Abstract
Aims
Individuals with spinal cord injury (SCI) exhibit neurogenic detrusor overactivity (NDO) causing high intravesicle pressures and incontinence. The first aim was to measure changes in maximum cystometric capacity (MCC) evoked by electrical stimulation of the dorsal genital nerve (DGN) delivered either continuously or conditionally (only during bladder contractions) in persons with SCI. The second aim was to use the external anal sphincter electromyogram (EMGEAS) for real-time control of conditional stimulation.
Methods
Serial filling cystometries were performed in nine volunteers with complete or incomplete supra-sacral SCI. Conditional stimulation was delivered automatically when detrusor pressure increased to 8–12 cmH2O above baseline. MCCs were measured for each treatment (continuous, conditional, and no stimulation) and compared using post- ANOVA Tukey HSD paired comparisons. Additional treatments in two subjects used the EMGEAS for automatic control of conditional stimulation.
Results
Continuous and conditional stimulation increased MCC by 63±73 mL (36±24%) and 74±71 mL (51±37%), respectively (p<0.05), compared to no stimulation. There was no significant difference between MCCs for conditional and continuous stimulation, but conditional stimulation significantly reduced stimulation time (174±154 s, or 27±17% of total time) as compared to continuous stimulation (469±269 s, 100% of total time, p<0.001). The EMGEAS algorithm provided reliable detection of bladder contractions (six of six contractions over four trials) and reduced stimulation time (21±8% of total time).
Conclusions
Conditional stimulation generates increases in bladder capacity while substantially reducing stimulation time. Furthermore, EMGEAS was successfully used as a real-time feedback signal to control conditional electrical stimulation in a laboratory setting.
Keywords: Neurogenic detrusor overactivity, Electromyogram, Neural prosthesis, Neuromodulation, Overactive bladder, Urinary incontinence, Biofeedback
1 Introduction
Urological complications following spinal cord injury (SCI), including neurogenic detrusor overactivity (NDO), bladder-urethral sphincter dyssynergia, and urinary retention, can result in frequent urinary tract infections, severe urinary incontinence, kidney infections, and long-term renal damage (1,2). Common management of NDO, including intermittent self-catheterization and anticholinergic medication, remains inadequate due to recurrent urinary tract infections and dose-limiting side effects (3–5).
Neuromodulation is an alternative treatment for NDO. Electrical activation of pudendal afferents suppresses bladder activity and promotes continence (6–13). Continuous stimulation of afferents in the dorsal genital branch (DGN) of the pudendal nerve increases maximum cystometric capacity (MCC) in persons with SCI (9,11,13). Conditional stimulation, applied only during bladder contractions, may be more effective than continuous stimulation by minimizing habituation of spinal reflexes (9, 14,15), and in cats, MCC was significantly larger with conditional stimulation than with continuous stimulation (16). Despite previous studies of the efficacy of conditional stimulation (6, 9, 23, 24), there are few data directly comparing conditional and continuous stimulation. Kirkham and colleagues compared conditional and continuous stimulation, but only one protocol directly compared the two types of stimulation on the same day in the same patient (n=3) and the use of serial, rather than randomized trial order may have influenced the results (9). The current study tested for a significant difference between continuous, conditional, and no stimulation using a randomized trial order study design in human participants with SCI. Further, the potential benefit of conditional stimulation is counterbalanced by the requirement for a control signal to determine when the bladder is contracting. Previous studies used bladder pressure, electroneurogram, or electromyogram (EMG) to detect nascent bladder contractions (16–19).
The long term goal of our research is to develop a fully implanted electrical device to treat NDO and restore continence in persons with SCI. The first objective of this study was to quantify the effects of continuous and conditional electrical stimulation of pudendal dorsal genital afferents (DGN) on MCC in persons with SCI. The second objective was to demonstrate real-time closed-loop control of conditional stimulation using the EMG from the external anal sphincter (EMGEAS) as the control signal.
2 Methods
2.1 Subject Selection
The study protocol was approved by the Institutional Review Board, and written informed consent was obtained. Inclusion criteria were neurologically stable complete or incomplete suprasacral SCI >1 yr and documented NDO; exclusion criteria were implanted electronic device, history of pelvic surgery, acontractile bladder, urethral stricture/obstruction or severe prostatic hypertrophy determined by their urologic physician, perineal inflammation, or low bladder compliance.
2.2 Instrumentation
A Foley catheter (Bardex Foley, 12 Fr, 5 cc balloon, Coude tipped, C.R. Bard, Inc., Covington, GA) was placed into the bladder, inflated to occlude the bladder neck, and the bladder was drained. A pressure catheter (BPC-4L, Life-Tech, Inc., Stafford, TX), inserted with the Foley, was connected to an external pressure transducer (Deltran I, Utah Medical Products, Inc., Midvale, UT). A balloon catheter (Life-Tech RPC-9) was placed in the rectum and connected to a second pressure transducer, and detrusor pressure (Pdet) was calculated as intravesicle pressure minus rectal (abdominal) pressure. Pressure signals were amplified (1000x) and low-pass filtered (f=40Hz, ETH-255, iWorx/CB Sciences, Inc., Dover, NH), and sampled (5kHz, DAQPad-6016, National Instruments, Austin, TX). The EMGEAS was recorded using a bipolar pair of wire electrodes (1512A-M, Life-Tech) inserted ~ 2–3 cm deep into the EAS, both on the same side ~ 2 cm lateral to the anal opening, and a self-adhesive reference electrode (Empi 9000 series, 1.25 in round) placed on the outer thigh. The EMGEAS was pre-amplified (50x, C-ISO-255, iWorx), further amplified (1000x) and band-pass filtered (3Hz-1kHz, ETH-255), and sampled (5kHz, DAQPad-6016). All data were further processed, displayed, and recorded on a computer (Dell Latitude D610, 1.86 GHz) using custom software developed with LabVIEW (National Instruments). For stimulation, a self-adhesive surface electrode patch (Empi 9000, 1.25 in diameter, St. Paul, MN) was placed over the genital branches of the pudendal nerve (dorsal base of penis in males (20), just cranial to clitoris in females (21)), with the counter electrode (Empi 5000, 2.75×4 in rectangle) on one hip. Charge-balanced biphasic regulated current 200 μs rectangular stimuli were delivered using an isolated, battery-powered electrical stimulator (300PV, Empi).
2.3 Experimental Protocol
The bladder was filled at 10–40 mL/min (dependent on maximum capacity in prior urodynamic evaluations and held constant for each subject) until MCC was reached (defined as infused volume at time of sustained bladder contraction, urine leakage, or 700 mL infused). In this study, a contraction was considered sustained if Pdet was ≥ 40 cmH2O for 20 s.
Two repetitions of three treatment types (no stimulation, continuous stimulation, and conditional stimulation) were performed, with the first treatment always no stimulation to determine baseline MCC and check for exclusion criteria (e.g., low compliance), the second treatment always continuous stimulation or conditional stimulation, and the remaining trials conducted in random order. If time permitted, additional trials were performed with newly randomized treatments. Stimulation amplitude was set at twice the pudendo-anal (PA) reflex threshold, or at 80 mA if no reflex was observed (22). During continuous stimulation treatments, low frequency (10 or 15 Hz) continuous stimulation was applied. Stimulation during conditional treatments was applied automatically when Pdet increased to 8–12 cmH2O, or when the EMGEAS, acting as a surrogate detector for detrusor contractions, exceeded a threshold (see below). Stimulation remained on for ten seconds then turned off for two seconds, and this cycle continued while Pdet remained above the threshold. An additional ten seconds of stimulation were delivered after Pdet decreased below threshold. At least five minutes elapsed between trials.
In two subjects, two additional conditional stimulation treatments were conducted using the EMGEAS to control the delivery of stimulation. The EMGEAS signal was rectified, low-pass filtered, and compared with an adaptive threshold determined from an offset and time-lagged version of the rectified EMGEAS (18). The parameters of the adaptive threshold algorithm including the signal low-pass filter time constant (0.5 s), the threshold low-pass filter time constant (2.0 s), and the threshold offset (2x) were determined by running the algorithm during the no stimulation cystometries performed earlier in each subject.
2.4 Data Analysis
For each subject, MCCs were averaged within each treatment (at least two per treatment), and the average cystometric capacities (ACC) were compared using ANOVA (level of significance α=0.05) with subject as a random factor to remove inter-subject variation in absolute bladder volumes (6,9). Post-ANOVA pair-wise comparisons were made using Tukey’s Honestly Significant Differences test with protected level of significance α=0.05. Spearman’s Rank was used to test for a correlation between MCC and trial order. Student’s t-tests were used to compare stimulation times and to test for effects of stimulation carry-over (see Results). All p-values less than 0.05 were considered significant.
3 Results
3.1 Subject Characteristics
Informed consent was obtained from 13 subjects (11 male) who met medical and demographic criteria (Table I). Subjects had a mean age of 55 ± 11 yr at the time of study, and injury levels ranged from C5 to L2. Four of 13 subjects were excluded after completing the initial control cystometry (Subjects P1, P7, and P10 for acontractile bladders; Subject P9 for low bladder compliance). The remaining nine subjects completed the study, and there were no episodes of autonomic dysreflexia or other adverse events during the study.
TABLE I.
Subject Characteristics
| ID | Sex | Age (yr) | Injury Level, Severity | Injury Date | Injury Cause | Normal Voiding Method | Medications (stopped>48 h) |
|---|---|---|---|---|---|---|---|
| (P1) | M | 34 | T10, Complete | 1983 | Gunshot | CIC | None |
| P2 | F | 41 | T8/T9, Complete | 1986 | Fall | CIC | None |
| P3 | F | 37 | T12/L1, Incomplete | 2001 | TM/CES | Straining | Oxytrol patch |
| P4 | M | 57 | T4, Complete | 1996 | Accident | CC | Baclofen |
| P5 | M | 63 | T2, Incomplete | 1998 | Iatrogenic | CIC | Oxybutinin |
| P6 | M | 59 | T8, Complete | 1984 | Gunshot | CIC | Oxybutinin |
| (P7) | M | 62 | C5, Incomplete | 1986 | Accident | CIC | None |
| P8 | M | 62 | T10/T12, Complete | 1999 | Fall | CIC | None |
| (P9) | M | 57 | T10, Complete | 1995 | Accident | CIC | None |
| (P10) | M | 53 | T3, Complete | 2005 | Gunshot | IC | Diazepam |
| P11 | M | 66 | L1, Complete | 1966 | Accident | CC | None |
| P12 | M | 71 | T12/L1, Incomplete | 1965 | Accident | CIC | Ditropan |
| P13 | M | 51 | T12/L2, Complete | 1977 | Accident | CC | None |
TM/CES - Transverse Myelitis with Cauda Equina Syndrome; CIC - clean intermittent catheterization; CC - condom catheter; IC - indwelling catheter. Parentheses around subject ID denote subject exclusion (see text).
3.2 Cystometric Capacity
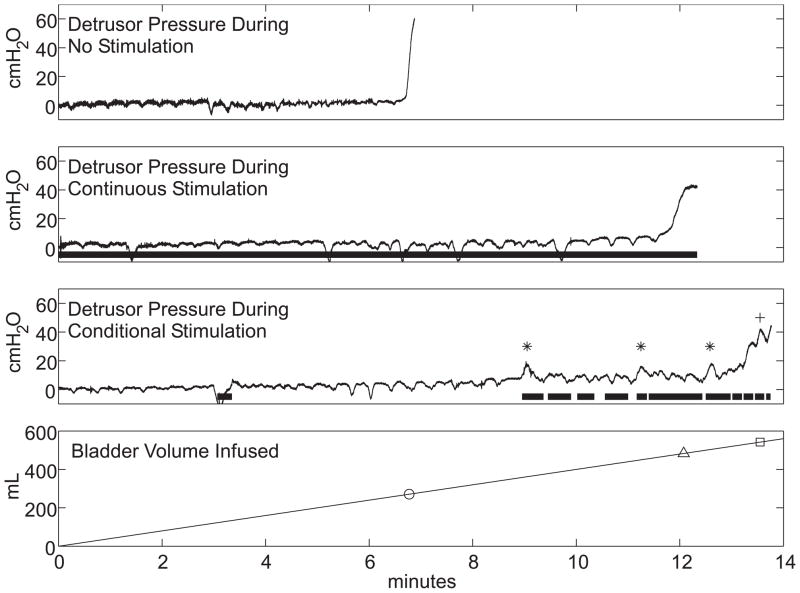
Example cystometrograms recorded during no stimulation, continuous stimulation, and conditional stimulation trials are shown in Figure 1. During bladder filling without stimulation, Pdet remained low until a sustained bladder contraction occurred at 271 mL. With continuous stimulation, Pdet remained low for a longer period of time before a sustained contraction occurred, resulting in a larger MCC of 483 mL. Conditional stimulation applied at the onset of each contraction suppressed three of four contractions and increased MCC to 534 mL.
Fig. 1.
Cystometrograms in a person with spinal cord injury with no stimulation, continuous stimulation, and intermittent stimulation of the dorsal genital nerve. Top three traces display detrusor pressure during no, continuous, and conditional stimulation treatments, with cystometric capacities marked on the bottom trace of infused volume (circle: no stimulation, 271 mL; triangle: continuous stimulation, 483 mL; square: conditional stimulation, 534 mL). The thick horizontal lines denote the delivery of 15 Hz stimulation, asterisks denote stimulation-suppressed contractions, and cross denotes unsuppressed contraction during the conditional stimulation treatment. The transient dips in detrusor pressure were caused by fluctuations in abdominal pressure (not shown).
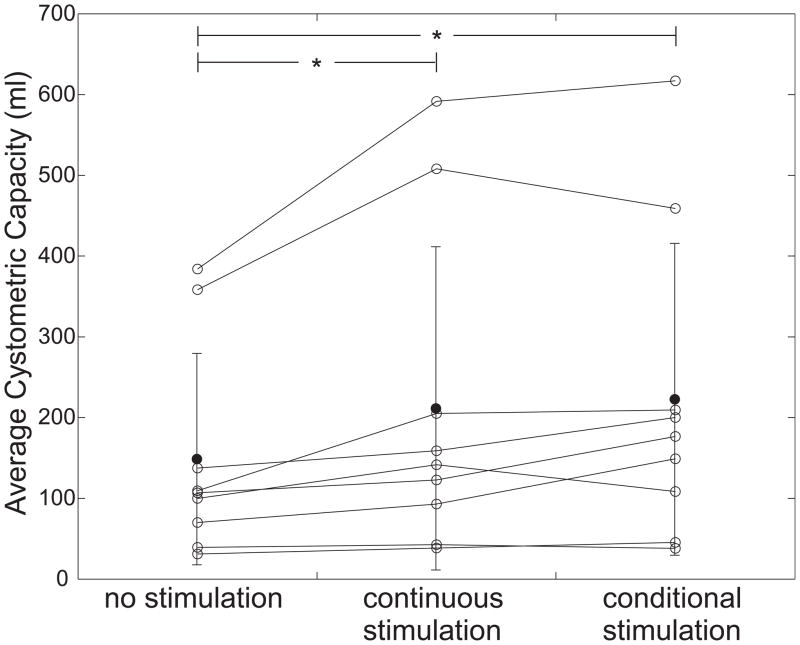
For each subject, MCCs were averaged within each treatment type (at least two per treatment), and these averages (ACC) were compared. Mean ACCs ± SD were 149 ± 131 mL (no stimulation), 211 ± 200 mL (continuous), and 223 ± 193 mL (conditional) from a total of 58 trials among nine subjects (Fig. 2). Compared to no stimulation treatments, continuous stimulation increased mean ACC by 42%, while conditional stimulation increased mean ACC by 50% over no stimulation and by 6% over continuous stimulation. Among the 12 conditional stimulation trials displaying one or more suppressed contractions, mean volume increase between the first contraction and MCC was 70±63 mL (129±196%). ACC was dependent on trial type (p<0.05, ANOVA with subject as a random factor). Furthermore, ACCs from continuous and conditional stimulation were significantly larger than those from no stimulation, but ACCs from continuous and conditional stimulation showed no significant difference (post hoc Tukey HSD pair-wise comparisons with α=0.05).
Fig. 2.
Average cystometric capacity during cystometry with no stimulation, continuous stimulation, and conditional stimulation. Open points are individual subjects, solid points are population means, and error bars show standard deviations. Asterisks denote significant difference between groups.
ACC during continuous stimulation was greater than ACC during no stimulation in all nine subjects. One subject displayed a decrease in conditional stimulation ACC compared to no stimulation (−3%), and the remaining eight displayed increases (range 8–113%). In six of nine subjects, ACC during conditional stimulation trials was greater than ACC during continuous trials by an average of 31±25% (range 2–60%), but this proportion (67%) was not different than chance (p=0.25, Sign test).
Compared to continuous stimulation, conditional stimulation decreased stimulation time from 469±269 s or 100% of total time to 174±154 s or an average of 27±18% (two-tailed t-test on stimulation time, p<0.001). In five of the 15 conditional stimulation trials triggered by pressure, stimulation remained on during the latter part of the trial because detrusor pressure remained above threshold.
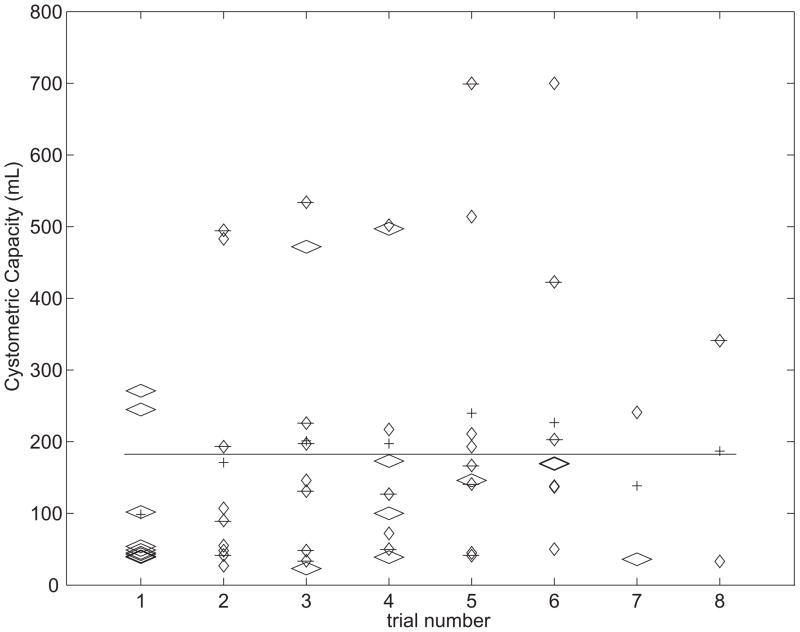
Trial order could have confounding effects on MCC including an increase in capacity during repeated baseline cystometries and/or “carry-over” effects of prior stimulation (9). Trial order was randomized, and did not correlate with MCC (Spearman’s rank, p=0.749 for all trials, p=0.802 for no stimulation trials only; Fig. 3). Further, a two-tailed t-test comparing MCCs of the first baseline trials to all other no stimulation trials was not significant (p=0.331), as were similar tests comparing trials of the same type preceded by either continuous or conditional stimulation trials (trial k+1 = no stimulation: p=0.106; continuous: p=0.860; conditional: p=0.152).
Fig. 3.
Analysis of cystometric capacity by trial number (1=first trial, 2=second trial, etc.). The plot displays cystometric capacities for each trial (diamonds: wide, no stimulation; narrow, continuous stimulation; barred, conditional stimulation), the mean cystometric capacity within each trial number group (crosses), and the overall mean (line). There was no significant correlation between cystometric capacity and trial number (Spearman’s rank). Furthermore, the average cystometric capacity during the first trials (always baseline) was not significantly different from the average of other no stimulation trials (Student’s t-test).
3.3 Suppressed Contractions
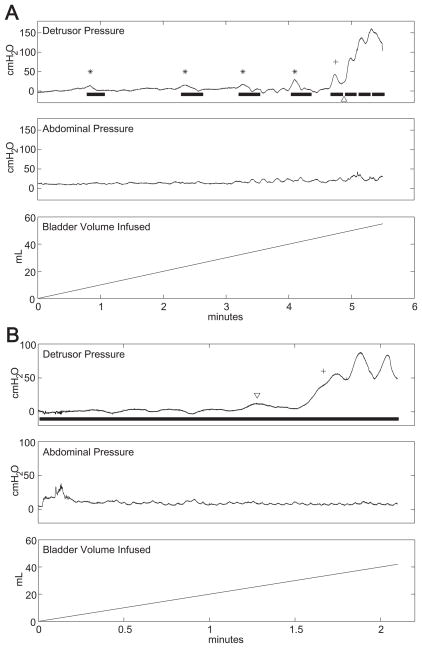
Of the 23 conditional stimulation trials using either pressure or EMGEAS, 12 trials displayed one or more suppressed contractions (52%). Among these, the median number of suppressed contractions per trial was three (range one to seven). Figure 4A displays a conditional stimulation trial in which four distension-evoked detrusor contractions were suppressed with conditional stimulation. A fifth contraction was suppressed during stimulation and then developed into a sustained contraction after stimulation was switched off. Bouts of stimulation during the contraction resulted in phasic relaxations of the detrusor, but were unable to reduce Pdet below 40 cmH2O. There were two transient contractions during 19 no stimulation trials and three transient contractions during 20 continuous stimulation trials (Fig. 4B).
Fig. 4.
A: Example cystometrogram during a conditional stimulation treatment displaying four suppressed detrusor contractions (asterisks). The fifth contraction (cross) was not suppressed completely, possibly due to the pause in stimulation (arrowhead). B: Transient detrusor contractions (arrowhead) appeared in two of 20 continuous stimulation treatments and in one control treatment. An uncontrolled detrusor contraction (cross) determined the cystometric capacity (33 mL @ second 100).
Mean peak Pdet during suppressed contractions was 22 ± 12 cmH2O (range 10–50 cmH2O). The five suppressed contractions with peak pressures between 40 and 50 cmH2O subsided within 20 seconds, did not cause leakage, and therefore did not constitute reaching cystometric capacity.
3.4 Closed-Loop Control Using EMGEAS
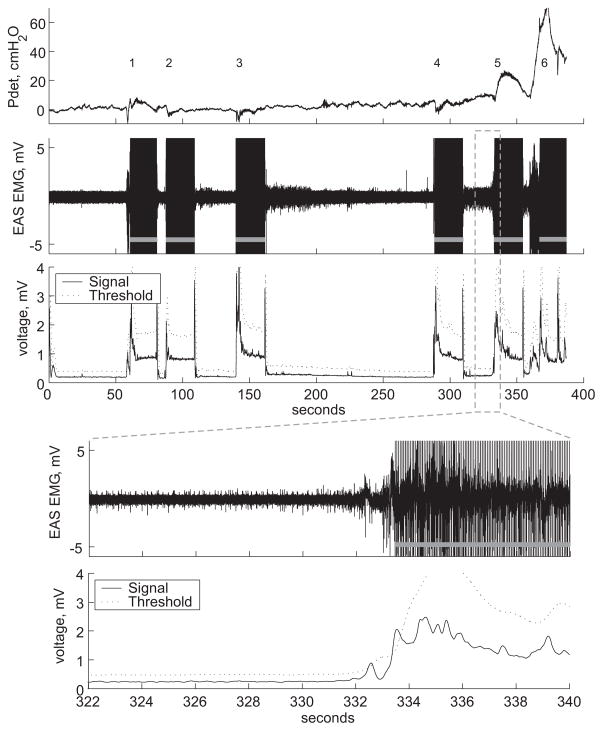
EMG-based conditional stimulation trials were conducted twice in each of two subjects (P12, P13). An adaptive threshold algorithm was used to detect nascent hyper-reflexive detrusor contractions from the EMGEAS in real-time and trigger conditional stimulation of the DGN. The adaptive threshold algorithm successfully detected all true contractions. After each detection, the stimulator turned on automatically for 20 s, then turned off for a time-out no-stimulation period of 5 s. An example is shown in Figure 5: four false positive detections occurred before two true positive detections and this resulted in 27% stimulation time during the 6.5 minute trial. Stimulation delivered in response to the first true detection reduced Pdet to below 10 cmH2O and suppressed the contraction, but a subsequent detrusor contraction began within the time-out period and the second contraction was not suppressed by stimulation and resulted in MCC. Detection of the contraction successfully occurred immediately after the time-out expired, but stimulation was unable to arrest the contraction. Across all trials, a total of six true positive and 26 false positive detections occurred over 28 minutes of cumulative trial time, resulting in 22% stimulation. Delay from the time Pdet reached 10 cmH2O to detection was −4.3 ± 2.2 s (detection occurred first in all five cases) and Pdet at detection was 2.4 ± 1.9 cmH2O. The detection of a sixth contraction was delayed by 5.9 s, due to the time-out, and Pdet at detection was 55 cmH2O.
Fig. 5.
Closed-loop electrical control of continence. An adaptive threshold algorithm was used to detect hyper-reflexive detrusor contractions and trigger conditional stimulation (gray bars) in a real-time implementation of closed-loop control of urinary continence. Four false positive (1, 2, 3, 4) and two true positive detections (5, 6) were triggered by the algorithm. Magnification displays the external anal sphincter electromyogram (EAS EMG), detection signal, and adaptive threshold during the fifth detection. Pdet: detrusor pressure.
4 Discussion
Serial cystometries were conducted in persons with SCI to quantify the effects of continuous and conditional electrical stimulation of the DGN on cystometric capacity. Average cystometric capacities (ACC) were significantly greater with both conditional stimulation (50% increase) and continuous stimulation (42% increase), and although conditional stimulation reduced stimulation time by 73% compared to continuous stimulation, it was not less effective at increasing ACC. In contrast to previous studies in humans (6,9,19), this study directly compared the efficacy of conditional stimulation to both continuous and no stimulation within the same subjects and sessions. The results provide further evidence that conditional stimulation is effective in increasing cystometric capacity in persons with SCI.
Conditional stimulation increased cystometric capacity by 50% over no stimulation. Although absolute increases in volumes were small (74 ± 71 mL), a large percentage increase in bladder capacity should translate to a proportional increase in time between necessary bladder evacuations. Similar increases of 66% (6) and 53% (23) were reported by Dalmose et al. and Hansen et al., respectively. In contrast, Kirkham et al. reported a 144% increase using 60 s stimulus trains (9). Shorter stimulation durations in the Dalmose study and stimulation cycling in the present study may explain the differences in efficacy.
Although some investigators have used physiologic filling (23,24), they were only able to perform two or three trials per experiment due to the slow rate of natural diuresis (8 mL/min, range 2–14 mL/min (24); 8.7 ± 4.2 mL/min (23)). In this study, a physiologic fill rate was traded for randomly ordered repeated measures, and the rate of 10–40 mL/min used was lower than used in other studies employing serial cystometries (6,11). Carry-over effects of repeated fills or stimulation can affect subsequent MCCs (9) but our analysis revealed that trial order was not a significant factor determining MCC.
A median of three suppressed contractions occurred among 52% of conditional stimulation trials. Dalmose et al. reported a mean of 7.8 inhibited contractions per trial in ten conditional stimulation trials performed in a similar study (6). One possible reason for the low number of inhibited contractions in the present study is that stimulation did not always remain on until the contraction had fully subsided (see Fig. 4A, arrowhead). The two-second pause in stimulation that occurred periodically when the pressure remained high (5 s during EMG detection time-out) may have allowed Pdet to continue to increase. Using one minute closed-loop stimulation durations, Kirkham and colleagues reported that contractions took an average of 41 seconds to return to 10% of the pre-contraction baseline pressure (9). It is likely that cycling diminished the effectiveness of conditional stimulation for trials in which contractions did not decrease below the pressure threshold within the stimulation duration (10–20 s). Cycling was included to mimic the behavior of the closed-loop EMG algorithm, since due to large stimulation artifacts, the EMGEAS was not monitored during stimulation. Lengthening the minimum stimulation duration may further increase cystometric capacity by increasing the number of successfully inhibited contractions. Another possible reason for the low number of inhibited contractions is that inadvertent automatic stimulation due to fluctuations in abdominal pressure during some conditional treatments (i.e., false positives) may have delayed the occurrence of distension-evoked contractions. Therefore, both short-term inhibitory carry-over effects (9) and suppressed nascent contractions may have contributed to increases in MCC during conditional treatments.
Conditional stimulation increased ACC by 6% compared to continuous stimulation trials while using 73% less stimulation, and two-thirds of subjects exhibited increases in ACC of up to 60% during conditional versus continuous stimulation. A similar study also found a non-significant 5% increase in mean ACC with conditional stimulation, even though trial types were not randomized and the number of subjects was low (9). Furthermore, four of six subjects undergoing protocols designed to compare the two stimulation types displayed larger ACCs with conditional stimulation (9), and the present study revealed an equivalent ratio of six of nine subjects. Wenzel and colleagues reported a similar decrease in stimulation during closed-loop trials in cats (16), and conditional stimulation used less stimulation compared with the Medtronic Interstim, which employs a 70–90% duty cycle (25). Non-conditional intermittent stimulation may be an effective alternative to conditional stimulation to reduce stimulation time while preserving efficacy of continence control. Open-loop intermittent stimulation has the advantage of not requiring detection of a nascent bladder contraction and feedback control, but because inhibitory stimulation must be delivered soon after a hyper-reflexive contraction begins in order to be effective (16), intermittent stimulation may not be effective at suppressing contractions that begin during the “off” phase."
Four of nine subjects exhibited a PA reflex (mean threshold 37 ± 11mA). Average percent increase in open-loop ACC over control for these subjects was 50% (±30%, range 15–87%), compared to 25% (±13%, range 8–42%) for those not exhibiting a reflex. Although not significant (p<0.20, Wilcoxon Rank-Sum Test), this difference reflects the expected increase in stimulation efficacy with robust sacral spinal reflexes. The PA reflex is not necessarily a physiological prerequisite for effective neuromodulation, but may be a convenient criterion for choosing subjects most likely to respond to pudendal nerve stimulation. In this study, the PA reflex was tested with 2 Hz stimulation, but lower frequency stimulation may have improved the response rate (26).
Given the variability of MCCs within subjects and assuming a true conditional stimulation effect size in the general SCI population of about 5% over continuous stimulation, this study was not adequately powered (low sample size) to detect a significant difference between the two treatments. Regardless of any true difference, an average increase of 5% is not clinically significant, and it remains unclear what factors (e.g., injury level, severity, baseline MCC, etc.) are predictive of the relative efficacy of conditional and continuous stimulation (6).
Persons with incontinence resulting from SCI also lose the ability to empty the bladder. Electrical stimulation of pudendal afferents can also facilitate efficient bladder emptying (27–29), and a future prosthesis may use pudendal nerve stimulation for both continence and micturition. Detrusor-sphincter-dyssynergia presents a challenge to producing efficient bladder emptying, and although pudendal afferent stimulation produces efficient voiding in chronic SCI cats (30), whether this will translate to humans with SCI remains uncertain. Conditional stimulation may reduce continence-promoting carry-over effects and allow more efficient voiding when stimulation is changed from continence-promoting (e.g., low frequency) to micturition-promoting (e.g., high frequency).
The EMGEAS was used to detect hyper-reflexive bladder contractions and trigger conditional stimulation during real-time electrical control of conditional stimulation. An adaptive threshold algorithm successfully detected all true contractions, and five of six were detected before Pdet rose above 10 cmH2O. False positive detections also occurred, and some of these coincided with fluctuations in abdominal pressure or with coughing. Although a formal optimization of the adaptive threshold parameters was not performed, such optimization is feasible (18). Given the robust detection of hyper-reflexive contractions without formal parameter optimization, such optimization may reduce false positives and further reduce stimulation time. The similar performance of two different methods for real-time detection of hyper-reflexive contractions using EMG signals in this and a previous study (19) strongly supports the utility of this approach. Also, EMG signals can be accessed for clinical research using minimally invasive techniques, while a nerve cuff electrode implanted on the pudendal nerve may provide similar information for a fully implanted system (18).
5 Conclusions
Conditional and continuous stimulation were equally effective at increasing the MCC in persons with SCI, but conditional stimulation substantially reduced stimulation time compared to continuous stimulation. The benefit of conditional electrical control of urinary continence may be the ability to decrease the amount of stimulation needed to maintain continence. The combination of increased bladder capacity with reduced stimulation may prove to make closed-loop control of urinary continence the best therapeutic option in persons with SCI. Furthermore, the demonstration of feasibility in using the EMGEAS signal for real-time closed-loop control may support future efforts in developing a neural prosthesis for closed-loop electrical control of urinary continence.
Acknowledgments
This work was financially supported by National Institutes of Health grant R01 NS050514.
Footnotes
All work was performed at Duke University, Durham, NC
References
- 1.Shingleton WB, Bodner DR. The development of urologic complications in relationship to bladder pressure in spinal cord injured patients. J Am Paraplegia Soc. 1993;16:14–17. doi: 10.1080/01952307.1993.11735878. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Rivas DA, Chancellor MB. Urodynamics of spinal cord injury. Urol Clin North Am. 1996;23:459–473. doi: 10.1016/s0094-0143(05)70325-6. [DOI] [PubMed] [Google Scholar]
- 3.Petersen T, Nielsen JB, Schroder HD. Intravesical capsaicin in patients with detrusor hyper-reflexia--a placebo-controlled cross-over study. Scand J Urol Nephrol. 1999;33:104–110. doi: 10.1080/003655999750016078. [DOI] [PubMed] [Google Scholar]
- 4.Schurch B. What is new in detrusor-sphincter dyssynergia treatment in spinal cord injury patients? NeuroRehabilitation. 1998;10:267–279. [Google Scholar]
- 5.Staskin DR. Hydroureteronephrosis after spinal cord injury. Effects of lower urinary tract dysfunction on upper tract anatomy. Urol Clin North Am. 1991;18:309–316. [PubMed] [Google Scholar]
- 6.Dalmose AL, Rijkhoff NJ, Kirkeby HJ, Nohr M, Sinkjaer T, Djurhuus JC. Conditional stimulation of the dorsal penile/clitoral nerve may increase cystometric capacity in patients with spinal cord injury. Neurourol Urodyn. 2003;22:130–137. doi: 10.1002/nau.10031. [DOI] [PubMed] [Google Scholar]
- 7.Groen J, Amiel C, Bosch JL. Chronic pudendal nerve neuromodulation in women with idiopathic refractory detrusor overactivity incontinence: results of a pilot study with a novel minimally invasive implantable mini-stimulator. Neurourol Urodyn. 2005;24:226–230. doi: 10.1002/nau.20131. [DOI] [PubMed] [Google Scholar]
- 8.Janez J, Plevnik S, Suhel P. Urethral and bladder responses to anal electrical stimulation. J Urol. 1979;122:192–194. doi: 10.1016/s0022-5347(17)56323-5. [DOI] [PubMed] [Google Scholar]
- 9.Kirkham AP, Shah NC, Knight SL, Shah PJ, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord. 2001;39:420–428. doi: 10.1038/sj.sc.3101177. [DOI] [PubMed] [Google Scholar]
- 10.Shah N, Edhem I, Knight S, Craggs M. Acute suppression of detrusor hyper-reflexia with detrusor-sphinctor dyssynergia by electrical stimulation of the dorsal penile nerve in patients with a spinal injury. Eur Urol. 1998;33(suppl 1):60. [Google Scholar]
- 11.Vodusek DB. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodyn. 1986;5:381–389. [Google Scholar]
- 12.Jezernik S, Grill WM, Sinkjaer T. Detection and inhibition of hyperreflexia-like bladder contractions in the cat by sacral nerve root recording and electrical stimulation. Neurourol Urodyn. 2001;20:215–230. doi: 10.1002/1520-6777(2001)20:2<215::aid-nau23>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Kirkham AP, Knight SL, Craggs MD, Casey AT, Shah PJ. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord. 2002;40:272–281. doi: 10.1038/sj.sc.3101278. [DOI] [PubMed] [Google Scholar]
- 14.Granat MH, Heller BW, Nicol DJ, Baxendale RH, Andrews BJ. Improving limb flexion in FES gait using the flexion withdrawal response for the spinal cord injured person. J Biomed Eng. 1993;15:51–56. doi: 10.1016/0141-5425(93)90093-e. [DOI] [PubMed] [Google Scholar]
- 15.Floeter MK, Gerloff C, Kouri J, Hallett M. Cutaneous withdrawal reflexes of the upper extremity. Muscle Nerve. 1998;21:591–598. doi: 10.1002/(sici)1097-4598(199805)21:5<591::aid-mus5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Wenzel BJ, Boggs JW, Gustafson KJ, Grill WM. Closed loop electrical control of urinary continence. J Urol. 2006;175:1559–1563. doi: 10.1016/S0022-5347(05)00657-9. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel BJ, Boggs JW, Gustafson KJ, Creasey GH, Grill WM. Detection of neurogenic detrusor contractions from the activity of the external anal sphincter in cat and human. Neurourol Urodyn. 2006;25:140–147. doi: 10.1002/nau.20204. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel BJ, Boggs JW, Gustafson KJ, Grill WM. Detecting the onset of hyper-reflexive bladder contractions from the electrical activity of the pudendal nerve. IEEE Trans Neural Syst Rehabil Eng. 2005;13:428–435. doi: 10.1109/TNSRE.2005.848355. [DOI] [PubMed] [Google Scholar]
- 19.Hansen J, Borau A, Rodriguez A, Vidal J, Sinkjaer T, Rijkhoff NJ. Urethral sphincter EMG as event detector for neurogenic detrusor overactivity. IEEE Trans Biomed Eng. 2007;54:1212–1219. doi: 10.1109/TBME.2007.890739. [DOI] [PubMed] [Google Scholar]
- 20.Yang CC, Bradley WE. Peripheral distribution of the human dorsal nerve of the penis. 1998;159:1912–1916. doi: 10.1016/S0022-5347(01)63194-X. discussion 1916–1917. [DOI] [PubMed] [Google Scholar]
- 21.O’Connell HE, Sanjeevan KV, Hutson JM. Anatomy of the clitoris. 2005;174:1189–1195. doi: 10.1097/01.ju.0000173639.38898.cd. [DOI] [PubMed] [Google Scholar]
- 22.Previnaire JG, Soler JM, Perrigot M, Boileau G, Delahaye H, Schumacker P, Vanvelcenaher J, Vanhee JL. Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength. Paraplegia. 1996;34:95–99. doi: 10.1038/sc.1996.17. [DOI] [PubMed] [Google Scholar]
- 23.Hansen J, Media S, Nohr M, Biering-Sorensen F, Sinkjaer T, Rijkhoff NJ. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol. 2005;173:2035–2039. doi: 10.1097/01.ju.0000158160.11083.1b. [DOI] [PubMed] [Google Scholar]
- 24.Fjorback MV, Rijkhoff N, Petersen T, Nohr M, Sinkjaer T. Event driven electrical stimulation of the dorsal penile/clitoral nerve for management of neurogenic detrusor overactivity in multiple sclerosis. Neurourol Urodyn. 2006;25:349–355. doi: 10.1002/nau.20170. [DOI] [PubMed] [Google Scholar]
- 25.Jezernik S, Craggs M, Grill WM, Creasey G, Rijkhoff NJ. Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurol Res. 2002;24:413–430. doi: 10.1179/016164102101200294. [DOI] [PubMed] [Google Scholar]
- 26.Uher EM, Swash M. Sacral reflexes: physiology and clinical application. 1998;41:1165–1177. doi: 10.1007/BF02239440. [DOI] [PubMed] [Google Scholar]
- 27.Yoo PB, Klein SM, Grafstein NH, Horvath EE, Amundsen CL, Webster GD, Grill WM. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn. 2007;26:1020–1023. doi: 10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]
- 28.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J Neural Eng. 2006;3:43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- 29.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 30.Tai C, Wang J, Wang X, Roppolo JR, de Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol Urodyn. 2007;26:879–86. doi: 10.1002/nau.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]