles of the Th1-asssociated IL-12–IFNγ inflammatory axis and the new, Th17-associated IL-23–IL-17 axis in multiple autoimmune and chronic inflammatory diseases, including IBD. Because IL-12 and IL-23 are heterodimeric cytokines that share the IL-12p40 subunit, and the majority of studies pre-dating IL-23 and Th17 that examined the role of IL-12 in IBD pathogenesis targeted the common IL-12p40 subunit, it was unclear whether the observed disease-ameliorating effects were due to IL-12, IL-23, or both. This, coupled with increases of both Th1 and Th17 cells in inflamed colons of both mice and humans, suggested that Th17 cells might also be key players in IBD.
In both the CD45RBhi T cell transfer and IL-10–deficient models of colitis, early studies showed that development of colitis in both models was almost completely abrogated by early treatment with IFNγ-blocking antibody, consistent with a Th1-mediated pathogenesis (Berg et al., 1996; Powrie et al., 1994). In one report, disease did not develop in the transfer model if CD4+ T cells were isolated from IFNγ-deficient donors (Ito and Fathman, 1997). In support of a Th1 pathogenesis, transfer of T cells deficient for the Th1-associated transcription factor, T-bet, failed to induce disease, whereas adoptive transfer of T-bet–transduced T cells resulted in colitis in recipient mice (Neurath et al., 2002). Further, T cells deficient in the Th1-associated differentiation factor, STAT4, produced substantially reduced disease following transfer (Simpson et al., 1998). In conflict with a strictly Th1 pathogenesis, however, the transfer of IFNγ-deficient CD4+CD45RBhi T cells did induce colitis in one study (Simpson et al., 1998), suggesting the involvement of either an IFNγ-independent Th1-type program or a distinct effector pathway. Consistent with the latter observation, H. hepaticus-induced colitis developed in mice doubly-deficient for IL-10 and IFNγ comparable to that of IL-10-deficient mice (Kullberg et al., 2001), demonstrating that IFNγ was dispensable for disease onset.
As in mouse IBD models, CD was initially postulated to result from a dysregulated Th1 response, based on elevated frequencies of IFNγ- and T-bet-expressing CD4+ T cells in diseased intestinal tissues (Fuss et al., 1996; Matsuoka et al., 2004; Neurath et al., 2002). Increased serum IFNγ was also detected in CD patients, but not in UC patients or normal controls (Beltran et al., 2009). Moreover, expression of both chains of the IL-12 receptor (IL12Rβ1 and IL-12Rβ) and the IL-18R, features of mature Th1 cells, were up-regulated on lamina propria CD4+ T cells of CD patients and enabled enhanced production of IFNγ when the cells were activated ex vivo with IL-12 and IL-18 (Okazawa et al., 2002). Finally, although antibody blockade of IFNγ has shown limited effectiveness in IBD patients, consistent with its limited effectiveness in established disease in mice, treatment with anti-human ‘IL-12’ (in fact, an IL-12p40 mAb that neutralizes both IL-12 and IL-23) has shown substantial efficacy in active CD (Mannon et al., 2004). Although originally offered as evidence in support of a Th1 etiology of CD, recent mouse data demonstrating greater treatment efficacy by interruption of IL-23 rather than IL-12 signaling (see below), has weakened a strict Th1 link in CD.
Although findings implicating factors associated with the Th1 effector pathway in models of CD cannot be ignored, studies that have come in the wake of the discovery of Th17 have increasingly implicated a dominant role for Th17 cells in disease pathogenesis. In addition to the IFNγ-producing cells described in early studies, a sizeable fraction of CD4+ T cells recovered from mucosal compartments in diseased mice of CD45RBhi T cell transfer and IL-10–deficient models are a distinct subset of IL-17-producing CD4+ T cells, as well as some ‘double-producers’ that express both IL-17 and IFNγ (Izcue et al., 2008; Yen et al., 2006). Importantly, adoptive transfers of intestinal bacteria-reactive Th17 cells into immunodeficient recipients induced more severe colitis than comparable transfers of Th1 cells, and induced disease at far lower cell doses (Elson et al., 2007), analogous to earlier findings in a model of EAE (Langrish et al., 2005). Treatment with an IL-23p19 monoclonal antibody inhibited colitis development when administered at the time of transfer, but also suppressed ongoing disease and resulted in the depletion of the transferred Th17 effectors, indicating that IL-23 is required to sustain the pathogenic Th17 population (Elson et al., 2007). Further, deletion of the gene encoding IL-23p19, but importantly, not IL-12p35, inhibited spontaneous colitis in IL-10-deficient mice, proving that IL-23, and not IL-12, is necessary for the spontaneous colitis that develops in this model (Yen et al., 2006). Moreover, treatment with IL-23 accelerated disease onset in RAG-deficient recipients of memory T cells harvested from IL-10-deficient mice. Collectively, these data strongly implicate IL-23-dependent Th17 effector mechanisms in the development of intestinal inflammation.
Other animal studies have recently examined possible contributions of additional components of the Th17 pathway in IBD pathogenesis. Antagonism of IL-6 receptor signaling, or transfer of cells deficient for interferon regulatory factor (IRF)-4 — a transcription factor implicated in Th17 differentiation — blocked induction of colitis in a T cell transfer model. Interestingly, colitis that was indistinguishable from that induced by wild type T cells developed in RAG-deficient recipients of naïve T cells isolated from Il17a−/−, Il17f−/−, or Il22−/− mice (Izcue et al., 2008; Leppkes et al., 2009). However, transfer of Il17f−/− T cells with concomitant neutralization of IL-17A resulted in reduced disease severity, as did transfer of RORγt-deficient naïve T cells, suggesting that RORγt-directed IL-17A and IL-17F redundantly drive inflammation in this model (Leppkes et al., 2009). This is at apparent odds with earlier experiments showing that T-bet deficiency, IFNγ deficiency, or blockade of IFNγ is sufficient to prevent colitis in this model (Berg et al., 1996; Neurath et al., 2002; Powrie et al., 1994), and with a recent report demonstrating enhanced kinetics of wasting disease in recipients of Il17a−/− T cells compared to recipients of wild-type T cells (O’Connor et al., 2009). This latter study suggests a regulatory role for IL-17A early in disease development and the authors propose that this is achieved via suppression of IFNγ production from Th1 effectors, implicating Th1 cells as key culprits in disease onset, consistent with earlier reports. However, none of the histological parameters of experimental colitis were different between recipients of wild type and Il17a−/− T cells and thus any IL-17-mediated protection appears to be readily overcome by the early Th1 burst.
Pre-dating discovery of the Th17 lineage, elevated expression of IL-17A by CD3+ lymphocytes and CD68+ macrophages or monocytes was found in intestinal tissues of CD and UC patients compared to normal patients, or patients with infectious or ischaemic colitis (Fujino et al., 2003). Although IL-17A+ lymphocytes were readily detectable in both CD and UC lesions, the frequencies were higher in CD, and correlated with disease activity and serum IL-17A. A different picture emerged when intestinal transcripts of Il17f, the other IL-17 family member produced by Th17 cells, were measured. Although Il17f mRNA was elevated in inflamed versus non-inflamed biopsies from the same CD patient, it was substantially higher in inflamed lesions from UC patients in comparison to CD patients (Seiderer et al., 2008). However, further work will be necessary to examine the contribution of IL-17F in human IBD.
Whether Th1 cells, Th17 cells, or both are required for experimental colitis continues to be contentious as there is experimental evidence supporting the pathogenicity of each population, although this varies between models and in some instances, between laboratories studying the same models. Nevertheless, it is clear that cells competent for expression of both IFNγ and IL-17 are detectable at all stages of disease in T cell-dependent mouse models, as well as in human CD. The fact that early, but not late blockade of IFNγ prevents disease suggests that IFNγ might be more critical for disease onset than for its persistence. Alternatively, it is possible that other T-bet– and STAT4–dependent aspects of the Th1 pathway can compensate for critical functions of IFNγ. T-bet is believed to promote the pathogenic potential of both Th1 and Th17 in other models of autoimmunity, such as EAE (Yang et al., 2009), and might function in a similar manner in IBD. Finally, recent studies by our group and others have demonstrated developmental plasticity in the Th17 pathway that enables Th17 cells to divert to IFNγ production in the context of colitis (see below) (Annunziato et al., 2007; Lee et al., 2009b; Lexberg et al., 2008), offering the possibility of a pathogenetic mechanism that involves IL-17– and IFNγ–producing T cells that arise from a common developmental pathway.
Ulcerative colitis: a role for Th2 and Th2-like cells?
In contrast to CD, the absence of elevated IFNγ in UC patients fueled early speculation that perhaps the other T effector lineage then known (Th2) might be involved in disease pathogenesis (Niessner and Volk, 1995). However, direct data in support of a Th2 etiology were lacking. In fact, IL-4-expressing T cells were reduced in IBD patients compared to healthy controls, although IL-5 was shown to be increased in UC patients compared to CD patients and normal controls (Fuss et al., 1996). It was later found that whereas there were no major differences in Il4 and Il13 mRNA expression between UC patients with inactive disease and controls, both transcripts were elevated in active UC lamina propria cells. Interestingly, in studies that corroborated the elevated IL-5 and IL-13 secretion by lamina propria cells from UC patients, it was also shown that the major source of the increased IL-13 was a population of CD1d-restricted NKT cells (Fuss et al., 2004).
Despite the on-going association of UC with Th2-type responses — or at least Th2 cytokines, mechanisms involved in the pathogenesis of UC have been limited by the paucity of suitable animal models. The oxazolone challenge model was originally characterized as an acute, Th2-dependent model that displayed certain hallmarks of UC, with the caveat that, unlike UC, colitis in this model resolves spontaneously and does not recur (Boirivant et al., 1998). The early inflammatory infiltrate in this model is characterized by IL-4-, IL-5-, and IL-13-secreting CD4+ T cells, and importantly, systemic administration of anti-IL-4 suppresses disease (Boirivant et al., 1998). Confirming the importance of effector T cell differentiation in this model, mice deficient for IRF-4 develop minimal disease (Mudter et al., 2008), although a caveat here is the recent association of IRF-4 with Th17 development in addition to Th2 development (Brustle et al., 2007). In this regard, disease development is also impeded in the absence of IL-6, a known inducer of Th2 and Th17 cell differentiation (Weigmann et al., 2008).
In subsequent studies of a more chronic variant of the oxazalone model, it was found that an IL-4-dependent early phase of intestinal inflammation was superseded by an IL-13-dominated late phase. The major source of IL-13 was identified as a ‘non-classical’ NKT cell that is restricted by the MHC molecule, CD1d, but unresponsive to the invariant NKT ligand, α-galactosylceramide (Fuss et al., 2004). Accordingly, depletion of NK and NKT cells prior to oxazolone treatment, as well as inhibition of CD1d antigen presentation to NKT cells, prevented the development of disease (Heller et al., 2002). It is still unknown how IL-13 is induced in this model, except that CD1d-restricted antigen presentation is required. It is also unknown how early induction of IL-4 is linked to disease development and progression.
Although not readily detectable at steady state, Th2 cell responses are rapidly induced in the intestines of mice infected with parasitic worms. Most models involve immune-mediated worm expulsion and resolution of inflammation in contrast to the chronic relapsing and remitting inflammation associated with UC. However, these studies have identified key mechanisms that contribute to the initiation and resolution of type 2 inflammation in intestinal tissues, and although it is still unknown whether any of these mechanisms contribute to the Th2-type responses associated with UC, mechanistic insights from these studies could prove informative.
Worm infections result in activation of intestinal epithelial cells (IEC) to produce thymic stromal lymphopoietin (TSLP), which promotes parasite-specific Th2 responses in mucosal tissues (Zaph et al., 2007). TSLP-activated DCs upregulate the Th2-attracting chemokines thymus and activation-regulated chemokine (TARC or CCL17) and MDC macrophage-derived chemokine (MDC or CCL22) and induce Th2 differentiation (Soumelis et al., 2002; Zhou et al., 2005), suggesting that TSLP directly drives Th2 responses. However, Th2 cells still develop in the absence of TSLP signaling, especially if IFNγ is neutralized (Ramalingam et al., 2009; Taylor et al., 2009). Thus, elevated expression of Th1 and Th17 associated cytokines in parasite-infected Tslpr−/− mice, and the resultant defect in Th2 responses, suggest that TSLP might be more critical for suppression of IL-12- and IL-23-dependent responses in the intestine, thereby allowing for the initiation of type 2 immunity (Taylor et al., 2009; Zaph et al., 2007).
IL-25 is constitutively expressed in the intestines, contingent on the presence of a commensal flora, and is therefore more highly expressed in the large intestines than any other tissue (Zaph et al., 2008). IL-25 treatment induces heightened expression of the Th2 effector cytokines IL-5, and IL-13 (Fort et al., 2001), both prominent in human UC, and IL-25 was recently shown to inhibit IL-23-dependent responses in the colon (Zaph et al., 2008). Thus, IL-25 and TSLP appear to perform redundant and non-redundant roles in type 2 immune responses to parasite infection. In this regard, it was recently reported that TSLP induced by intestinal helminth infection promotes the rapid recruitment of basophils, which act both as the primary APC and source of IL-4 to initiate Th2 responses (Perrigoue et al., 2009), establishing a unique role for basophils in type 2 intestinal inflammation. Although it is unclear at present whether IL-25 and TSLP contribute to oxazalone-induced colitis, have links to UC pathogenesis, or promote development and function of non-classical NKT cells, or whether basophils are central to immune pathogenesis, these studies suggest novel mechanisms to be explored in the context of Th2-type intestinal inflammation and IBD.
Immunological ‘flip-floppers’: lineage plasticity of effector and regulatory T cells and possible implications for IBD
Several recent studies have unveiled a previously unappreciated feature of CD4+ T cell biology that promises to impact IBD immune pathogenesis — lineage plasticity among effector and regulatory T cell lineages (Lee et al., 2009a; Zhou et al., 2009). CD4+ T cells expressing both Foxp3 and RORγt have been detected in the normal intestines of both mice and humans, residing alongside subsets that express one or the other of these factors (Miyara et al., 2009; Yang et al., 2008a; Zhou et al., 2008). It is currently unknown whether these ‘dual-expressors’ represent the earliest precursors of Th17 or Treg cells, each of which have been shown in vitro to express both RORγt and Foxp3 prior to extinction of the opposing factor (Zhou et al., 2008), whether these cells represent fully differentiated Foxp3+ Treg cells transitioning to Th17 cells in response pro-inflammatory cytokines, or both. However, this finding raises the intriguing possibility that CD4+ T cells that differentiate in or localize to the intestines retain developmental and functional flexibility to facilitate adjustment to this dynamic environment. The transition of Tregs to Th17 progeny might not be unique, as a recent study provides evidence that Tregs might also acquire Th1 effector features in response to Th1 polarizing cytokine conditions (Wei et al., 2009). It is currently unclear whether similar transitions might occur in the inflamed intestines, and if so, the implications for regulatory control in IBD.
If the developmental plasticity of Tregs in the intestines remains largely speculative, that of Th17 cells is not. Th17 cells defined by an IL-17F reporter transgene could diverge into distinct progeny contingent upon the balance of TGF-β, IL-23 and IL-12 (Lee et al., 2009b). Importantly, the transition of a subset of Th17 precursors to Th1-type cells was associated with the development of colitis following transfer of Th17 cells into immunodeficient recipients, resulting in a population of intestinal effector T cells with mixed Th17 and Th1 phenotypes similar to that found in CD45RBhi transfer controls. In a complementary study, it was found that memory Th17 cells isolated from the mesenteric lymph nodes of normal mice were resistant to transition to Th1 cells (Lexberg et al., 2008), implying that under certain conditions the Th17 program becomes fixed, through mechanisms yet to be defined. These findings might explain the paired coexistence of Th1 and Th17 type cells in intestinal tissues —at homeostasis and in IBD — without invoking independent, parallel development of Th1 and Th17 lineages.
Flexibility in the late Th17 developmental program appears to be shared in man. CD4+ T cells isolated from the intestinal mucosa of CD patients demonstrated distinct subsets of Th1 and Th17 cells, as well as IL-17 and IFNγ double-expressing CD4+ T cells (Annunziato et al., 2007), analogous to findings in mouse models of CD, and Th17 and ‘Th17-Th1’ cells cloned from intestinal isolates were diverted to a Th1 phenotype in response to IL-12. More recently, the expression of the NKT cell marker CD161 has been linked to Th17 cells present in healthy and chronically-inflamed patients (Cosmi et al., 2008; Kleinschek et al., 2009). Notably, CD161 also identifies a sizeable fraction of IFNγ-producers (even larger than the IL-17+ subset). Thus, although initially proposed as a marker for human Th17 cells, CD161 marks precursors that, in response to IL-1 and IL-23 — conditions that favor development of human Th17 cells — also give rise to an important, distinct population of IFNγ+ cells, suggesting that this marker might be common to precursors predisposed to both Th17 and Th1 phenotypes in response to Th17-promoting signals (Cosmi et al., 2008). Thus, it appears that differentiating and early effector Th17 cells are phenotypically unstable and responsive to multiple stimuli that define their late developmental path. The impact of these phenomena on IBD development and persistence are likely to be important, but remains to be defined.
Concluding Remarks
There are multiple pathophysiologic pathways that can culminate in the diseases that we recognize as CD or UC. Animal studies have established that dysregulated effector T cell responses to the commensal flora can be causative in IBD, and likely represent a final, common immunopathogenetic mechanism in most, if not all, forms of IBD, irrespective of the inciting events that promote them. Remarkably, evidence for conserved recognition of a group of immunodominant commensal microbial antigens that is shared by a subset of patients with CD and mice with IBD, particularly bacterial flagellins, provides evidence supporting a common, microbiota-driven disease mechanism in human IBD (Lodes et al., 2004). Given that the pool of microbial antigens encoded by collective genome of commensal microbiota far outstrips that encoded by the host genome, and co-exists in the host from soon after birth until death, it is remarkable that IBD is not more common. It is a testament to the robustness of normal barrier, innate and regulatory adaptive immune responses to the microbiota that these antigens are largely sequestered in the gut lumen, and that homeostasis in the intestinal tract is in fact the norm.
Alternatively, given the antigenic diversity and immune stimulatory potential of the commensal microbiota, and its intrinsic feeding of the extracellular antigen processing pathways linked to MHC II and certain non-classical MHCs (eg, CD1d), it is perhaps not surprising that each of the well-defined CD4+ effector T cell lineages (Th1, Th2 and Th17), as well as NKT effectors, have been implicated in some form of IBD. The challenge now before us will be to better define which effector pathway(s) is active in specific forms of IBD, indeed, in individual patients, and to determine how they might be best brought under control.
As reflected in the studies highlighted herein, even in genetically defined animal models there remains considerable controversy over which effector responses are involved and how, even if there is consensus over the central role played by effector T cells. Although the power of animal models, and in particular, the in-bred, genetically defined — and genetically manipulable — mouse, has proven invaluable in getting us this far, it is likely that animal models will play an increasingly supportive role going forward. Perhaps ironically, increasingly it is the remarkably out-bred human that holds the key to best unraveling the pathogenetic mechanisms important in the complex, polygenic diseases encompassed within IBD. In this post-genomic era, the development of high-throughput technologies for defining micro-genetic variations in the outbred human population has rapidly accelerated discovery of, or confirmation of, important innate and adaptive immune pathways that predispose to intestinal inflammation — precisely because the more random assortment of gene in outbred populations permit the linking of susceptibility haplotypes that are rare or nonexistent in in-bred populations. Thus, in the wake of the report of the first two genes linked to IBD through genome-wide association studies (GWAS), one representing the innate arm (NOD2-CARD15) and one with direct connections to the adaptive arm (IL23R), the number and diversity of susceptibility or protective genetic variations identified in human IBD cohorts is rapidly accelerating (Cho, 2008; Shih et al., 2008), and genes and pathways that were not immediately forthcoming from animal studies are being identified (eg, the autophagy pathway genes, ATG16L1 and IRGM). Continued efforts in this vein should ultimately lead to more patient-specific therapies, as the range of disease-susceptibility (and resistance) genes are more finely mapped, and technologies for efficient, cost-effective patient screening advance. Indeed, it is likely that immunologists, not just IBD patients, will also reap the benefits of this information explosion, by identifying central immunomodulatory genes and pathways heretofore unknown.
Acknowledgments
The authors thank members of the Weaver lab for helpful comments and discussions and Gloria Gaskins for administrative assistance. We offer our apologies to colleagues whose work could not be adequately discussed or cited due to space limitations. This work was supported by the UAB Mucosal HIV and Immunobiology Center and grants from the NIH (AI35783, AI57956 and DK071176, to C.T.W.) and the Crohn’s and Colitis Foundation of America (to C.L.M. and C.T.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- Artis D, Kane CM, Fiore J, Zaph C, Shapira S, Joyce K, Macdonald A, Hunter C, Scott P, Pearce EJ. Dendritic cell-intrinsic expression of NF-kappa B1 is required to promote optimal Th2 cell differentiation. J Immunol. 2005;174:7154–7159. doi: 10.4049/jimmunol.174.11.7154. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, Frick J, Galle PR, Autenrieth I, Neurath MF. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran CJ, Candia E, Erranz B, Figueroa C, Gonzalez MJ, Quera R, Hermoso MA. Peripheral cytokine profile in Chilean patients with Crohn’s disease and ulcerative colitis. Eur Cytokine Netw. 2009;20:33–38. doi: 10.1684/ecn.2009.0142. [DOI] [PubMed] [Google Scholar]
- Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- Fuss IJ, Strober W. The role of IL-13 and NK T cells in experimental and human ulcerative colitis. Mucosal Immunol. 2008;1(Suppl 1):S31–33. doi: 10.1038/mi.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA Limits Regulatory T Cell Conversion and Is a Natural Adjuvant of Intestinal Immune Responses. Immunity. 2008 doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fathman CG. CD45RBhigh CD4+ T cells from IFN-gamma knockout mice do not induce wasting disease. J Autoimmun. 1997;10:455–459. doi: 10.1016/s0896-8411(97)90152-9. [DOI] [PubMed] [Google Scholar]
- Ivanov II, de Frutos LR, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosiewicz MM, Nast CC, Krishnan A, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Kozaiwa K, Cominelli F. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn’s disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, Gorelick PL, Cheever AW, Sher A. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun. 2001;69:4232–4241. doi: 10.1128/IAI.69.7.4232-4241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009a;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009b;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Lexberg MH, Taubner A, Forster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Okabe Y, Setoyama H, Takayama K, Ohtsuka J, Funahashi H, Imaoka A, Okada Y, Umesaki Y. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–78. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Inoue N, Sato T, Okamoto S, Hisamatsu T, Kishi Y, Sakuraba A, Hitotsumatsu O, Ogata H, Koganei K, et al. T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn’s disease. Gut. 2004;53:1303–1308. doi: 10.1136/gut.2003.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- Maynard CL, Hatton RD, Helms WS, Oliver JR, Stephensen CB, Weaver CT. Contrasting roles for all-trans retinoic acid in TGF-beta-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J Exp Med. 2009;206:343–357. doi: 10.1084/jem.20080950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, Bhan AK. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Mudter J, Amoussina L, Schenk M, Yu J, Brustle A, Weigmann B, Atreya R, Wirtz S, Becker C, Hoffman A, et al. The transcription factor IFN regulatory factor-4 controls experimental colitis in mice via T cell-derived IL-6. J Clin Invest. 2008;118:2415–2426. doi: 10.1172/JCI33227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyori A, Nagakura Y, Ichikawa K. Piroxicam accelerates development of colitis in T-cell receptor alpha chain-deficient mice. Eur J Pharmacol. 2009;615:241–245. doi: 10.1016/j.ejphar.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009 doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor W, Jr., Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Garra A, McEvoy LM, Zlotnik A. T-cell subsets: chemokine receptors guide the way. Curr Biol. 1998;8:R646–649. doi: 10.1016/s0960-9822(07)00413-7. [DOI] [PubMed] [Google Scholar]
- Okazawa A, Kanai T, Watanabe M, Yamazaki M, Inoue N, Ikeda M, Kurimoto M, Ishii H, Hibi T. Th1-mediated intestinal inflammation in Crohn’s disease may be induced by activation of lamina propria lymphocytes through synergistic stimulation of interleukin-12 and interleukin-18 without T cell receptor engagement. Am J Gastroenterol. 2002;97:3108–3117. doi: 10.1111/j.1572-0241.2002.07107.x. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Ramalingam TR, Pesce JT, Mentink-Kane MM, Madala S, Cheever AW, Comeau MR, Ziegler SF, Wynn TA. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol. 2009;182:6452–6459. doi: 10.4049/jimmunol.0900181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Muller W. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jurgens M, Schmechel S, Konrad A, et al. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14:437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- Shih DQ, Targan SR, McGovern D. Recent advances in IBD pathogenesis: genetics and immunobiology. Curr Gastroenterol Rep. 2008;10:568–575. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Shah S, Comiskey M, de Jong YP, Wang B, Mizoguchi E, Bhan AK, Terhorst C. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J Exp Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, Elson CO, Bedigian H, Birkenmeier EH. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology. 1994;107:1726–1735. doi: 10.1016/0016-5085(94)90813-3. [DOI] [PubMed] [Google Scholar]
- Taguchi T, McGhee JR, Coffman RL, Beagley KW, Eldridge JH, Takatsu K, Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990;145:68–77. [PubMed] [Google Scholar]
- Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, Braun J, Targan SR. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy AE, Zaph C, Du Y, Taylor BC, Guild KJ, Hunter CA, Saris CJ, Artis D. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–2044. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008a;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ’reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008b;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann B, Lehr HA, Yancopoulos G, Valenzuela D, Murphy A, Stevens S, Schmidt J, Galle PR, Rose-John S, Neurath MF. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. J Exp Med. 2008;205:2099–2110. doi: 10.1084/jem.20072484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Finotto S, Kanzler S, Lohse AW, Blessing M, Lehr HA, Galle PR, Neurath MF. Cutting edge: chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF- plus IFN-gamma-producing CD4+ T cells that respond to bacterial antigens. J Immunol. 1999;162:1884–1888. [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008a;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008b;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]