Abstract
Background and Purpose
Conventional analysis of vascular prevention trials assigns equal weight to disparate vascular events of composite endpoint at variance with public’s perception of their differential impact on health outcome. This study was to apply the disability-adjusted life year (DALY) metric for differential weighting of individual vascular endpoints in trial analyses.
Methods
DALY values for the most common major endpoints in vascular prevention trials, nonfatal myocardial infarction (MI), nonfatal stroke, and vascular death, were derived using World Health Organization Global Burden of Disease Project methodology. The standardized DALYs for each event were applied to recent major primary and secondary vascular prevention trials and to hypothetical model trials.
Results
Standardized DALYs lost were 7.63 for nonfatal stroke, 5.14 for nonfatal MI, and 11.59 for vascular death. In the published trials analyses, direction of treatment effects was consistent between DALY and standard event analysis, but rank order of treatment effect changed for 10 of 18 trials. The DALY analysis also enabled to provide number-needed-to-treat values to gain one DALY: 2.1 for anticoagulation in atrial fibrillation, 2.7 for carotid endarterectomy in symptomatic stenosis, and 4.7 for clopidogrel added to aspirin in acute coronary syndrome. Hypothetical trial analyses demonstrated that the DALY metric more finely discriminates treatment effects.
Conclusions
Compared with a nonfatal MI, a nonfatal stroke causes a 1.48-fold greater loss and vascular death a 2.25-fold greater loss of DALY. DALY analysis integrates these valuations in a summary metric reflecting the net impact of therapy on patient and societal health, complementing conventional endpoint analyses.
Keywords: DALY, vascular disease, clinical trial, treatment effect
Introduction
Diseases and their treatment can influence many organs in diverse ways. Consequently, in all medical specialties, composite endpoints are increasingly used in randomized clinical trials. Composite endpoints capture the number of patients who have one or more of several events of interest. By incorporating a range of important endpoints in a single metric, composite endpoints can index the overall impact of therapeutic interventions and reduce sample size requirements. However, composite endpoints have well-recognized limitations that arise from the common practice of weighting all endpoint components equally, irrespective of their relative impact on the life of the patient. If positive results are driven by less salient endpoints, the trial may give the misleading impression of broad benefit. If treatment exerts differential benefit and harm on different endpoint components, a treatment may reduce the net number of events but actually worsen global health-related quality of life. Many clinical trialists and statisticians have recognized the desirability of differential weighting of clinical trial endpoints, but a widely acceptable weighting method has not been advanced.
In vascular disease prevention trials, this tension has given rise to two opposing approaches in endpoint selection: the organ-specific and multi-organ paradigms. The organ-specific approach asserts that endpoints should focus on the same vascular bed as the presenting event, since recurrent events are likely to cluster in that vascular bed.1 Including events outside of the presenting vascular bed may dilute measurement of a desired effect on the target organ at greatest risk.2 A weakness of the organ-specific argument is that, even if less frequent, events outside the initially symptomatic vascular bed are clinically relevant, and accumulate as time goes by.3 In contrast, the multi-organ paradigm employs composite outcomes, such as the first occurrence of nonfatal stroke, transient ischemic attack, nonfatal myocardial infarction (MI), angina, or vascular death, but has generally weighted each of these disparate events equally.
The disability-adjusted life year (DALY) metric, which the World Health Organization Global Burden of Disease Project (WHO-GBDP) developed to measure the global burden of diseases,4 is a promising metric to weight components of composite endpoints in clinical trials. The DALY method converts hundreds of health conditions into a uniform, patient-centered metric of healthy life years lost, by quantifying years lost due to premature mortality and optimum health years lost due to living with disability.
The objective of this study was to develop standardized DALY values for the most common endpoints assessed in vascular disease prevention trials, nonfatal stroke, nonfatal MI, and vascular death; apply these values to completed vascular prevention trials to quantify the efficacy of existing prevention treatments; and compare DALY measures to conventional measures of clinical efficacy.
Methods
Trial selection and Data collection
For the analysis, we selected pivotal primary and secondary prevention trials of antiplatelets, statins, anti-hypertensives, and surgery, including: 1) diverse treatment interventions in diverse target populations, 2) recent trials of major clinical importance, and 3) a subset of trials in which tested treatments exerted opposite direction effects on coronary and cerebrovascular endpoint events (as these pose special difficulty to traditional endpoint analysis). Technical inclusion criteria were: 1) randomized controlled trial, 2) individual event numbers for nonfatal stroke, nonfatal MI, vascular death, and composite endpoint (nonfatal stroke, nonfatal MI, and vascular death) separately stated or could be directly estimated from published tables, 3) more than 6-month follow-up, and 4) trial reported in English.
For each trial, we abstracted data for event numbers and annual event rates for nonfatal stroke, nonfatal MI, vascular death, and composite endpoint. For event numbers, if more than one outcome event occurred in one patient, only the first event was included (Supplementary material 1, http://stroke.ahajournals.org).
DALY Formulas
A DALY measures the total amount of optimal life years lost from a disease process, whether from premature mortality or from incapacity associated with nonfatal conditions. Formally, DALYs are derived from the formula DALY=YLL+YLD, where YLL is the years of life lost due to premature death and YLD is the years of healthy life lost due to disability.
YLL and YLD are derived by the following formulas:4, 5
K: age-weighting modulation factor (K=1 or 0), β: parameter from age weighting function (β=0.04 or 0), r: discount rate (r=0.03 or 0), C: constant (C=0.1658), A: age of death, L: life expectancy of general population at age A, DW: disability weight, Av: age at vascular event, Ld: duration of disability (=life expectancy after vascular event at age Av)
As in the WHO-GBDP, we applied an 3% annual discount rate (r=0.03) and age-weighting (K=1, β=0.04): DALY[3,1]. The discount rate is the standard health policy modeling assumption that values a year of healthy life gained in the future less than a year of healthy life gained in the immediate present. The age-weighting is another health policy assumption that assigns different values to different years of life, higher in young adult ages than in infancy or old ages. The values of age-weighting modulation factor, age-weighting function, constant, and discount rate were derived by the WHO-GBDP from extensive computational modeling.
Life expectancy estimation
In this study-level analysis, we did not have available individual patient ages at time of each endpoint vascular event. Instead, we employed a standardized DALY value for each event type, derived by applying a standard age to all vascular events. In the primary analysis, we set this age to 60. In sensitivity analyses, we varied this assumption to ages 50 and 70. Life expectancies at ages 50, 60, and 70 for MI survivors and general population were taken from the Framingham Heart Study (FHS) cohort, as were life expectancies for stroke survivors at ages 60 and 70.6 The life expectancy of stroke survivors at age 50, not provided in the FHS, was assigned 11.4 years (3 years shorter than that of MI survivors), based on the FHS observations of the stroke and MI survivors in 50, 60 and 70 year olds.
In the FHS, at age 60, the life expectancy for a stroke survivor is 8.9 years (men and women combined), for an MI survivor 11.2 years, and for general population 22.25 years. Accordingly, the age of death was determined as 60 for vascular death, 68.9 for stroke survivors and 71.2 for MI survivors.
While the life expectancy of general population at age 60 could be taken from the FHS, those of age at 68.9 and 71.2 were not provided in the FHS.6 As the life expectancies of the FHS general population are quite close to those of US white population,7 we employed those values: 16.36 years (women and men combined) at age 68–69 and 14.62 at age 71–72 (Figure 1).
Figure 1.
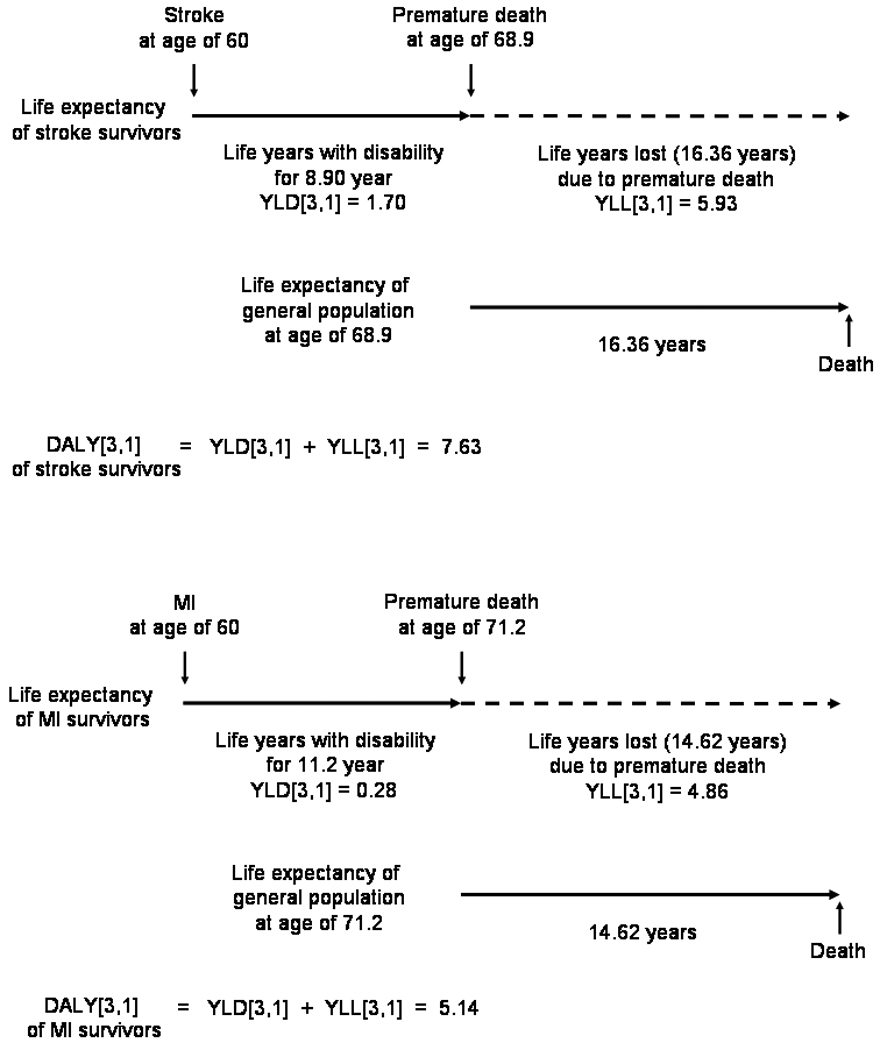
Life expectancies and DALYs for stroke and MI survivors
Disability weights for each vascular event
We employed the specific disability weight (DW) provided in the WHO-GBDP for the chronic post-stroke state of 0.266 and vascular death of 1.0.8 For the chronic post-MI state, the WHO-GBDP does not currently provide a unified DW, but one can be derived from the WHO-GBDP specific DWs for the two most common disabling sequelae of MI, chronic heart failure (CHF, DW=0.201) and angina pectoris (AP, DW=0.124).8 However, these conditions occur in only a subset of post-MI patients, and even among these only intermittently. Since the DW of WHO-GBDP was originally derived under the assumption of chronic and persistent symptom and sign, only the disabling days should be counted in deriving a unified DW of chronic post-MI state. Thus, we employed density values that were derived from days of symptom occurrence divided by 365 days. Accordingly, the DW for MI can be derived by the following formula:
PCHF or AP: proportion of MI survivors experiencing CHF or AP, DensityCHF or AP: days per year that these individuals experience CHF or AP (days divided by 365 days).
From the literature review, we projected PAP as 19.8% and PCHF as 16.9%.9, 10 For density values, literature review suggested post-MI angina frequencies of 1.2% for daily angina, 3.0% for weekly angina (angina symptom per 2–7 days: 104.3 days per year), and 15.6% for angina attacks less than one day per week for 4 weeks (angina attack per 8–28 days: 20.3 days per year), yielding a DensityAP of 0.14. For CHF, DensityCHF was assigned 1.0 under the assumption of daily symptoms or restriction in daily activities. From these, the DWMI was determined as 0.037.
By applying the values of life expectancies and DWs to the above formulas, we converted the outcomes of nonfatal stroke, nonfatal MI, and vascular death into DALYs (Supplementary material 2, http://stroke.ahajournals.org).
To compare the DALY approach with the conventional equal-weighted event analysis, values of number needed to treat (NNT) for one year were derived: NNTevent, for preventing one event; NNTDALY=100/[DALY saved for 100 patient-years]), for preventing one DALY loss (Supplementary material 3, http://stroke.ahajournals.org).
Hypothetical model trials analysis
To further illustrate the perspectives afforded by DALY analysis, we also assessed DALYs saved in six hypothetical model trials demonstrating two different patterns of treatment effect (effective only for stroke prevention or MI prevention) in three different populations (population at equal risk for stroke and MI, stroke-prone population, and MI-prone population). For this analysis, we explored the effect of removing age-weighting only (DALY[3,0]) and removing both age-weighting and future discounting (DALY[0,0]) (Supplementary material 4, http://stroke.ahajournals.org).
Results
Trials characteristics
Based on expert consensus, we selected 18 trials: 11 secondary stroke prevention trials, four secondary prevention trials in coronary heart disease (CHD), and three primary prevention trials: nine trials testing antithrombotics, 3 statins, 3 anti-hypertensives, 2 estrogens, and 1 surgery (Table 1, full names of trials in Supplementary material 5, http://stroke.ahajournals.org). Table 1 shows that patients in stroke trials had higher stroke rates and treatments had a greater impact on stroke prevention, while patients in CHD trials had higher MI rates and treatments had a greater impact on MI prevention.
Table 1.
Characteristics of included trials
| Trial | Intervention ( A/C) |
Population | Randomised patients (A/C) |
FU (years) |
Mean age (years) |
Female (%) |
Annual rate (%/year), A/C |
||
|---|---|---|---|---|---|---|---|---|---|
| Stroke† | MI | Composite endpoint |
|||||||
| Stroke trials |
1380±1059/ 1377±1056 |
2.9±1.0 | 64.9±3.4 | 42.2±18.9 |
4.47±2.03/ 6.25±3.16 |
1.53±0.79/ 1.59±0.67 |
6.79±2.79/ 8.42±3.51 |
||
| EAFT | WFR/PLC | Stroke + | 225/214 | 2.3 | 70.5 | 43.5 | 3.94/11.85 | 3.16/2.96 | 8.28/15.56 |
| UK-TIA | ASA 300 mg/PLC | Stroke | 806/814 | 4.0 | 59.5 | 29.0 | 2.84/3.32 | 2.50/2.71 | 5.89/6.45 |
| TASS | TCP/ASA | Stroke | 1529/1540 | 3.3 | 63.2 | 35.0 | 3.60/4.55 | 1.36/1.18 | 5.77/6.55 |
| CAPRIE_Stroke | CLP/ASA | Stroke | 3233/3198 | 1.9 | 64.7 | 37.0 | 5.20/5.65 | 0.73/0.85 | 7.15/7.71 |
| ESPS-2 | DP-ASA/ASA | Stroke | 1650/1649 | 2.0 | 66.6 | 42.3 | 4.76/6.25 | 1.06/1.18 | 8.24/9.73 |
| ESPRIT | DP-ASA/ASA | Stroke | 1363/1376 | 3.5 | 63.0 | 35.0 | 2.40/3.05 | 0.96/1.33 | 3.31/4.27 |
| WASID | Warfarin/ASA | Stroke | 289/280 | 1.8 | 62.8 | 40.0 | 9.41/11.50 | 2.22/1.39 | 13.11/13.08 |
| SPARCL | ATV/PLC | Stroke | 2365/2366 | 4.9 | 62.5 | 41.0 | 2.51/2.95 | 0.77/1.14 | 3.16/3.86 |
| PROGRESS | Perindopril±IDP/PLC | Stroke | 3051/3054 | 3.9 | 64.0 | 30.0 | 2.70/3.80 | 0.97/1.30 | 4.10/5.50 |
| WEST | Estrogen/PLC | Stroke | 337/327 | 2.8 | 71.5 | 100.0 | 6.68/6.12 | 1.59/1.86 | 9.33/8.85 |
| NASCET | CEA/Medical | Stroke | 328/331 | 2.0 | 65.5 | 31.5 | 5.18/9.67 | NR | 6.40/11.03 |
| CHD trials |
5037±1829/ 5046±1834 |
3.0±2.0 | 61.3±1.9 | 24.4±9.2 |
0.88±0.45/ 1.05±0.49 |
3.96±2.50/ 5.21±3.29 |
6.26±4.25/ 7.87±5.24 |
||
| CURE | CLP+ASA/ASA | CHD | 6259/6303 | 0.75 | 64.2 | 38.5 | 1.61/1.89 | 6.96/9.11 | 12.50/15.64 |
| TRITON− | PRG+ASA/CLP+ASA+ | CHD | 6813/6795 | 1.2 | 61.0 | 26.0 | 0.91/0.88 | 5.89/7.82 | 7.81/9.65 |
| CARE | Pravastatin/PLC | CHD | 2081/2078 | 5.0 | 59.0 | 14.0 | 0.51/0.76 | 1.57/2.12 | 2.71/3.61 |
| TNT | ATV 80mg/ATV 10mg | CHD | 4995/5006 | 4.9 | 61.0 | 19.0 | 0.49/0.66 | 1.41/1.78 | 2.00/2.56 |
|
Primary prevention trials |
5209±2493/ 5071±2322 |
4.5±0.6 | 68.6±5.6 | 63.7±29.9 |
0.63±0.24/ 0.80±0.42 |
1.08±0.80/ 1.21±1.08 |
2.04±1.00/ 2.36±1.40 |
||
| HOPE | Ramipril/PLC | High risk | 4645/4652 | 4.5 | 66.0 | 26.7 | 0.75/1.08 | 2.20/2.72 | 3.11/3.95 |
| SCOPE | Candersartan/PLC | HT, elderly | 2477/2460 | 3.7 | 76.4 | 64.5 | 0.85/1.11 | 0.67/0.61 | 2.30/2.58 |
| WHI | Estrogen/PLC | General | 8506/8102 | 5.2 | 63.3 | 100.0 | 0.29/0.21 | 0.37/0.30 | 0.70/0.55 |
Stroke (or MI) includes fatal and nonfatal stroke (or MI). CAPRIE_Stroke: stroke subgroup, Mean or median FU, A/C: active/control groups. Stroke and MI included nonfatal and fatal events. PLC: placebo, ASA: aspirin, TCP: ticlopidine, CLP: clopidogrel, DP: dipyridamole, IPD: indapamide, ATV: atorvastatin, CEA: carotid endarterectomy, PRG: prasgurel
DALYs lost for individual vascular events
Table 2 shows the calculated DALYs[3,1] lost for each vascular event at various ages. For the primary analysis, setting vascular events at age 60, the DALYs[3,1] lost due to nonfatal stroke, nonfatal MI, and vascular death were 7.63, 5.14, and 11.59, respectively. The contributions of YLL to total DALY lost were 77.9% for nonfatal stroke and 94.6% for nonfatal MI. Changing the average age of vascular events to 50 magnified the DALYs lost across all vascular event categories and changing to 70 reduced them.
Table 2.
DALYs[3,1] for individual vascular events
| YLD[3,1] | YLL[3,1] | DALY[3,1] | |
|---|---|---|---|
| Age 60 | |||
| Nonfatal stroke | 1.69 | 5.94 | 7.63 |
| Nonfatal MI | 0.28 | 4.86 | 5.14 |
| Vascular death | 0 | 11.59 | 11.59 |
| Age 50 | |||
| Nonfatal stroke | 2.58 | 7.91 | 10.49 |
| Nonfatal MI | 0.42 | 6.31 | 6.73 |
| Vascular death | 0 | 16.79 | 16.79 |
| Age 70 | |||
| Nonfatal stroke | 1.00 | 4.06 | 5.06 |
| Nonfatal MI | 0.16 | 3.69 | 3.85 |
| Vascular death | 0 | 7.24 | 7.24 |
YLL: the years of life lost due to premature death; YLD: the years of healthy life lost due to disability
DALY[3,1]: both future discounting and age weighting applied
DALYs saved by individual trials
Table 3 shows the DALYs saved for individual vascular events from each trial treatment per 100 patient-years of intervention. The DALYs saved for composite endpoint of nonfatal stroke, nonfatal MI, and vascular death by individual trial treatments ranged from −6.47 to 46.96: from losing 6.47 years to saving 46.96 years of healthy life.
Table 3.
DALYs[3,1] saved for a 100 patient-years treatment in each trial
| Population | Composite endpoint |
Nonfatal stroke | Nonfatal MI | Vascular death | |
|---|---|---|---|---|---|
| Group 1 | |||||
| EAFT | Stroke | 46.96 | 61.46 | 4.32 | −18.81 |
| NASCET | Stroke | 37.62 | 30.78 | NR | 6.84 |
| CURE | CHD | 21.42 | 2.75 | 10.83 | 7.84 |
| Group 2 | |||||
| ESPS-2 | Stroke | 11.58 | 11.36 | −0.15 | 0.38 |
| TRITON-TIMI 38 | CHD | 9.17 | 0.01 | 9.75 | −0.59 |
| PROGRESS | Stroke | 8.79 | 6.74 | 1.56 | 0.49 |
| ESPRIT | Stroke | 8.51 | 3.91 | 0.46 | 4.13 |
| HOPE | Primary prevention | 7.70 | 1.56 | 0.90 | 5.24 |
| CARE | CHD | 6.65 | 2.20 | 2.07 | 2.38 |
| TASS | Stroke | 5.84 | 6.06 | NR | −0.23 |
| Group 3 | |||||
| SPARCL | Stroke | 4.48 | 2.45 | 1.91 | 0.13 |
| TNT | CHD | 4.10 | 1.14 | 1.47 | 1.49 |
| CAPRIE_Stroke | Stroke | 4.16 | 3.53 | 0.38 | 0.24 |
| UK-TIA | Stroke | 2.98 | 3.94 | 1.20 | −2.16 |
| SCOPE | Primary prevention | 2.69 | 1.91 | −0.31 | 1.09 |
| Group 4 | |||||
| WHI | Primary prevention | −1.11 | −0.60 | −0.34 | −0.17 |
| WEST | Stroke | −5.53 | 2.09 | −0.89 | −6.73 |
| WASID | Stroke | −6.47 | 15.38 | −1.41 | −20.45 |
NR: not reported
Based on the magnitude of DALYs saved for the composite endpoint, the trials were coarsely classified into four groups: 1) trials with the greatest DALYs saved of about 20 or more (3 trials), 2) trials saving 5–12 DALYs (6 trials), 3) trials with a relatively small DALY gain of less than 5 (6 trials), and 4) trials showing DALYs lost (treatment harm, 3 trials).
All stroke and CHD secondary prevention trials nominally reduced nonfatal recurrent events in the initially presenting vascular bed. However, seven trials (five stroke trials, one CHD trial, one primary prevention trial) showed a discrepant treatment effects on other vascular events. Overall, two stroke trials and one primary prevention trial showed net harm (DALYs lost).
NNTevent, NNTDALY, and Comparison of treatment effects
NNT values per event and per DALY are shown in Table 4. Whether a trial treatment was beneficial or harmful was consistent between the DALY and composite events conventional analyses. However, applying DALYs substantially changed the rank order of treatment effect magnitude compared to the conventional approach: only eight out of 18 trials keep their original ranks (Supplementary Table 1. http://stroke.ahajournals.org). While the trial with the greatest benefit (anticoagulation for secondary stroke prevention in AF) was consistent between the DALY and conventional approaches, the most harm was observed in anticoagulation for symptomatic intracranial stenosis with DALY approach and estrogen for secondary stroke prevention with the conventional approach.
Table 4.
Comparison of NNTDALY[3,1] and NNTevent analysis for treatment efficacies
| Composite endpoint | Nonfatal stroke | Nonfatal MI | Vascular death | |||||
|---|---|---|---|---|---|---|---|---|
| NNTDALY | NNTevent | NNTDALY | NNTevent | NNTDALY | NNTevent | NNTDALY | NNTevent | |
| Stroke trials | ||||||||
| EAFT | 2.1 | 13.8 | 1.6 | 12.4 | 23.2 | 119.0 | −5.3 | −61.6 |
| NASCET | 2.7 | 21.6 | 3.3 | 24.8 | NR | NR | 14.6 | 169.4 |
| ESPS-2 | 8.6 | 67.1 | 8.8 | 67.2 | −648.7 | −3334.4 | 265.7 | 3079.6 |
| PROGRESS | 11.4 | 71.4 | 14.8 | 113.1 | 64.2 | 330.1 | 203.5 | 2358.3 |
| ESPRIT | 11.8 | 104.3 | 25.6 | 194.9 | 218.0 | 1120.6 | 24.2 | 280.4 |
| TASS | 17.1 | 129.0 | 16.5 | 125.9 | NR | NR | −440.3 | −5103.1 |
| SPARCL | 22.3 | 142.3 | 40.9 | 311.9 | 52.4 | 269.3 | 796.7 | 9233.6 |
| CAPRIE_Stroke | 24.1 | 179.2 | 28.3 | 215.9 | 263.8 | 1356.1 | 408.3 | 4731.6 |
| UK-TIA | 33.6 | 177.5 | 25.4 | 193.7 | 83.2 | 427.8 | −46.2 | −535.9 |
| WEST | −18.1 | −208.6 | 47.7 | 364.3 | −112.4 | −577.8 | −14.9 | −172.2 |
| WASID | −15.5 | −4538.8 | 6.5 | 49.6 | −71.1 | −365.4 | −4.9 | −56.7 |
| CHD trials | ||||||||
| CURE | 4.7 | 31.8 | 36.4 | 277.7 | 9.2 | 47.5 | 12.8 | 147.8 |
| TRITON-TIMI 38 | 10.9 | 54.1 | 11963.8 | 91284.0 | 10.3 | 52.7 | −170.7 | −1977.8 |
| CARE | 15.0 | 111.5 | 45.5 | 346.9 | 48.3 | 248.3 | 42.0 | 486.5 |
| TNT | 24.4 | 177.3 | 88.0 | 671.3 | 68.0 | 349.0 | 67.1 | 778.1 |
|
Primary prevention trials |
||||||||
| HOPE | 13.0 | 120.3 | 64.1 | 489.2 | 111.1 | 571.1 | 19.1 | 221.3 |
| SCOPE | 37.1 | 351.3 | 52.3 | 399.0 | −324.6 | −1668.4 | 91.8 | 1064.0 |
| WHI | −90.1 | −627.8 | −166.5 | −1270.6 | −295.2 | −1517.4 | −588.0 | −6814.6 |
NR: not reported
Sensitivity analysis
Treatment effects were magnified by changing age at vascular events to 50 and reduced by changing to 70, but their ranks remained generally stable whether age 60, 50, or 70 were employed (Supplementary Table 1 and 2, http://stroke.ahajournals.org).
Hypothetical trials
Table 5 shows the comparison of hypothetical trials in terms of NNTevent and NNTDALY. Compared to the conventional NNTevent, NNTDALY more finely discriminated the differences in treatment effects between 6 hypothetical settings. The discriminating power remained robust when age-weighting and both age-weighting and future discounting were removed.
Table 5.
Hypothetical trials analysis
| Population and intervention | Event number, control/intervention |
NNT for composite endpoint |
||||||
|---|---|---|---|---|---|---|---|---|
| Non-fatal Stroke |
Non-fatal MI |
Vascular death |
NNTevent | NNTDALY[3, 1] |
NNTDALY[3, 0] |
NNTDALY[0, 0] |
||
| Population at equal risk for Stroke and MI |
||||||||
| Stroke effective | 160/96 | 160/160 | 100/84 | 50 | 5.9 | 3.9 | 2.6 | |
| MI effective | 160/160 | 160/96 | 100/84 | 50 | 7.8 | 4.9 | 3.0 | |
| Population at unequal risk for Stroke and MI |
||||||||
| Stroke prone population, age 60 | ||||||||
| Stroke effective | 160/96 | 80/80 | 80/64 | 50 | 5.9 | 3.9 | 2.6 | |
| MI effective | 160/160 | 80/48 | 80/72 | 100 | 15.6 | 9.7 | 6.1 | |
| MI prone population, age 50 | ||||||||
| Stroke effective | 80/48 | 160/160 | 80/72 | 100 | 8.5 | 6.6 | 3.9 | |
| MI effective | 80/80 | 160/96 | 80/64 | 50 | 5.7 | 4.2 | 2.3 | |
For each hypothetical trial, assumptions of age onset, event rate, and relative risk reduction by treatment were described in supplementary text.
DALY[3,0]: age-weihting removed, DALY[0,0]: both future discounting and age weighting removed
Discussion
This study provides standardized DALY values for the most common components of composite endpoints used in contemporary vascular prevention trials and demonstrates that treatment effects of diverse vascular prevention trials can be presented as a summary patient-centered metric of healthy life years gained or lost. Formal analysis indicated that the major vascular events routinely assessed in prevention trials are not of equal importance. Rather, compared with a nonfatal myocardial infarct, a nonfatal stroke causes a 1.48-fold greater loss of disability-adjusted life years and vascular death a 2.25-fold greater loss.
A fundamental principle of evidence-based medicine is that assessment of a treatment effect should encompass all the outcomes a treatment might alter, in proportion to the degree they are valued by the patient and society. Consequently, the desirability of weighting components of composite endpoints in clinical trials has been widely recognized.11, 12 Several weighting approaches have been suggested, but none to date has captured general allegiance.12–15 Most prior weighting algorithms were derived using informal methods and thin theoretical foundations, limiting their acceptance. In contrast, the DALY approach has a strong methodological framework (person trade-off analyses by internationally representative health providers) and a rich theoretical grounding. It has already achieved wide acceptance as a tool for international health policy analysis and decision-making, by the WHO and many health planning authorities. This extensive acceptance in arenas external to clinical trial design gives DALYs credibility and authority for porting into clinical trial planning and analysis.
Another health adjusted life year metric, the quality-adjusted life year (QALY), is also an attractive candidate for weighting outcome endpoints and already has been employed in cost-effectiveness analyses of clinical trial results. Compared with QALYs, DALY analysis has some advantages. QALYs are derived from patients or healthy individuals with a limited experience of diverse disease states, employing a wide variety of techniques.16 As a result, considerable variation across studies occurs in the quality of life weights assigned to the same health states. In contrast, DALYs are derived from broadly experienced health professionals in a single, explicit, and broad-based disease comparison framework, resulting in internally coherent weightings for a broad range of conditions.
The functional limitations that vascular events can impose on an individual’s remaining life are critically important to patients, families, and society. However, conventional composite endpoint analysis, counting the number of acute events, provides only a one-time, snapshot metric that fails to capture the lifelong impact of disease. In contrast, the DALY metric reflects the disparity across the individual vascular events in both event-related premature mortality and reduced human flourishing during an individual’s remaining years of life.
The different contributions of disability and premature mortality to the valuations in our study are noteworthy. The disability weight assigned to the chronic post-stroke state was seven times higher than that for the chronic post-MI state, reflecting the greater frequency and disabling impact of neurologic deficits after stroke than functionally limiting CHF and chronic angina after MI. Consequently, years lost due to disability contributed substantially, 22.1%, to the total DALYs lost for nonfatal stroke events. In contrast, years lost due to disability only accounted for 5.4% of the loss of optimal health for nonfatal MI, with premature mortality exerting the overwhelming effect.
Weighting the components of composite endpoints allowed us to derive a single DALY value that summarized for diverse vascular prevention treatments each intervention’s net impact on patient health. For treatments that had opposing direction effects on different endpoints, a widely recognized challenge to interpretation of composite endpoints, the DALY resolved and quantified the overall benefit or risk of intervention. Across all treatments, the DALY metric allowed comparisons to be made of the relative degree of benefit delivered by diverse therapies. The greatest benefits were seen in secondary prevention trials, in which enrolled patients are at highest risk for new events; carotid endarterectomy in symptomatic carotid stenosis, anticoagulation for secondary prevention in atrial fibrillation, and clopidogrel added on to aspirin in the first year after acute coronary syndrome.
Concurrent validity for the DALY findings is provided by the general consistency of benefit and harm shown with conventional composite endpoint analysis and DALY analysis. In addition, the DALY analysis permitted a more refined discrimination of interventional efficacy. Across all trials, number needed to treat values to gain 1 healthy life year were nearly an order of magnitude lower than those needed to avert one vascular event. In the hypothetical trial models, DALY analysis demonstrated more clearly than conventional analysis the variation in degree of benefit obtained when an intervention effective for a specific event is used in patients at high or low risk for that event.
This study has limitations. We analyzed the three most common and important endpoints used in vascular prevention trials. Additional work is needed to derive and apply to clinical trials DALY values for other endpoints, including unstable angina, transient ischemic attack, and hospitalizations for revascularization procedures.
We performed an analysis of study-level, not individual patient-level, clinical trial data. The most precise application of DALY analysis to clinical trials would use patient-level data, allowing life expectancy calculations to take into account age, sex, country of residency, and other specific characteristics of each patient.17 However, often only study-level data are available for this analysis. For such settings, a standardized DALY for a prototypical age is useful to convert event counts into DALY values.
An additional limitation the current analysis is that it focuses on only the first postrandomization vascular event, not all events that may eventually occur. The DALY values we used only partially capture recurrent events, through reduced life expectancy values, but a more comprehensive depiction of treatment effect would be provided by actual ascertainment and disability weighting of all postrandomization events, not just first events. When patient-level data of subsequent events is available, the DALY metric could directly index the impact of these subsequent events. For example, if a patient had an MI at age 60, and then a stroke at 65, and then die at 70, and the life expectancy of general population at age 70 is 15.35 (from the Framingham Heart Study6) years, the DALY lost of this patient would be the sum of the YLD lost to nonfatal MI for 10 years, YLD to nonfatal stroke for 5 years, and YLL to 15.35 years of premature death (Supplementary Table 3, http://stroke.ahajournals.org).
In trial analyses, we used the same patient ages for MI, stroke, and vascular death events. This approach contrasts with epidemiologic observations showing that MIs tend to occur in younger individuals than strokes. However, this study measured the burden of stroke and MI in clinical trial populations, and the ages at which MI and stroke occur in a given trial tend to be much closer than in the general population. Life expectancy assumptions were based on Framingham data which had published salient life expectancy data of post-stroke, post-MI, and general population. The results are therefore most applicable to Caucasian populations, but the underlying method can be generalized to other populations whenever salient life-expectancy data are available. Our analyses assumed the same disability weights of non-fatal stroke (or MI) across all trials. This assumption does not always hold. For example, strokes experienced by an atrial fibrillation population tend to be more disabling than strokes in other populations.18 Data on event severity were generally not available for the trials we analyzed. When available, incorporating event severity data will permit more refined DALY estimates of treatment effect.17, 19
While conventional event rate analysis of treatment effect is based on direct observations, health adjusted life year metrics (i.e., QALY, DALY), inevitably require several assumptions when determining life expectancies after each vascular event and quantifying health state values. However, equal weighting to different outcomes of different impact on health should be reappraised as reflected in recent debates on the findings of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).20 In CREST, quality-of-life analyses among survivors at 1 year indicate that stroke had a greater adverse effect on a broad range of health-status domains than did myocardial infarction, consonant with our findings. That the general DALY approach has undergone extensive validation by the World Health Organization and is an accepted foundation for planning of global health policies supports its being considered a valuable technique evaluating treatment effects of vascular prevention trials.
The present study demonstrates that the detailed and nuanced WHO-GBDP DALY framework can be applied to weight composite endpoint events in cardiovascular trials. The DALY approach, as an integrated, patient-centered outcome metric, complements conventional endpoint analyses and delineates more clearly the summary impact of a therapy on patient and societal health.
Supplementary Material
Acknowledgments
Acknowledgements and Funding
This work was supported by a grant (A060171) of the Korea Health 21 R&D project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (K.-S.H.), National Heart Lung and Blood Institute (GCF), NIH-NINDS Award P50 NS044378 (JLS), and an American Heart Association PRT Outcomes Research Center Award (JLS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
KSH, LKA, SLS, GCF, and JLS participated in the study conception and design. KSH, LKA, SLS, GCF, and JLS performed the literature search and study selection. KSH, LKA, SLS extracted data. KSH and JLS performed the statistical analyses. KSH and JLS wrote the first draft of the manuscript. All authors contributed to the revision of the manuscript.
Disclosure
K.-S.H. is involved in the design and a site investigator of multicenter clinical trials sponsored by Korea Otsuka and Boryung; received lecture honoraria from Sanofi-Aventis (modest). JLS is a scientific consultant regarding trial design and conduct to AGA Medical, CoAxia (all modest); has received lecture honoraria from Boehringer Ingelheim (modest); is a site investigator in the NIH IRIS, ARUBA, CREST, and SAMMPRIS multicenter clinical trials for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled, has served as a site investigator in a multicenter trial sponsored by AGA Medical for which the UC Regents received payments based on the clinical trial contract for the number of subjects enrolled; and is funded by NIH-NINDS Awards P50 NS044378 and U01 NS 44364. GCF has received research funding by the National Heart Lung and Blood Institute (significant), served as a consultant for Novartis and Pfizer (modest) and honorarium from Bristol Myers Squibb, Sanofi-Aventis, Astra-Zeneca, Merck-Schering Plough, and Pfizer (significant) and is an employee of the University of California, which holds a patent on retriever devices for stroke. SLS has received lecture honoraria from EKR Therapeutics and Sanofi- Aventis.
References
- 1.Vickrey BG, Rector TS, Wickstrom SL, Guzy PM, Sloss EM, Gorelick PB, Garber S, McCaffrey DF, Dake MD, Levin RA. Occurrence of Secondary Ischemic Events Among Persons With Atherosclerotic Vascular Disease. Stroke. 2002;33:901–906. doi: 10.1161/hs0402.105246. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW. Choice of endpoints in antiplatelet trials: Which outcomes are most relevant to stroke patients? Neurology. 2000;54:1022–1028. doi: 10.1212/wnl.54.5.1022. [DOI] [PubMed] [Google Scholar]
- 3.Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year survival after first-ever stroke in the perth community stroke study. Stroke. 2003;34:1842–1846. doi: 10.1161/01.STR.0000082382.42061.EE. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJ. The Global Burden of Disease, ed. C. J. L. Murray and A. D. Lopez, Vol. 1 of Global Burden of Disease and Injury Series. Cambridge, MA: Harvard University Press; 1996. Rethinking DALYs; pp. 1–98. [Google Scholar]
- 5.Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy and Planning. 2001;16:326–331. doi: 10.1093/heapol/16.3.326. [DOI] [PubMed] [Google Scholar]
- 6.Peeters A, Mamun AA, Willekens F, Bonneux L. A cardiovascular life history. A life course analysis of the original Framingham Heart Study cohort. Eur Heart J. 2002;23:458–466. doi: 10.1053/euhj.2001.2838. [DOI] [PubMed] [Google Scholar]
- 7.Arias E. United States Life Tables 2004. National Vital Statistics Reports. 2007;56:1–40. [PubMed] [Google Scholar]
- 8.WHO. [Accessed 4/02/2009];Global Burden of Disease 2004 Update: Disability Weights for diseases and conditions. 2008 www.who.int/healthinfo/global_burden_disease/GBD2004_DisabilityWeights.pdf)
- 9.Maddox TM, Reid KJ, Spertus JA, Mittleman M, Krumholz HM, Parashar S, Ho PM, Rumsfeld JS. Angina at 1 year after myocardial infarction: prevalence and associated findings. Arch Intern Med. 2008;168:1310–1316. doi: 10.1001/archinte.168.12.1310. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y Committee ftAHAS, Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 11.Montori VM, Permanyer-Miralda G, Ferreira-Gonzalez I, Busse JW, Pacheco-Huergo V, Bryant D, Alonso J, Akl EA, Domingo-Salvany A, Mills E, Wu P, Schunemann HJ, Jaeschke R, Guyatt GH. Validity of composite end points in clinical trials. Bmj. 2005;330:594–596. doi: 10.1136/bmj.330.7491.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira-Gonzalez I, Busse JW, Heels-Ansdell D, Montori VM, Akl EA, Bryant DM, Alonso-Coello P, Alonso J, Worster A, Upadhye S, Jaeschke R, Schunemann HJ, Permanyer-Miralda G, Pacheco-Huergo V, Domingo-Salvany A, Wu P, Mills EJ, Guyatt GH. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. Bmj. 2007;334:786. doi: 10.1136/bmj.39136.682083.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Califf RM, Harrelson-Woodlief L, Topol EJ. Left ventricular ejection fraction may not be useful as an end point of thrombolytic therapy comparative trials. Circulation. 1990;82:1847–1853. doi: 10.1161/01.cir.82.5.1847. [DOI] [PubMed] [Google Scholar]
- 14.Hallstrom AP, Litwin PE, Weaver WD. A method of assigning scores to the components of a composite outcome: an example from the MITI trial. Control Clin Trials. 1992;13:148–155. doi: 10.1016/0197-2456(92)90020-z. [DOI] [PubMed] [Google Scholar]
- 15.Neaton JD, Gray G, Zuckerman BD, Konstam MA. Key issues in end point selection for heart failure trials: composite end points. J Card Fail. 2005;11:567–575. doi: 10.1016/j.cardfail.2005.08.350. [DOI] [PubMed] [Google Scholar]
- 16.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hong KS, Saver JL. Years of disability-adjusted life gained as a result of thrombolytic therapy for acute ischemic stroke. Stroke. 2010;41:471–477. doi: 10.1161/STROKEAHA.109.571083. [DOI] [PubMed] [Google Scholar]
- 18.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 19.Hong KS, Saver JL. Quantifying the value of stroke disability outcomes: WHO global burden of disease project disability weights for each level of the modified Rankin Scale. Stroke. 2009;40:3828–3833. doi: 10.1161/STROKEAHA.109.561365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


