Abstract
The 17-residue N-terminus (httNT) directly flanking the polyQ sequence in huntingtin (htt) N-terminal fragments plays a crucial role in initiating and accelerating the aggregation process that is associated with Huntington’s disease (HD) pathogenesis. Here we report on magic-angle-spinning solid state NMR studies of the amyloid-like aggregates of an htt N-terminal fragment. We find that the polyQ portion of this peptide exists in a rigid, dehydrated amyloid core that is structurally similar to simpler polyQ fibrils and may contain anti-parallel β-sheets. In contrast, the httNT sequence in the aggregates is composed in part of a well-defined helix, which likely also exists in early oligomeric aggregates. Further NMR experiments demonstrate that the N-terminal helical segment displays increased dynamics and water exposure. Given its specific contribution to the initiation, rate and mechanism of fibril formation, the helical nature of httNT and its apparent lack of effect on the polyQ fibril core structure seem surprising. The results provide new details about these disease-associated aggregates, and also provide a clear example of an amino acid sequence that greatly enhances the rate of amyloid formation while itself not taking part in amyloid structure. There is an interesting mechanistic analogy to recent reports pointing out the early stage contributions of transient intermolecular helix-helix interactions in the aggregation behavior of various other amyloid fibrils.
Keywords: Amyloid fibrils, solid-state NMR, magic-angle-spinning
Introduction
At least nine human diseases are associated with mutations that extend the length of a polyQ-encoding CAG repeat in a corresponding disease protein1. Since neurons in brain tissue from these diseases exhibit polyQ-rich aggregates2, and since polyQ peptides in vivo3 and in vitro4,5 exhibit repeat length dependent aggregation kinetics, there is considerable interest in the role of protein aggregation in the disease mechanisms. The only common element in these disease proteins, the expanded polyQ sequence, in isolation aggregates by a nucleated growth mechanism without any non-amyloid intermediates6–8. While some polyQ flanking sequences do not alter this fundamental mechanism9–11, in other cases the flanking sequence can play a dramatic role by kinetically overriding the normal polyQ nucleation mechanism10,12,13. The nature of the flanking sequences also has direct consequences for the aggregate morphology and observed toxicity14–16. In the protein huntingtin (htt), which is responsible for Huntington’s disease (HD), a short and moderately hydrophobic 17 amino acid N-terminal sequence (httNT) flanking the polyQ sequence plays an enormous role in stimulating aggregation and altering the aggregation mechanism10,17,18. In N-terminal fragments similar to the likely toxic proteolysis products19 of the htt protein, the httNT sequence dramatically enhances aggregation rates in vivo20 and in vitro10,17, apparently by mediating the rapid formation of spherical oligomers with the httNT segment at their core10. Interestingly, most other protein sequences that form amyloid also appear to spontaneously assemble in vitro via the intermediate formation of similar oligomeric structures21–23.
Understanding this mechanism and its products in greater detail may reveal new approaches for intervention in aggregate formation in HD and other disorders. In spite of several experimental10,17,18 and computational24,25 studies, much remains to be learned. Central questions include the structural transformations from monomer to oligomer to fibril for each of the separate domain segments, and how these domains might interact to further affect mechanism and rate. Fibrils formed by polyQ peptides without flanking domains are known to consist of β-sheet structure26,27, analogous to other amyloids. The mechanisms by which flanking sequences can modulate polyQ aggregation mechanisms and/or rates are varied. A polyPro segment C-terminal to polyQ slows aggregation, apparently by altering the distribution of various polyQ conformations in the low molecular weight, soluble ensemble9,28,29. In several other cases, the flanking sequence instead aids or initiates polyQ aggregation by virtue of its own propensity for amyloid formation. In the disease protein ataxin-3 (AT-3), the large Josephin domain initiates aggregation by forming an amyloid structure itself, which then directly or indirectly propagates into the polyQ segment12. Similarly, in an artificial polyQ protein formed by fusion of polyQ to cellular retinoic acid binding protein (CRABP), CRABP aggregates first, followed by polyQ amyloid formation30. In both cases, the Josephin and CRABP domains alone have an independent folded conformation, but are capable of aggregating into amyloid fibrils under appropriate conditions.
As an aggregation-assisting domain, the httNT segment has some interesting differences to these two cases, both in terms of its innate structure and its ability to form amyloid in isolation. The httNT sequence by itself exhibits tediously slow aggregation kinetics10 and, on aggregation, forms oligomeric structures, similar to the intermediates of htt N-terminal fragment aggregation, but fails to progress to amyloid structure. In terms of its structure, simulations31 and structure-propensity calculations17 predict httNT to exist in an α-helical conformation, but a sequence-based analysis of intrinsic structure indicates only a modest tendency toward order10. Experimentally, monomeric httNT exhibits no stable secondary structure by solution NMR10, but displays a degree of α-helicity in CD spectra10,16,25. There is little direct information on the structural transformations undergone by httNT during amyloid formation by httNT-containing polyQ sequences. Mutations within httNT designed to diminish α-helical propensity diminish the aggregation of htt exon1 mutants17 as well as some httNT targeting functions in the cell16. Independent of its initial conformation, it would be reasonable to expect that httNT might become incorporated into the β-stranded amyloid structure along with the polyQ elements. Thus, this is consistent with observations of transformations of native structure to amyloid β-structure (e.g. seen in the Josephin and CRABP domains, as well as other proteins32). This expectation is also in line with simulations suggesting a conversion of α- to β-structure within httNT as a result of, or even a prerequisite for, amyloid formation24. Experimental data that seem consistent with a transition into β-structure include fluorescence data indicating that residues 11 and 17 within httNT in httNT-polyQ peptides become much more solvent-excluded as oligomers transition into amyloid fibrils 10.
Based on the use of magic-angle-spinning (MAS) solid-state (ss)NMR techniques33 augmented by FTIR and electron microscopy (EM), we report here on the structure of fibrillar aggregates formed by the htt N-terminal fragment httNTQ30P10K2 (Figure 1). Incorporation of 13C,15N-labels into residues within the N-terminal segment of this construct permitted site-specific characterization of residues within both the httNT and polyQ domains. This revealed a β-sheet structure for polyQ residues and for residues at the httNT-polyQ boundary, with spectroscopic signatures for the former that indicated a strong resemblance to glutamines in simple polyQ peptide fibrils. Surprisingly, our data also clearly indicated an α-helical segment in the N-terminal portion of the httNT sequence, which shows increased mobility and exposure to water compared to the rigid β-sheet core of the fibrils. FTIR suggests that a helical conformation is also likely the dominant secondary structural feature of the initially formed httNT oligomers. Thus, our data suggest that the httNT sequence exerts its dramatic ability to enhance the rate of formation of β-rich amyloid fibrils without either relinquishing its own initial α-helical structure or greatly influencing the β-sheet structure in the polyQ-rich core of the fibril.
Figure 1.
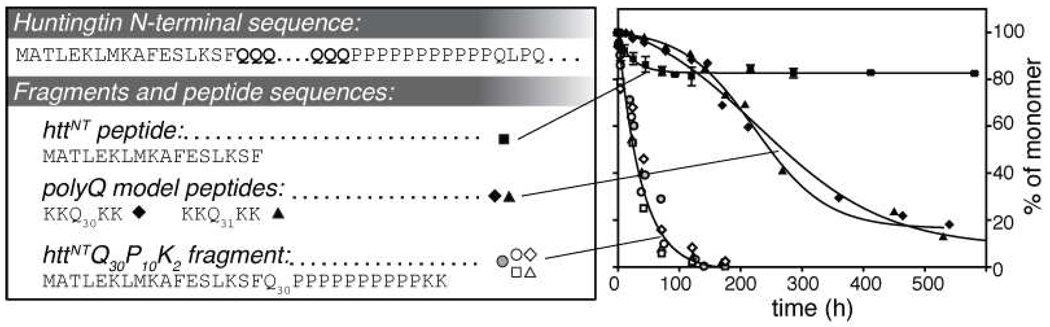
Aggregation kinetics and peptide sequences. Aggregation kinetics were followed by a HPLC-based sedimentation assay, starting from disaggregated monomeric peptide. Labeled (open symbols) and unlabeled (grey circles, R2 = 0.9912, SD= ±3.59) httNTQ30P10K2 (at ~10 µM) show accelerated aggregation relative to peptide lacking httNT, e.g. 10 µM K2Q31K2 (black triangles, R2 = 0.9909, SD= ±4.02) or K2Q30K2 (with [13C,15N-Gln6]; black diamonds, R2 = 0.9926, SD= ±3.16). Note the qualitative difference in the initial rate regime. By itself, 10µM httNT (black squares, R2 = 0.9534, SD= ±1.5169) shows only limited amount of aggregation.
Materials and Methods
Synthesis and fibril formation
Fmoc-protected 13C- and/or 15N-labeled amino acids were from Cambridge Isotope Laboratories (Andover, MA) and Isotec (Sigma-Aldrich, St. Louis, MO). Site-specifically labeled peptides were prepared by solid-phase peptide synthesis by the W.M. Keck Facility at Yale University (see also Table S2 in the Supporting Information). Crude peptide was purified in-house, disaggregated and fibrillized as described previously10,34. Briefly, fibril formation took place in PBS buffer at 37 °C and was monitored via HPLC-based sedimentation assays. The morphology of the mature fibrils was examined via TEM, using a FEI Tecnai 12 electron microscope (Hillsboro, OR), employing negative staining with 1% uranyl acetate.
Fourier transform infrared spectroscopy
Mature aggregated samples were studied by FTIR, using methods analogous to those described previously10. The FTIR samples were prepared by resuspension into 3µl of PBS of a pellet obtained by centrifugation at 20,817 × g for 45 min. The pelleted material was characterized using an MB series spectrophotometer (ABB Bomem, Quebec City, QC, Canada) and PROTA software from Biotools Inc, (Jupiter, FL). The FTIR data are reported as second-derivative spectra as calculated using the PROTA software.
Magic-angle-spinning solid-state NMR spectroscopy
After washing with 10mM sodium phosphate buffer, the mature fibrils were packed into MAS rotors (Bruker Biospin, Billerica, MA) by centrifugation, keeping them hydrated and unfrozen at all times. Unless indicated otherwise, NMR experiments were performed using a wide-bore Bruker Avance I spectrometer operating at 600 MHz 1H Larmor frequency (14.3 T) using Bruker 3.2mm MAS EFree HCN probes. Some data were acquired on an Avance II spectrometer with a 800 MHz 1H Larmor frequency (19 T). The sample temperature was controlled with cooled gas, but samples remained unfrozen. 2D 13C-13C experiments relied on 1H-13C cross polarization (CP) followed by DARR mixing35 for the 13C-13C transfers, with mixing times between 8 and 100ms, and 83–100 kHz 1H TPPM decoupling36 during acquisition and evolution. Additional 13C SQ-DQ 2D experiments at 12kHz MAS and ω0,H/2π=600 MHz employed SPC53 to generate DQ coherence37. Inter-residue distance measurements in sample p4 (httNTQ30P10K2 labeled with 13C’-Leu7, 13Cβ-Ala10, 13C’-Phe17) were performed at 600 MHz (1H frequency) using a 4mm Bruker MAS HCN solenoid probe, employing PDSD 13C-13C recoupling. Water-filtered 1H-13C CP experiments that eliminate rigid 1H signals via a T2-relaxation filter and incorporate a 1H-1H spin diffusion period, were performed analogous to previously published methods (see also Figure S5 in the supporting information)38,39. Spectra were processed and analyzed with the aid of the NMRPipe, Sparky and CCPNMR/Analysis programs40–42. Indirect external referencing of the 13C shifts relative to aqueous DSS was done based on the 13C shifts of adamantane43. Secondary shift calculations involved subtraction of random coil shifts reported by Zhang et al44. Additional experimental details are listed in Table S3 in the SI.
Results
Aggregation kinetics and morphology
Consistent with previous results, the presence of the httNT segment in the httNTQ30P10K2 construct strongly enhances its rate of aggregation compared to polyQ model peptides of an equivalent length (Figure 1). Transmission electron microscopy (TEM) shows that the morphology of the httNTQ30P10K2 fibrils is similar to fibrils formed by analogous constructs with different polyQ lengths10, but appears to differ from the polyQ peptide fibrils (Figure 2). The polyQ peptides form ribbon-like fibrils of variable width, whereas the fibrils formed by httNTQ30P10K2 are more well-defined. As noted previously10, the httNT segment itself is much less prone to aggregate, but does form a limited amount of an oligomeric aggregate, as shown in both Figure 1 and Figure 2. The morphology of these httNT aggregates resembles that of the characteristic oligomeric intermediates observed early in the aggregation of httNTQ30P10K2 and other amyloid fibrils. As expected, the incorporation of 13C and 15N-labeled residues in various peptides does not noticeably affect the fibril morphology or aggregation kinetics (Figure 1; compare open symbols and grey circles), even without seeding with pre-existing aggregates.
Figure 2.
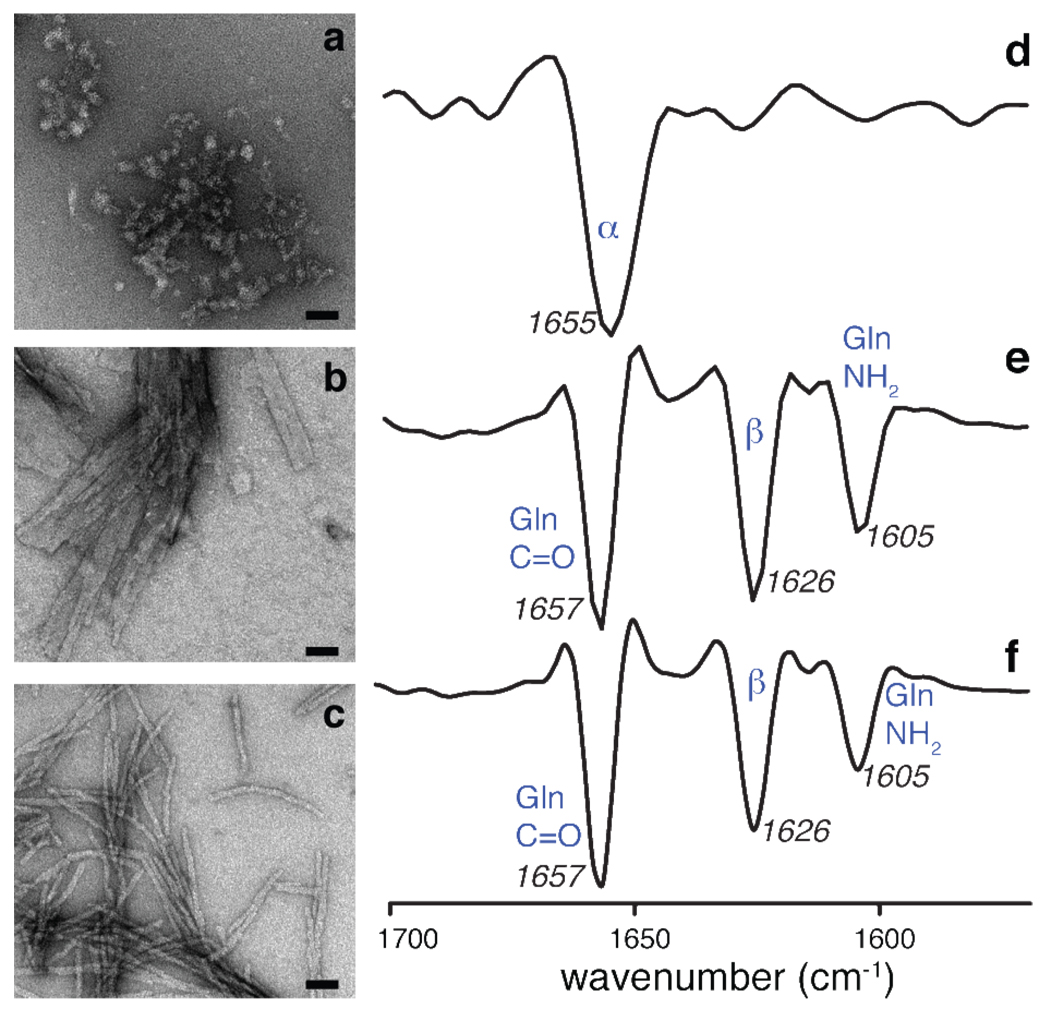
Aggregate morphology and secondary structure. Negatively-stained transmission electron micrographs (left) and second-derivative FTIR data (right) of (a,d) httNT peptide aggregates, (b,e) mature fibrils formed by the polyQ model peptide K2Q31K2, and (c,f) httNTQ30P10K2. The TEM scale bars indicate a length of 50 nm. The httNT FTIR spectrum indicates substantial α-helical content, whereas that of the polyQ fibrils is dominated by the Gln signals and indicates β-sheet secondary structure10. Despite morphological differences by TEM, the httNTQ30P10K2 FTIR data strongly resemble the polyQ results. No helical signals can be unequivocally identified due to the overlap between the Gln signals and the helical band. Band assignments: (d) 1655 cm−1: α-helix; (e,f): 1657 cm−1: Gln side chain C=O stretch; 1626 cm−1: β-sheet structure; 1605 cm−1: Gln side chain NH2 deformation45,46.
Secondary structure analysis of aggregates by FTIR
The secondary structure content of the aggregated peptides was examined by FTIR spectroscopy. The results (Figure 2) show that the oligomeric aggregates formed by the isolated httNT peptide are predominantly α-helical in structure. Note that this is in contrast to the behavior of the monomeric peptide, which was previously found to be largely unstructured by solution NMR10. It is well known that simple polyQ model peptides aggregate into β-sheet rich fibrillar aggregates26,47. Amide I FTIR data on the K2Q31K2 fibrils are consistent with this, showing strong bands characteristic of β-sheet46 as well as bending and stretching modes associated with the glutamine side chain48. The FTIR spectrum of the httNTQ30P10K2 fibrils is dominated by the same bands, as previously reported for related constructs10. Unfortunately, a complete overlap of the α-helix and glutamine side chain bands of the Amide I, as well as the limited ability of FTIR to give segment specific information, prevents us from extracting information on the secondary structure of httNT in these aggregates. Keeping in mind this ambiguity about the structure of the httNT segment in the fibrils and the limited resolution of the technique, our FTIR data are unable to show any structural difference between httNTQ30P10K2 and K2Q31K2 fibrils (even in the respective polyQ domains).
SSNMR chemical shift assignment
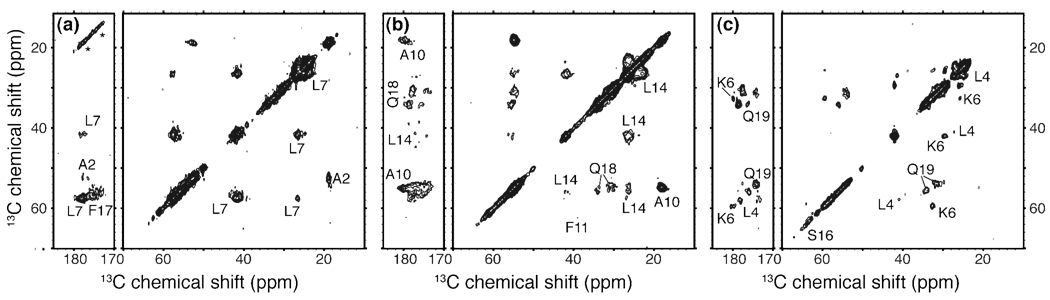
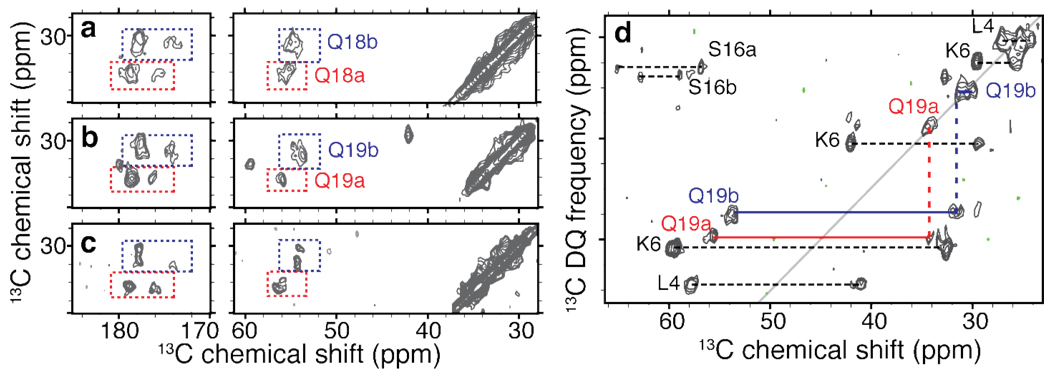
To obtain the required site-specific structural information we applied MAS ssNMR to study fibrils prepared from three differently labeled peptides, featuring U-13C,15N-labeled residues in selected positions (ref. Table S2 in the SI): Ala2, Leu7, Phe17 (sample p1); Ala10, Phe11, Leu14, Gln18 (sample p2); and Leu4, Lys6, Ser16, Gln19 (sample p3). 2D 13C-13C DARR experiments35 were primarily used to assign the 13C resonances (Table S1 in the S.I.). Representative 2D spectra with 25ms mixing time are shown in Figure 3. We observed single resonances for many of the labeled sites (Ala2, Leu4, Lys6, Leu7, Ala10, Phe11, Leu14). However, for several sites multiple signals were observed, most noticeably for the labeled glutamines at positions 18 and 19. The 2D DARR spectra show twice the cross-peaks expected for a single labeled glutamine, as highlighted in Figure 4a–b. Intriguingly, these doubled sets of resonances are nearly identical between Gln18 and Gln19. These observations indicate that both residues feature two distinct conformations with approximately equal intensity. In addition, within each conformer the chemical shifts of the glutamine Cβ and Cγ appear nearly identical. As these shift patterns are somewhat unusual, we obtained further evidence for this from 2D spectra that correlate single-quantum (SQ) and double-quantum (DQ) frequencies, employing SPC53 DQ mixing37 (e.g. Figure 4d). Such spectra lack diagonal peaks (including the signals due to natural abundance 13C sites), facilitating the identification of cross-peaks between nuclei with (nearly) identical shifts. Generally one expects pairs of peaks that bracket a pseudo-diagonal (shown in gray), reflecting directly bonded pairs of sites. If both carbons have identical frequencies, they show up as a single peak on this line, and we indeed observe this for the Gln19 Cβ and Cγ resonances of both forms. Analogous data were obtained for Gln18 (not shown). In addition to the doubling of the glutamine signals, we also observe doubling in Ser16 and Phe17, with the former being clearly visible in Figure 4d. Note that we currently lack the data to know how the two forms for these sequential “doubled” residues (marked ‘a’ and ‘b’ in the figures) correlate to each other.
Figure 3.
2D 13C-13C ssNMR spectra using 25 ms DARR mixing, providing 1–2 bond transfers. Measurements on samples p1–p3 are shown in panels (a)–(c), with (a) obtained at 800MHz 1H frequency and 16 kHz MAS, and (b, c) at 600 MHz 1H frequency and 10 and 13 kHz MAS, respectively. Spinning side bands are marked with asterisks. For each spectrum aliphatic-to-carbonyl (left) as well as the intra-aliphatic (right) spectral regions are shown.
Figure 4.
Doubling and similarity of glutamine resonances. (a,b) 2D 13C-13C DARR spectral regions highlighting the Gln18 and Gln19 resonances in httNTQ30P10K2 samples p2 and p3. For each residue we observe two distinct sets of resonances, marked as ‘a’ (red) and ‘b’ (blue). The cross-peak patterns suggest nearly identical chemical shifts for the Cβ and Cγ sites in each case. Panel (c) shows the virtually identical chemical shifts of a singly labeled glutamine within K2Q30K2 fibrils (U-13C,15N-Gln6 - the fourth residue within the polyQ). (d) 2D 13C-13C SQ-DQ spectrum for sample p3 (using 1.5 ms SPC53 mixing at 12kHz MAS and ω0,H/2π=600 MHz), showing analogously color-coded Gln19 cross peaks. Doubling appears specific to the β-sheet structure: singular sets of resonances are observed for helical residues (e.g. Leu4), doubling can also be seen for Ser16.
Secondary structure identification
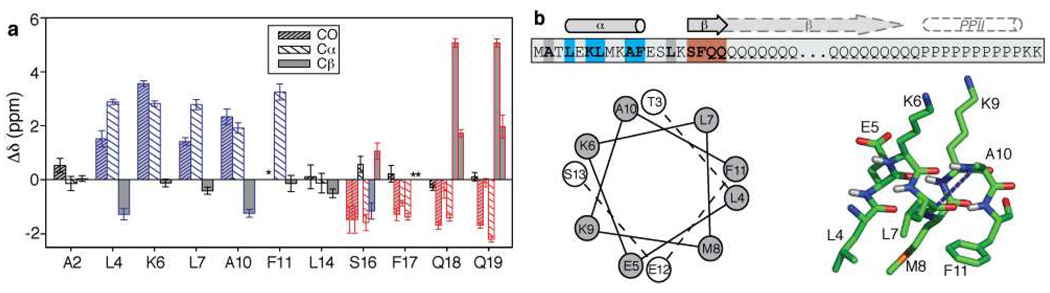
The observed chemical shifts were used to identify the secondary structure of the labeled residues via an approach that compares the shifts to those of the amino acids in defined secondary structures49. Figure 5 shows a graphical representation of the secondary shifts of the labeled residues, calculated through subtraction of random coil chemical shifts44, along with a schematic showing how the secondary structure elements map onto the primary structure. Illustration of the consensus CSI and the Cα-Cβ chemical shift differences (which are insensitive to referencing differences) can be found in the SI (Figure S1). Residues 4, 6, 7, 10, and 11 are α-helical in structure. This is indicative of an amphipathic helix, as illustrated in Figure 5b. Residues 16–19 are predominantly in a β-sheet conformation, with one exception in that one of the two Ser16 forms is neither clearly β-sheet nor α-helical. Residues Ala2 and Leu14 also lack a defined secondary structure, thereby delimiting the length of the helical segment within the httNTdomain. Our identification of the helix was further supported by the observation of an inter-residue (i → i+3) contact consistent with a helical rather than β-sheet conformation. A 2D 13C-13C proton-driven spin diffusion (PDSD) experiment on a sample (p4) specifically labeled in the 13Cβ of Ala10 and 13C’ of Leu7 yielded a cross-peak that is indicative of a distance of no more than 6Å, consistent with the presence of these two residues within an α-helix (Figure S2). We also tentatively identified the background 13C signals due to the unlabeled prolines in the polyPro segment and found them to be similar to those reported for a PPII helical conformation50 (see Figure S3).
Figure 5.
Site-specific secondary structure assignment of httNTQ30P10K2 fibrils. (a) Overview of secondary shifts (Δδ) of the labeled C’, Cα and Cβ sites, revealing helicity (blue) in residues spanning the residues 4–11, whereas β-sheet structure (red) is seen in residues 16–19. Residues 2 and 14 match neither β-sheet nor a-helical conformations (black). Asterisks mark unobserved labeled sites. Bars are split for sites where two conformations were detected (based on doubled chemical shifts). (b) Schematic representation of observed secondary structure alongside the primary sequence (top), with the labeled residues in bold and color-coded as in (a). Bottom: helical wheel view of the helical segment (observed helicity spans the residues in grey) and a schematic illustration of the amphipathic helix, showing the distribution of hydrophobic residues (front and down) and charged residues (upward) with arbitrary side-chain conformations (prepared using PyMOL, Schrödinger, LLC). The blue dashed line indicates the observed inter-residue contact between Ala10-Cβ and Leu7-CO (Figure S2).
PolyGlutamine structure and mobility
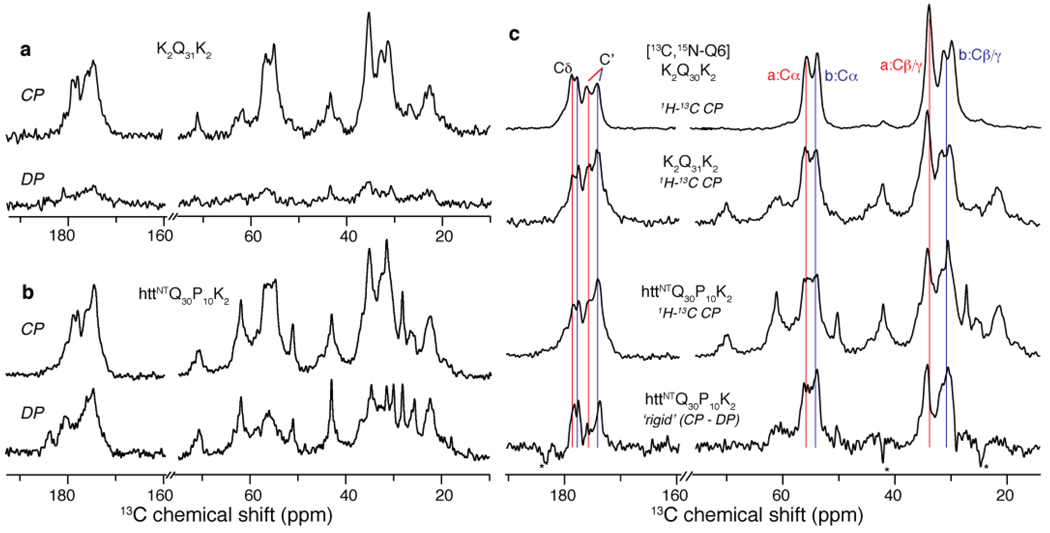
Given the striking similarity between the Gln18 and Gln19 conformations, we also probed the signals of a polyQ domain in fibrils of a polyQ peptide (K2Q30K2) that was labeled in position Gln6 (i.e. the fourth glutamine). The NMR signals of this residue, which was chosen to avoid putative hairpin turns51, showed doubling and chemical shifts that matched the data for Gln19 of httNTQ30P10K2 fibrils (Figure 4). To investigate whether these site-specific data reflect the polyQ domains as a whole, we also examined fully unlabeled fibrils of a K2Q31K2 and httNTQ30P10K2 fibrils. In the absence of labeling, site-specific assignment is difficult, but the observed natural abundance 13C signals in the 1D spectrum of K2Q31K2 (Figure 6a) are expected to predominantly represent a summation of all its combined glutamine signals. Interpretation of the httNTQ30P10K2 spectrum is not so straightforward, since it also includes numerous signals from the httNT and polyPro domains (Figure 6b). Fortunately, however, simple NMR experiments allow us to distinguish sites based on mobility differences. These measurements take advantage of the sensitivity of ssNMR experiments to local dynamics by comparing the observation of 13C magnetization in direct polarization (DP) experiments with signals obtained by 1H-13C CP. In a DP experiment with short inter-scan times, the signal of rigid 13C sites is attenuated due to their slow relaxation. In contrast, 1H-13C CP experiments disfavor mobile sites since the 1H to 13C transfer relies on the dipolar interaction, which is attenuated by dynamics. Indeed, in the K2Q31K2 fibrils, most signals are eliminated in the DP spectrum indicating an overall rigidity of the polyQ amyloid fibrils. In contrast, many resonances in the httNTQ30P10K2 fibrils show up strongly in the DP spectrum and are therefore much more mobile. Application of analogous experiments to the labeled peptides confirmed that these mobile sites are the non-β-sheet httNT residues, while the glutamine resonances are effectively ‘filtered’ out of the DP spectrum (see Figure S4).
Figure 6.
PolyQ amyloid core and the effect of the httNT domain. (a) 1H-13C CP (top) and direct 13C polarization (DP; bottom) of unlabeled K2Q31K2 fibrils. These rigid sites are largely absent from the DP spectrum due to the short recycle delay (3 s). (b) Analogous spectra for unlabeled httNTQ30P10K2 fibrils indicating increased mobility in many sites. (c) Subtraction of mobile sites in the DP spectrum for httNTQ30P10K2 from its CP spectrum (3rd row) reveals the most rigid sites in this sample (bottom). These sites match the pattern of rigid polyQ signals in unlabeled K2Q31K2 fibrils (2nd row), as well as the signal from the singly labeled Gln6 in K2Q30K2 (top), which is virtually identical to Gln19 in the httNTQ30P10K2 fibril core (Figure 4). Thus, the site-specifically labeled glutamine resonances are seemingly unaffected by the presence of httNT, and appear to be representative of the bulk of the glutamines in both httNTQ30P10K2 and simple polyQ fibrils.
Another way to highlight the most rigid sites is by simply subtracting the DP (mobile) signals from the CP spectrum. Applying this approach to the httNTQ30P10K2 fibrils yields a very specific set of resonances that strongly resemble not only the K2Q31K2 fibrils but also the peak positions and characteristic doubling of the labeled glutamine residues (Figure 6c). This indicates a strong resemblance between the molecular conformations of the polyQ domains in both the simple polyQ and httNTQ30P10K2 contexts and suggests that the characteristic structural features shared by all of the labeled glutamines also extend to the bulk of the polyQ domain.
Water accessibility
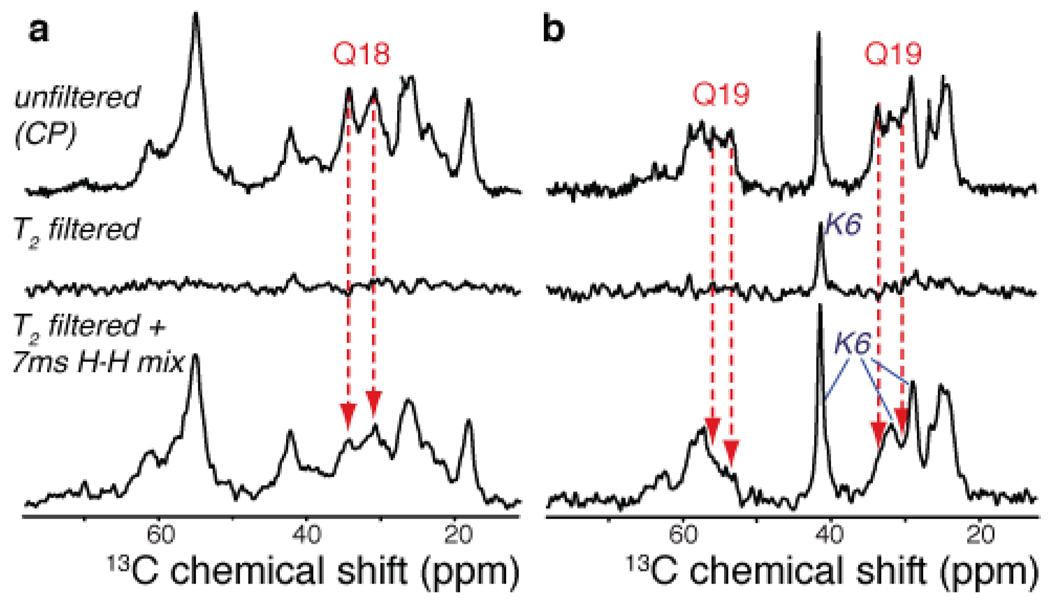
As a picture arises in which the N-terminal helix is mobile and seemingly not incorporated into the amyloid fibril proper, we explored the water-exposure of different parts of these fibrils via relaxation-filtered MAS NMR. This is an approach previously applied to membrane proteins and amyloid fibrils38,39,52. Following a T2 relaxation filter to select the highly mobile water protons, a 1H-1H diffusion period permits the monitoring of magnetization transfer back into the more rigid fibrils, observed as 13C signals following 1H-13C CP transfer. The water-based origin of the 1H polarization was confirmed using 2D 1H-13C spectra (not shown). The data on labeled samples p2 and p3 (Figure 7) reveal that residues in the httNT segment are polarized faster than either of the labeled glutamines. It also appears that the Gln19 sites are less accessible than Gln18. Even though a quantitative interpretation is not straightforward, since various polarization transfer mechanisms can be active in these experiments53, it is clear that the N-terminal helix is more accessible to water than either of the labeled glutamine residues.
Figure 7.
13C-detected water-filtered CP experiments on samples p2 (a) and p3 (b). The top row shows the 1H-13C CP signals in the absence of a T2 filter. A water-proton-selective T2 filter eliminates virtually all peptide signals (middle row). 7ms 1H-1H longitudinal mixing results in 1H polarization transfer from water into the fibrils (bottom). Gln18 and especially Gln19 signals remain suppressed in the water-filtered spectra (red arrows), whereas sites in the httNT helix are easily polarized (e.g., Lys6 in sample p3).
Discussion
N-terminal helicity
The first target in our investigation was the characterization of the conformation of the httNT segment in the mature amyloid fibrils. This short peptide element appears to initiate the aggregation mechanism of exon 1-like peptides, but only when attached to a polyQ sequence of significant length10. As a monomer in solution, the httNT segment by itself appears to exist as an ensemble of compact states that transiently sample α-helical conformations10,16,25. While enhanced helicity and self-association have been observed in certain contexts10,54, whether and how the structure of httNT changes as the aggregation reaction initiates and proceeds has been unclear. The general ability of the amyloid motif to recruit peptide segments into β-structure suggests that the httNT segment might similarly be drawn into the β-structure amyloid core as the polyQ domain adopts it β-stranded amyloid conformation, and recently published molecular dynamics simulations24 support this expectation.
Given the above context, our observations are of particular interest and somewhat surprising, since our ssNMR data on the fibrils clearly reveal helicity in residues spanning positions 4–11, which constitute an amphipathic helix (Figure 5b). HttNT as an amphipathic helix had previously been proposed as a functional unit, for instance as a membrane-binding targeting motif16,20. Amphipathicity and helicity have also been reported as essential for the httNT-dependent enhancement of fibrillization16,17, and to affect toxicity and nuclear accumulation16. However, it has been difficult to obtain solid structural data to support the existence of this α-helix in a biologically relevant setting. In this work, we provide the first direct experimental data demonstrating the presence and precise location of the α-helical segment in the context of the amyloid-like fibrils. The observed helical segment is bracketed by non-helical residues, clearly showing that the α-helix does not span the whole httNT, contrasting with earlier X-ray data54 and simulation results31. Instead, the observed α-helical segment appears to match well to experimental CD data suggesting 40–55% helical content10,16,25 and certain simulations17,31. Solution NMR experiments indicate that in the isolated httNT the helicity may be transient, unstable and most pronounced in the N-terminal few residues10.
Our FTIR data show that self-aggregation of the httNT peptide results in helical aggregates, suggesting that the helical structure is stabilized upon oligomerization. Through interacting with each other, the amphipathic httNT peptides may provide a much more hydrophobic environment than the monomer experiences in solution. This could significantly increase the stability of the helical conformation, in the oligomers and throughout the aggregation process. A recent X-ray study on a fusion construct consisting of a maltose-binding protein fused to the N-terminus of htt exon 1 (featuring 17 Gln residues in its polyQ domain) also revealed helicity in the httNT segment (and beyond).54 Interestingly, the crystal contacts in these crystals included httNT-httNT interactions in a httNT helical bundle, possibly analogous to the types of interacting involved in the early aggregates. A picture arises in which isolated, monomeric httNT has a limited propensity to attain a partial helical structure, but that this propensity may be modulated by interactions involving membranes, the polyQ domain, other httNT molecules, or other proteins.
Glutamine amyloid core structure
As expected, the FTIR data show the presence of high β-sheet content in the K2Q31K2 and httNTQ30P10K2 fibrils. Based on NMR on the labeled httNTQ30P10K2 fibrils, we also know that residues at the httNT-polyQ boundary (including Gln18 and Gln19) are in a β-sheet conformation. The observed rigidity and lack of water-exposure of the glutamines, which extends into the side chains, is consistent with their being in a restricted and dehydrated polar-zipper or steric-zipper-like motif55,56. Among the β-sheet residues at the C-terminal end of the httNT domain, we do see that the Phe17 side chains experience substantial mobility (see Figure S3 in the SI). However, such dynamics do not necessarily require or imply solvent exposure of the Phe rings57.
Both the FTIR and NMR data fail to show any obvious spectroscopic differences for the polyQ amyloid core of ‘simple’ polyQ fibrils compared to that of httNTQ30P10K2 fibrils. More convincingly, we observed strikingly similar patterns of chemical shifts for both Gln18 and Gln19 in httNTQ30P10K2 and for the Gln6 in K2Q30K2. Our data also suggest that most of the glutamine residues within the rigid polyQ core feature the same two conformations that are present in these two site-specifically labeled glutamines. It is thought that the fibrillar polyQ should incorporate tight turns connecting extended β-strands51, and it seems likely that the signals we observed reflect the residues that would occupy the β-strands.
One intriguing feature of all the β-sheet residues is that they present doubled sets of NMR resonances of roughly equivalent intensity. Multiplicity in amyloid fibril ssNMR signals is not uncommon and correlates to a heterogeneity of the molecular conformation within the sample. Polymorphism23 of the macroscopic fibril structure is one potential source. There are several reasons that argue against fibril polymorphism in this particular case. First, no sign of polymorphism has been detected by EM or other methods. Second, we see no sign of doubling for the signals of any of the helical residues, indicating that any polymorphism would have to be restricted to the β-sheet residues without having any consequences for the helical residues. Furthermore, we observe essentially identical spectroscopic signatures (including resonance doubling) for Gln residues in aggregates of both simple polyQ and httNTQ30P10K2 peptides; given the radically different kinetics and mechanisms by which these two types of polyQ-containing peptides aggregate10,11, it is highly unlikely that both peptides would independently grow into identical mixtures of polymorphic structures. Similarly, the relative intensity of the doubled peaks appears relatively invariant between samples, whereas one may expect more variation if these reflected different polymorphic aggregates, each forming according to its own mechanism-based kinetics.
On the other hand, a 1:1 peak ratio would seem inherently consistent with homogeneous samples of certain supramolecular motifs. Since parallel, in-register β-sheet structures inherently feature a single conformation and thus a single NMR signal for each residue56,58, it appears unlikely that the polyQ segment in the htt N-terminal fragment examined here exists in parallel, in-register β-sheets in the amyloid fibril. Other supramolecular assemblies are characterized by different numbers of resonances for each residue59. Thus, signal doubling might be due to a structural inequivalence of identical residues within the fibril assembly, as a consequence of anti-parallel and/or out-of-register arrangements. An anti-parallel assembly, as previously proposed based on X-ray diffraction studies of polyQ aggregates26,27, could indeed generate two distinct conformations for each residue, thus explaining the doubled resonances. Another alternate (but not mutually exclusive) explanation could involve a systematic or ‘random’ register shift of different peptides within the amyloid core, combined with a basic structure where alternating (odd vs even numbered) residues feature distinct side chain conformations. Such a pattern may be accommodated by the uniform polyQ sequence and the resulting out-of-register assembly could be expected to feature distinct sets of shifts for each sequence position (despite being quite uniform in its overall assembly).
Implications for the aggregation mechanism
In summary, we have clearly demonstrated the precise location of a well-defined α-helix within the mature fibrils and found evidence suggesting the presence of α-helical structure in httNT oligomeric aggregates. In the fibrils, the N-terminal residues adopt a water-exposed and somewhat mobile helix packed against the highly rigid and dehydrated amyloid core formed by the glutamine residues. This polyQ amyloid core seems highly repetitive in its molecular structure and shows striking similarities to the simple polyQ fibrils.
These observations complement previous experimental results on the earlier stages of the fibril formation by analogous polypeptides, which revealed an accelerated pathway that includes the formation of at least one oligomeric intermediate initiated via httNT interactions. Our FTIR data indicate a high level of α-helicity to be formed upon self-aggregation of the httNT peptides. The amphipathic helix that we identified in the mature fibrils may thus also be present upon the initial assembly of oligomeric intermediates. While there had been indirect evidence of such an amphipathic helix being involved17, structural data presented here explicitly reveal the existence of an α-helical httNT segment in both httNT oligomers and fibrils. These results argue against a model where the polyQ threshold length triggers fibril formation by permitting httNT to attain a β-conformation24.
Helicity in the oligomeric intermediates involved in amyloid formation is actually not unprecedented and has even been suggested to play a direct role in their formation60. However, in such cases, including two papers published while this manuscript was in review, maturation of the fibrils leads to a conversion into β-sheet structure60–64. The coincidence of our data on httNT-polyQ peptides with recently published data on the islet amyloid polypeptide (IAPP) is, in fact, remarkable: monomeric IAPP lacks a stable helix in isolation, helicity is present in the oligomeric aggregates, which mature into β-stranded amyloid fibrils, and mutations designed to interfere with helix-helix interactions can abolish amyloid formation63. Highly similar considerations were also reported for very short designed peptides 64. Our data provide a striking variation on this emerging theme, in which a helical element plays a critical role in the initial steps of amyloid formation, but without taking on β-sheet characteristics and without being incorporated into the amyloid core of the end stage fibrils.
An intriguing question is whether or how these observations affect the thinking about amyloid formation in a more general sense. For instance, the principles that have been applied in the design of computational models aimed at predicting amyloid formation rates from primary sequence information currently often reflect the compatibility of test sequences with a generic amyloid structural motif65,66. If there is indeed a critical role for helical conformations as well as non-local interactions (involving domains that never end up in an amyloid structure), then an over-reliance on compatibility with the final amyloid conformation may not fully capture the kinetics governing the formation process. In htt exon 1 aggregation it seems quite clear that the httNT segment provides an orders-of-magnitude boost in aggregation kinetics, as well as a dramatic change in mechanism (Thakur, 2009), while never itself engaging the cross-β amyloid motif. Naturally, it remains to be seen whether the mechanism by which httNT stimulates fibril formation is a rare oddity, or is a more common, but difficult to observe, phenomenon. Recent data on IAPP and other proteins (see above) lend some support to the latter point of view.
In terms of the mature amyloid core structure, the spectroscopic similarities between the polyQ domain within the htt N-terminal context and in the simple polyQ peptides indicate a resemblance in molecular structure. Given that amyloid structure can be very sensitive to the fibrillization conditions and mechanism, this would appear to imply that the polyQ β-sheet core in the htt-context might be formed along a similar pathway as occurs in the ‘simple’ polyQ systems. The role of the httNT may be mostly to bring the polyQ domains into close proximity, thus increasing the local concentration and permitting the fibril formation to start, without heavily modifying the resulting molecular structure. Note that the (local) polyQ concentration is known to have a large effect on the aggregation kinetics4,6,11. Thus, some of the structural and mechanistic lessons gleaned from studies of simple polyQ peptides may apply to features of htt fragments as well. This applies for instance to the observation that our NMR data show doubled chemical shifts specific to the β-sheet residues, raising doubts about assumptions of a parallel in-register conformation in the polyQ amyloid core18,29. Rather, it is perhaps more consistent with other arrangements like those proposed based on experimental data from simple polyQ fibrils26,27. As previously noted, unique supramolecular features may be facilitated by the uniform polyQ sequence, in contrast to amyloid fibrils with glutamine-rich, but more complex primary sequences67.
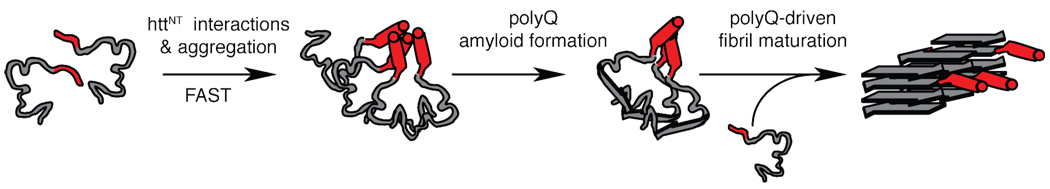
These considerations then lead to a schematic picture of the aggregation process as illustrated in Figure 8. Key to this proposed pathway is the distinction between the roles of the N-terminal segment and the polyQ domain in the initiation of aggregation and the subsequent (but structurally separate) formation of amyloid structure and maturation into fibrils. Once an amyloid core has formed (i.e., is nucleated), it is likely that continued growth of the fibrils no longer relies on the httNT-httNT interaction. From the NMR data it is nonetheless clear that the httNT helical conformation is shared by all peptides throughout the fibrils. Various questions remain, such as the detailed molecular structure of the amyloid core and the nature of potential httNT interactions with itself, the polyQ domain or the polyPro PPII helix. Nonetheless, these new experimental data have clearly delineated key features of the process in vitro, which may well play similar roles in the aggregation process in vivo and thus affect the onset of the disease.
Figure 8.
Schematic model of httNT-initiated aggregation process. (a) In solution, the monomeric species lacks a well-defined structure. The httNT segment may have some helical propensity, but appears to lack a stable α-helical structure. (b) The httNT segment initiates the aggregation process, mediated by httNT-httNT interactions resulting in oligomeric species featuring substantial helicity. (c) This leads to a much-increased local polyQ concentration and permits the formation of the amyloid core, involving the generation of β-sheet secondary structure within the polyQ stretch. (d) Upon maturation, a highly rigid and dehydrated amyloid core (consisting of the polyQ) is decorated with the N-terminal residues 4–11 persisting in an α-helix that is relatively mobile and solvent exposed. Not shown is the polyPro domain that forms an immobilized PPII helix in the mature fibrils.
Supplementary Material
ACKNOWLEDGMENT
We thank Józef Lewandowski and Rakesh Mishra for helpful discussions and Mike Delk for technical support. This work was funded in part from startup funds from the University of Pittsburgh to PvdW, and by the US National Institutes of Health R01 grant AG 019322 to RW.
Footnotes
Supporting Information Available. Table of chemical shift assignments, additional secondary structure analysis schematics, 2D 13C-13C spectrum of L7-A10 interresidue contact, additional 13C 1D spectra, dynamical NMR data on labeled peptides, table of labeled peptides and amounts, additional pulse sequence schematics and experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Ross CA. Neuron. 2002;35:819–822. doi: 10.1016/s0896-6273(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 2.Bates GP, Benn C. In: Huntington's Disease. Bates GP, Harper PS, Jones L, editors. Oxford, U.K.: Oxford University Press; 2002. pp. 429–472. [Google Scholar]
- 3.Morley JF, Brignull HR, Weyers JJ, Morimoto RI. Proc. Natl. Acad. Sci. USA. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherzinger E, Sittler A, Schweiger K, Heiser V, Lurz R, Hasenbank R, Bates GP, Lehrach H, Wanker EE. Proc. Natl. Acad. Sci. USA. 1999;96:4604–4609. doi: 10.1073/pnas.96.8.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S, Berthelier V, Yang W, Wetzel R. J. Mol. Biol. 2001;311:173–182. doi: 10.1006/jmbi.2001.4850. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Ferrone FA, Wetzel R. Proc. Natl. Acad. Sci. USA. 2002;99:11884–11889. doi: 10.1073/pnas.182276099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharyya AM, Thakur AK, Wetzel R. Proc. Natl. Acad. Sci. USA. 2005;102:15400–15405. doi: 10.1073/pnas.0501651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slepko N, Bhattacharyya AM, Jackson GR, Steffan JS, Marsh JL, Thompson LM, Wetzel R. Proc. Natl. Acad. Sci. USA. 2006;103:14367–14372. doi: 10.1073/pnas.0602348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharyya A, Thakur AK, Chellgren VM, Thiagarajan G, Williams AD, Chellgren BW, Creamer TP, Wetzel R. J. Mol. Biol. 2006;355:524–535. doi: 10.1016/j.jmb.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 10.Thakur AK, Jayaraman M, Mishra R, Thakur M, Chellgren VM, Byeon I-JL, Anjum DH, Kodali R, Creamer TP, Conway JF, Gronenborn AM, Wetzel R. Nat. Struct. Mol. Biol. 2009;16:380–389. doi: 10.1038/nsmb.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kar K, Jayaraman M, Sahoo B, Kodali R, Wetzel R. Nat. Struct. Mol. Biol. 2011 doi: 10.1038/nsmb.1992. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellisdon AM, Thomas B, Bottomley SP. J. Biol. Chem. 2006;281:16888–16896. doi: 10.1074/jbc.M601470200. [DOI] [PubMed] [Google Scholar]
- 13.Robertson AL, Bottomley SP. Curr. Med. Chem. 2010;17:3058–3068. doi: 10.2174/092986710791959800. [DOI] [PubMed] [Google Scholar]
- 14.Duennwald ML, Jagadish S, Muchowski PJ, Lindquist SL. Proc. Natl. Acad. Sci. USA. 2006;103:11045–11050. doi: 10.1073/pnas.0604547103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehay B, Bertolotti A. J. Biol. Chem. 2006;281:35608–35615. doi: 10.1074/jbc.M605558200. [DOI] [PubMed] [Google Scholar]
- 16.Atwal RS, Xia J, Pinchev D, Taylor J, Epand RM, Truant R. Hum. Mol. Genet. 2007;16:2600–2615. doi: 10.1093/hmg/ddm217. [DOI] [PubMed] [Google Scholar]
- 17.Tam S, Spiess C, Auyeung W, Joachimiak L, Chen B, Poirier MA, Frydman J. Nat. Struct. Mol. Biol. 2009;16:1279–1285. doi: 10.1038/nsmb.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebman SW, Meredith SC. Nat. Chem. Biol. 2010;6:7–8. doi: 10.1038/nchembio.279. [DOI] [PubMed] [Google Scholar]
- 19.Ratovitski T, Gucek M, Jiang H, Chighladze E, Waldron E, D'Ambola J, Hou Z, Liang Y, Poirier MA, Hirschhorn RR, Graham R, Hayden MR, Cole RN, Ross CA. J. Biol. Chem. 2009;284:10855–10867. doi: 10.1074/jbc.M804813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockabrand E, Slepko N, Pantalone A, Nukala VN, Kazantsev A, Marsh JL, Sullivan PG, Steffan JS, Sensi SL, Thompson LM. Hum. Mol. Genet. 2007;16:61–77. doi: 10.1093/hmg/ddl440. [DOI] [PubMed] [Google Scholar]
- 21.Serio TR, Cashikar AG, Kowal AS, Sawicki GJ, Moslehi JJ, Serpell L, Arnsdorf MF, Lindquist SL. Science. 2000;289:1317–1321. doi: 10.1126/science.289.5483.1317. [DOI] [PubMed] [Google Scholar]
- 22.Chiti F, Dobson CM. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 23.Kodali R, Wetzel R. Curr. Opin. Struct. Biol. 2007;17:48–57. doi: 10.1016/j.sbi.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Lakhani VV, Ding F, Dokholyan NV. PLoS Comput. Biol. 2010;6:e1000772. doi: 10.1371/journal.pcbi.1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson TE, Vitalis A, Crick SL, Pappu RV. J. Mol. Biol. 2010;396:1295–1309. doi: 10.1016/j.jmb.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma D, Shinchuk LM, Inouye H, Wetzel R, Kirschner DA. Proteins. 2005;61:398–411. doi: 10.1002/prot.20602. [DOI] [PubMed] [Google Scholar]
- 27.Sikorski P, Atkins E. Biomacromolecules. 2005;6:425–432. doi: 10.1021/bm0494388. [DOI] [PubMed] [Google Scholar]
- 28.Darnell G, Orgel JPRO, Pahl R, Meredith SC. J. Mol. Biol. 2007;374:688–704. doi: 10.1016/j.jmb.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Darnell GD, Derryberry J, Kurutz JW, Meredith SC. Biophys. J. 2009;97:2295–2305. doi: 10.1016/j.bpj.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ignatova Z, Thakur AK, Wetzel R, Gierasch LM. J. Biol. Chem. 2007;282:36736–36743. doi: 10.1074/jbc.M703682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley NW, Huang X, Tam S, Spiess C, Frydman J, Pande VS. J. Mol. Biol. 2009;388:919–927. doi: 10.1016/j.jmb.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fändrich M, Fletcher MA, Dobson CM. Nature. 2001;410:165–166. doi: 10.1038/35065514. [DOI] [PubMed] [Google Scholar]
- 33.Tycko R. Q. Rev. Biophys. 2006;39:1–55. doi: 10.1017/S0033583506004173. [DOI] [PubMed] [Google Scholar]
- 34.O'Nuallain B, Thakur AK, Williams AD, Bhattacharyya AM, Chen S, Thiagarajan G, Wetzel R. Methods Enzymol. 2006;413:34–74. doi: 10.1016/S0076-6879(06)13003-7. [DOI] [PubMed] [Google Scholar]
- 35.Takegoshi K, Nakamura S, Terao T. Chem. Phys. Lett. 2001;344:631–637. [Google Scholar]
- 36.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. J. Chem. Phys. 1995;103:6951–6958. [Google Scholar]
- 37.Hohwy M, Rienstra CM, Griffin RG. J. Chem. Phys. 2002;117:4973. [Google Scholar]
- 38.Kumashiro K, Schmidt-Rohr K, Murphy O, Ouellette K, Cramer W, Thompson LK. J. Am. Chem. Soc. 1998;120:5043–5051. [Google Scholar]
- 39.Andronesi OC, von Bergen M, Biernat J, Seidel K, Griesinger C, Mandelkow E, Baldus M. J. Am. Chem. Soc. 2008;130:5922–5928. doi: 10.1021/ja7100517. [DOI] [PubMed] [Google Scholar]
- 40.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 41.Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED. Proteins. 2005;59:687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- 42.Goddard TD, Kneller DG. SPARKY - NMR Assignment Program. 2006 ( http://www.cgl.ucsf.edu/home/sparky/)
- 43.Harris RK, Becker ED, De Menezes SMC, Granger P, Hoffman RE, Zilm KW. Magn. Reson. Chem. 2008;46:582–598. doi: 10.1002/mrc.2225. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Neal S, Wishart DS. J. Biomol. NMR. 2003;25:173–195. doi: 10.1023/a:1022836027055. [DOI] [PubMed] [Google Scholar]
- 45.Jayaraman M, Kodali R, Wetzel R. Protein Eng. Des. Sel. 2009;22:469–478. doi: 10.1093/protein/gzp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson M, Mantsch HH. Crit. Rev. Biochem. Mol. Biol. 1995;30:95–120. doi: 10.3109/10409239509085140. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Berthelier V, Hamilton JB, O'Nuallain B, Wetzel R. Biochemistry. 2002;41:7391–7399. doi: 10.1021/bi011772q. [DOI] [PubMed] [Google Scholar]
- 48.Venyaminov S, Kalnin NN. Biopolymers. 1990;30:1243–1257. doi: 10.1002/bip.360301309. [DOI] [PubMed] [Google Scholar]
- 49.Wishart DS, Sykes BD. J. Biomol. NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]
- 50.Kricheldorf H, Müller D. Int. J. Biol. Macromol. 1984;6:145–151. [Google Scholar]
- 51.Thakur AK, Wetzel R. Proc. Natl. Acad. Sci. USA. 2002;99:17014–17019. doi: 10.1073/pnas.252523899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huster D, Yao X, Hong M. J. Am. Chem. Soc. 2002;124:874–883. doi: 10.1021/ja017001r. [DOI] [PubMed] [Google Scholar]
- 53.Lesage A, Gardiennet C, Loquet A, Verel R, Pintacuda G, Emsley L, Meier BH, Böckmann A. Angew. Chem. Int. Ed. 2008;47:5851–5854. doi: 10.1002/anie.200801110. [DOI] [PubMed] [Google Scholar]
- 54.Kim MW, Chelliah Y, Kim SW, Otwinowski Z, Bezprozvanny I. Structure. 2009;17:1205–1212. doi: 10.1016/j.str.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perutz MF, Johnson T, Suzuki M, Finch JT. Proc. Natl. Acad. Sci. USA. 1994;91:5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson R, Sawaya MR, Balbirnie M, Madsen AØ, Riekel C, Grothe R, Eisenberg D. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bajaj VS, Van der Wel PCA, Griffin RG. J. Am. Chem. Soc. 2009;131:118–128. doi: 10.1021/ja8045926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van der Wel PCA, Lewandowski JR, Griffin RG. J. Am. Chem. Soc. 2007;129:5117–5130. doi: 10.1021/ja068633m. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen JT, Bjerring M, Jeppesen MD, Pedersen RO, Pedersen JM, Hein KL, Vosegaard T, Skrydstrup T, Otzen DE, Nielsen NC. Angew. Chem. Int. Ed. 2009;48:2118–2121. doi: 10.1002/anie.200804198. [DOI] [PubMed] [Google Scholar]
- 60.Abedini A, Raleigh DP. Protein Eng. Des. Sel. 2009;22:453–459. doi: 10.1093/protein/gzp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narayanan S, Walter S, Reif B. ChemBioChem. 2006;7:757–765. doi: 10.1002/cbic.200500382. [DOI] [PubMed] [Google Scholar]
- 62.Anderson VL, Ramlall TF, Rospigliosi CC, Webb WW, Eliezer D. Proc. Natl. Acad. Sci. USA. 2010;107:18850–18855. doi: 10.1073/pnas.1012336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu G, Prabhakar A, Aucoin D, Simon M, Sparks S, Robbins KJ, Sheen A, Petty SA, Lazo ND. J. Am. Chem. Soc. 2010;132:18223–18232. doi: 10.1021/ja1069882. [DOI] [PubMed] [Google Scholar]
- 64.Hauser CAE, Deng R, Mishra A, Loo Y, Khoe U, Zhuang F, Cheong DW, Accardo A, Sullivan MB, Riekel C, Ying JY, Hauser UA. Proc. Natl. Acad. Sci. USA. 2011;108:1361–1366. doi: 10.1073/pnas.1014796108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maurer-Stroh S, Debulpaep M, Kuemmerer N, Lopez de la Paz M, Martins IC, Reumers J, Morris KL, Copland A, Serpell L, Serrano L, Schymkowitz JW, Rousseau F. Nat Methods. 2010;7:237–242. doi: 10.1038/nmeth.1432. [DOI] [PubMed] [Google Scholar]
- 66.Goldschmidt L, Teng PK, Riek R, Eisenberg D. Proc. Natl. Acad. Sci. USA. 2010;107:3487–3492. doi: 10.1073/pnas.0915166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baxa U, Wickner RB, Steven AC, Anderson DE, Marekov LN, Yau W-M, Tycko R. Biochemistry. 2007;46:13149–13162. doi: 10.1021/bi700826b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.