Abstract
Reports of individuals with deletions of 1q24→q25 share common features of prenatal onset growth deficiency, microcephaly, small hands and feet, dysmorphic face and severe cognitive deficits. We report nine individuals with 1q24q25 deletions, who show distinctive features of a clinically recognizable 1q24q25 microdeletion syndrome: prenatal-onset microcephaly and proportionate growth deficiency, severe cognitive disability, small hands and feet with distinctive brachydactyly, single transverse palmar flexion creases, fifth finger clinodactyly and distinctive facial features: upper eyelid fullness, small ears, short nose with bulbous nasal tip, tented upper lip, and micrognathia. Radiographs demonstrate disharmonic osseous maturation with markedly delayed bone age. Occasional features include cleft lip and/or palate, cryptorchidism, brain and spinal cord defects, and seizures. Using oligonucleotide-based array comparative genomic hybridization, we defined the critical deletion region as 1.9 Mb at 1q24.3q25.1 (chr1: 170135865–172099327, hg18 coordinates), containing 13 genes and including CENPL, which encodes centromeric protein L, a protein essential for proper kinetochore function and mitotic progression. The growth deficiency in this syndrome is similar to what is seen in other types of primordial short stature with microcephaly, such as Majewski osteodysplastic primordial dwarfism, type II (MOPD2) and Seckel syndrome, which result from loss-of-function mutations in genes coding for centrosomal proteins. DNM3 is also in the deleted region and expressed in the brain, where it participates in the Shank-Homer complex and increases synaptic strength. Therefore, DNM3 is a candidate for the cognitive disability, and CENPL is a candidate for growth deficiency in this 1q24q25 microdeletion syndrome.
Keywords: Chromosome deletion 1q24-q25, intrauterine growth deficiency, proportionate short stature, microcephaly, cognitive deficiency, speech deficiency, dysmorphic features
INTRODUCTION
Most reports of deletions including 1q24 or 1q25 have been defined using traditional cytogenetic techniques [Schwanitz et al., 1977; de Pablo et al., 1980; Schinzel and Schmid, 1980; Moghe et al., 1981; Martin and Simpson, 1982; Taysi et al., 1982; Silengo et al., 1984; Beemer et al., 1985; Zaletaev et al., 1987; Lo et al., 1993; Takano et al., 1997; Melis et al., 1998; Pallotta et al., 2001; Okamoto et al., 2005; Kibe et al., 2011]. More recent cases have been specified more completely using molecular techniques or chromosomal microarrays [Franco et al., 1991; Chaabouni et al., 2006; Descartes et al., 2008; Nishimura et al., 2010]. A proximal 1q21–22→q25 deletion syndrome was originally described by Taysi et al. [1982] as consisting of severe pre- and postnatal growth and psychomotor retardation, microbrachycephaly, ear abnormalities, multiple hernias, external genital abnormalities, small hands and feet, incomplete transverse palmar creases, dysplastic nails, and fifth finger clinodactyly. Based on 4 patients, they also proposed that interstitial deletions of 1q24–25→q32 resulted in nonspecific features, with little overlap of the phenotypes except for severe growth deficiency, clinodactyly and abnormal ears in 3 of these 4 cases [Taysi et al., 1982]. As more patients have been described, it has been recognized that short stature with distinctive small hands and feet demonstrating characteristic radiographic findings are associated with 1q24q25 deletions [Descartes et al., 2008]. Our study characterizes the clinical features in nine individuals with 1q24q25 deletion defined by oligonucleotide-based array CGH (aCGH) to delineate a specific recognizable 1q24q25 microdeletion syndrome associated with the deletion of 13 known genes within this region. This syndrome includes prenatal onset growth deficiency with severe proportionate short stature and microcephaly, small hands and feet, distinctive facial characteristics and severe cognitive disability. Additionally, when deletions extend distally to include AT3/SERPINC1, the resulting diminished antithrombin 3 activity is a risk factor for thrombophilia.
CLINICAL REPORTS
Patient 1
This patient was born to a G3P3 28-year-old mother and her 32-year-old partner after an uncomplicated pregnancy, labor and delivery. He was delivered vaginally at term with birth weight 2500 g, −2.0 Standard Deviations below mean for age (−2.0 SD), length 45 cm (−2.5 SD) and congenital microcephaly noted on exam. An echocardiogram was normal. Neonatal complications included transient thrombocytopenia and feeding difficulties, which necessitated nasogastric feedings for the first 10 days of life. Brain MRI and karyotype were normal, 46,XY.
At age 8 weeks, weight, length and OFC were 3180 g (−2.9 SD), 50.6 cm (−3.0 SD) and 33 cm (−3.6 SD) respectively. Physical exam revealed epicanthal folds, mild hypertelorism, prominent nasal tip, low-set posteriorly angulated ears with thin helices, bilateral 5th finger clinodactyly, right single transverse palmar flexion crease, and right-sided cryptorchidism. At 6 months growth hormone, IGF1, IGFBP3, and thyroid function studies were all normal, and hand X-ray showed delayed carpal ossification. At age 8 months, PE tubes were placed for chronic otitis media. At age 10 months weight, length and OFC remained below the 3rd centile (−3.5 to −4.2 SD below mean for age), and physical exam revealed small hands with short, tapered fingers and hypotonia. He was started on growth hormone with no significant effect. Developmental milestones were delayed, and behavioral audiological testing at age 1 and 2/12 years revealed mild to moderate sensorineural hearing loss for at least one ear.
A skeletal survey at age 1 and 3/12 years showed disharmonious osseous maturation with severe carpal ossification delay and less epiphyseal delay on his hand X-ray. There was shortening of the 2nd and 5th middle phalanges, as well as the second distal phalanx. Skull radiograph showed a small sella turcica, and hip radiographs showed mild epiphyseal delay (small epiphyses) with mild coxa valga, and arms showed slight epiphyseal delay. At age 1 and 8/12 years dysmorphic facial features persisted (Figure 1B, Fig 2B, Fig 3A). Inner canthal distance was 25 mm (50th centile), interpupillary distance was 40 mm (<3rd centile), outer canthal distance was 70 mm (10th centile), and hands/feet were very small (Figure 4A and D) with hand length 7.2 cm, middle finger length 2.9 cm, palm width 4.3 cm (10th centile) and foot length 9.4 cm. (all ≪3rd centile except palm width). At age 2 and 1/12 years tympanometry revealed bilateral absent tympanic membrane mobility, small ear canals and middle ear volumes (0.5 cc for the right ear and 0.6 cc for the left ear) and persistent mild-moderate hearing loss. At 2 and 2/12 years, repeat brain MRI was normal.
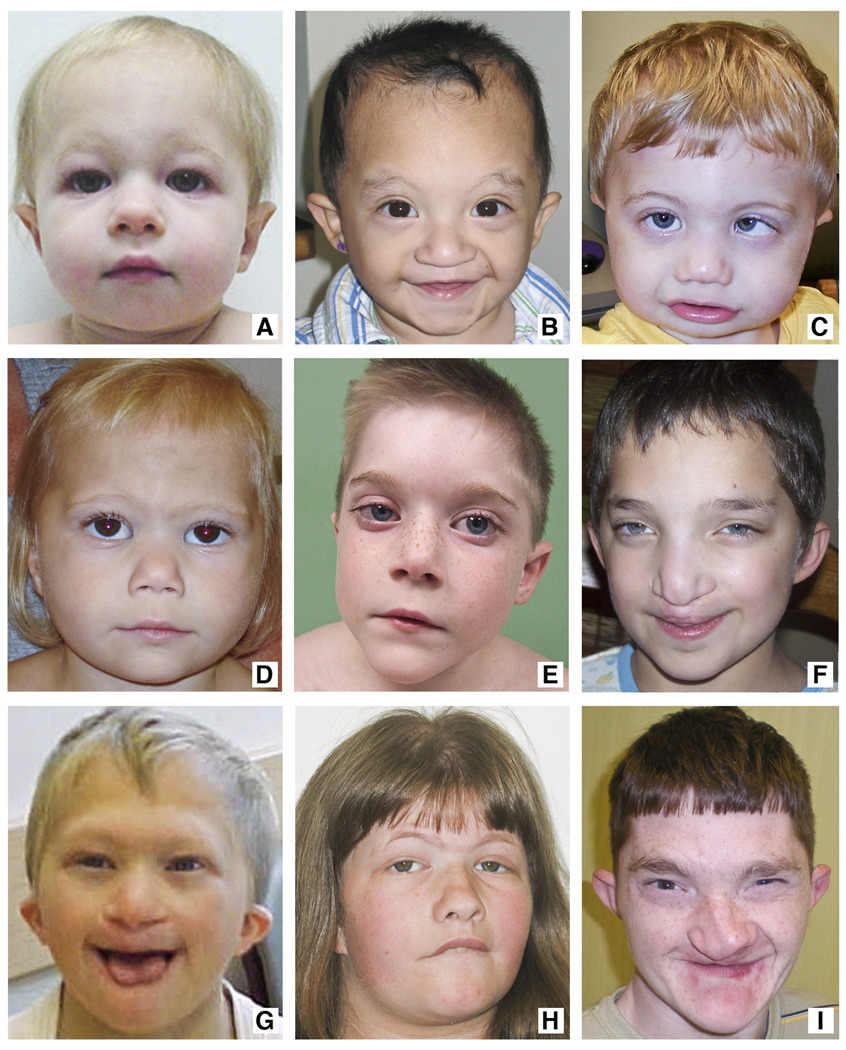
Figure 1.
Distinctive facial features include fullness to the upper eyelid, mild telecanthus, small ears with hypoplastic superior crus and ear lobes, short nose with broad nasal bridge, bulbous nasal tip, flared alae nasi, tented upper lip, down-turned corners of the mouth, and micrognathia. Patients’ facial features shown from youngest age (17 months) to oldest age (24 years). A. Patient 4, B. Patient 1, C. Patient 5, D. Patient 2, E. Patient 6, F. Patient 9, G. Patient 3, H. Patient 7, I. Patient 8.
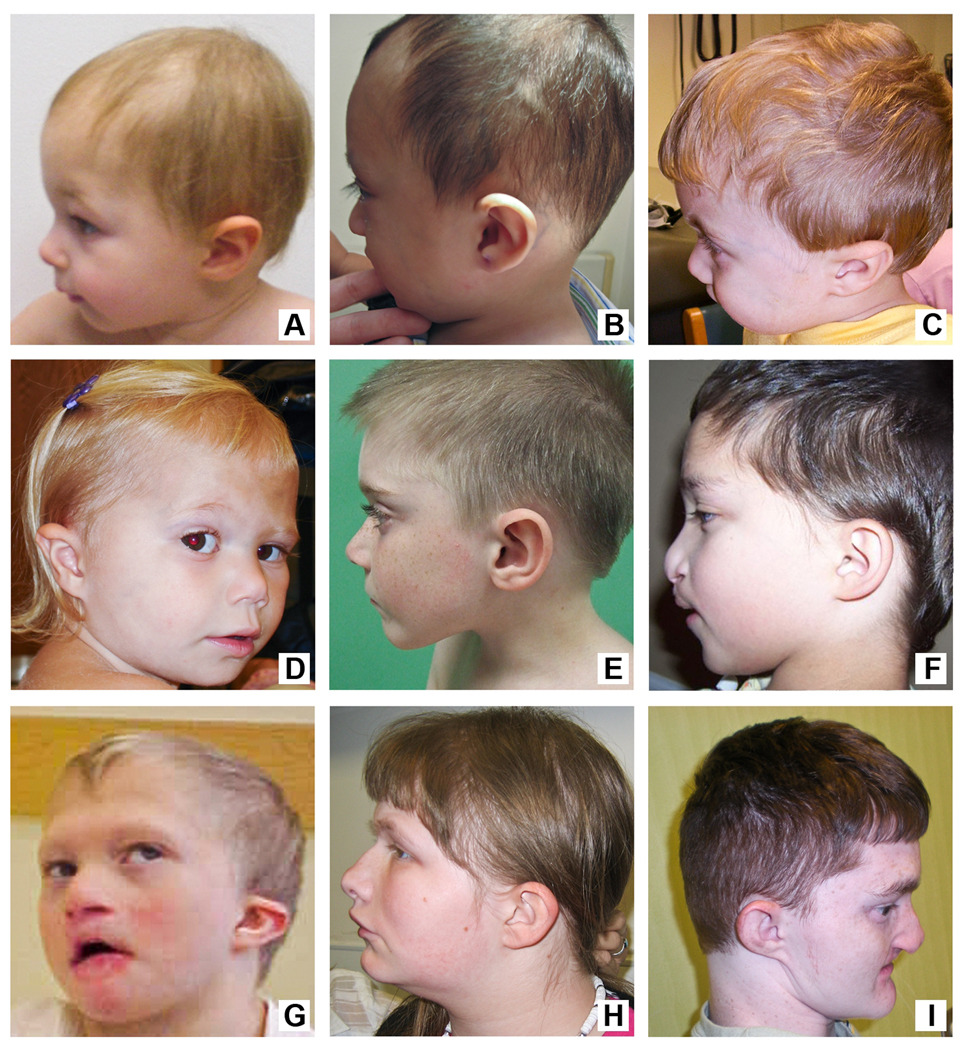
Figure 2.
On facial profile ears appear low-set and posteriorly oriented with relative prominence of the midface due to microcephaly and micrognathia. Patients’ facial features shown from youngest age (17 months) to oldest age (24 years). A. Patient 4, B. Patient 1, C. Patient 5, D. Patient 2, E. Patient 6, F. Patient 9, G. Patient 3, H. Patient 7, I. Patient 8.
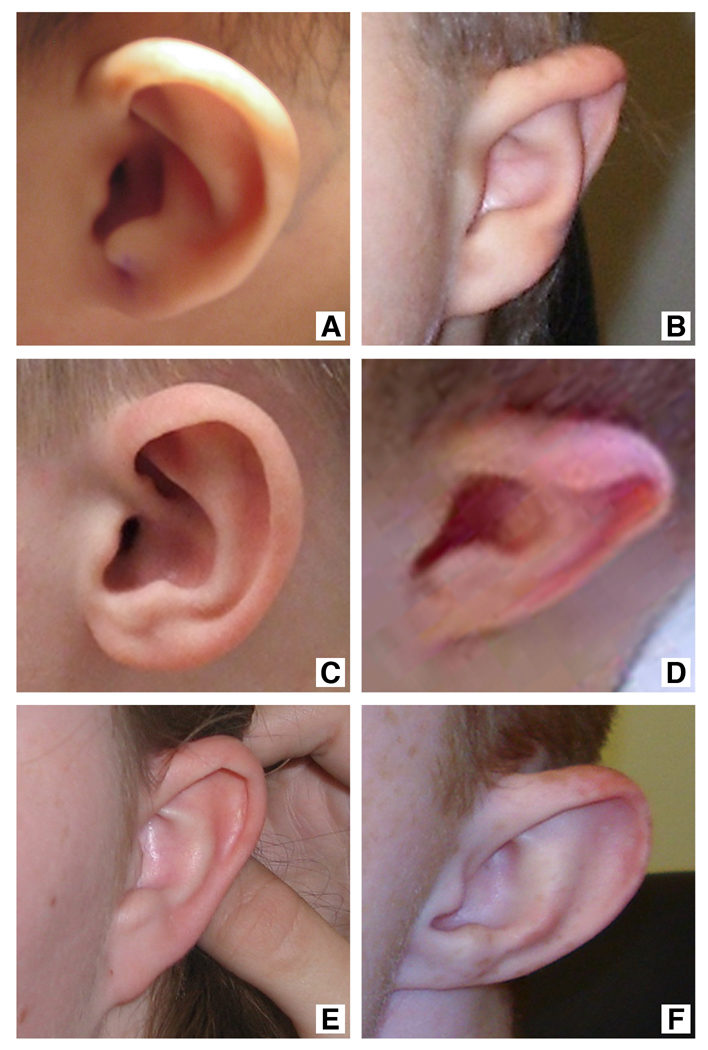
Figure 3.
Ears are usually small with hypoplastic superior crus and ear lobes. Images arranged from youngest to oldest patients. A. Patient 1, B. Patient 5, C. Patient 6, D. Patient 3, E. Patient 7, F. Patient 8.
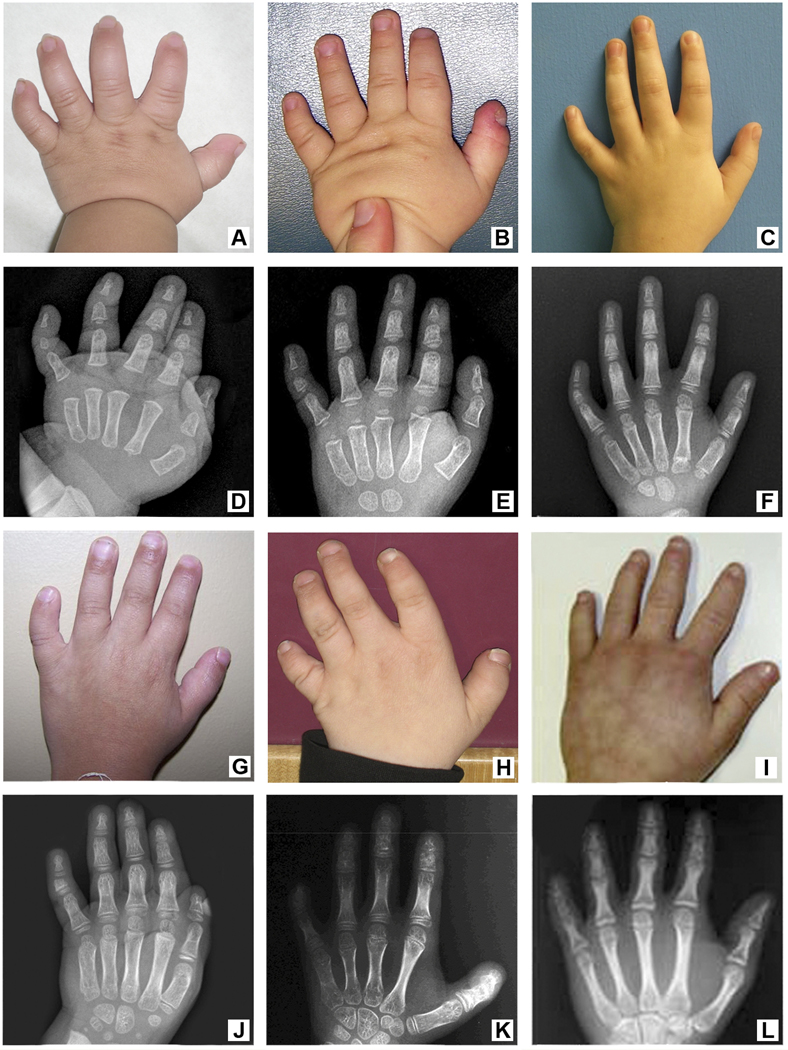
Figure 4.
Small hands with distinctive brachydactyly, short middle and distal phalanges, small nails, single transverse palm creases, and 5th finger clinodactyly. A. and D. Patient 1 Male hand X-ray at age 6 months showed delayed carpal ossification. B. and E. Patient 5 Male hand X-ray at age 30 months showed short 2nd, 3rd and 4th distal phalanges with severe delay in the bone age and disharmonic osseous maturation. C. and F. Patient 6 Male hand X-ray at 7 years, 5 months showed short 2nd and 5th middle phalanges with 5th finger clinodactyly. The 2nd middle phalanx showed a coned epiphysis, and bone age was severely delayed to 4 years 9 months. G. and J. Patient 9 Male hand X-ray at age 8 years, 8 months showed carpal and phalangeal ossification centers were markedly delayed to 5–6 years (3.6 SD below mean for age). H. and K. Patient 7 Female hand X-ray at age 12 years showed shortening of the 3rd, 4th and 5th metacarpals with milder shortening of the 2nd metacarpal. Proximal phalanges appeared normal, but there was shortening of the 2nd, 4th and 5th middle phalanges and shortening of the 2nd, 3rd, 4th and 5th distal phalanges. There was disharmonic osseous maturation with small carpal centers and short thumbs. I. and L. Hand from a 12-year-old girl with MOPD II and biallelic loss-of-function mutations in the centrosomal pericentrin gene (PCNT) showing similar radiographic features resulting in disharmonic osseous maturation (Figures courtesy of Anita Rauch with permission from Science).
Chromosomal microarray revealed a 12.48 Mb deletion at 1q24.3q25.2, which included deletion of AT3. Thrombophilia evaluation revealed low antithrombin 3 activity (52%, normal 80–130%), heterozygosity for MTHFR C677T, and negative for Prothrombin mutation G20210A or Factor V Leiden, with normal Protein C activity, and Proteins S activity.
Patient 2
This patient was born to a G3P3 20-year-old mother and her 22-year-old partner with no known consanguinity. A maternal half-brother died in the neonatal period. Pregnancy was complicated by exposure to cocaine and heroin, and the baby required treatment after birth for opioid withdrawal. The patient was delivered vaginally at 38 weeks with a weight 2600 g (−1.6 SD), length 45 cm (−2.8 SD) and OFC 31 cm (−2.4 SD). Neonatal exam revealed postaxial polydactyly of the right foot, which was surgically removed.
Physical exam at age 5 and 6/12 years revealed height 101 cm (−2.6 SD) and OFC 46.7 cm (−3.0 SD). Distinctive findings included: single transverse palmar flexion crease on the right, short and broad fingers and thumb bilaterally, bilateral clinodactyly of the fifth digits, and broad feet, with a scar from removal of her right postaxial polydactyly. Facial findings included upslanted palpebral fissures, periorbital fullness, persistent epicanthal folds, short nasal tip and long philtrum (Fig 1D, Fig 2D). Ears were small with hypoplastic, attached lobules and overfolded superior helices (Fig 2D). She had mild, generalized cutis marmorata, as well as generalized keratosis pilaris. Although the patient was hypotonic, tendon reflexes were brisk and symmetric bilaterally. She had severe speech delay at age 5 and 6/12 years, with only a few single words.
A skeletal survey at 11 months showed delayed carpal bone age with 5th finger clinodactyly. The right foot showed right postaxial polydactyly with a thickened/fused 5th metatarsal. Hips showed coxa valga, legs showed slight delay of epiphyseal ossification, and skull X-ray revealed an Inca bone (a large Wormian bone formed at the junction of sagittal and lambdoid suture, a normal variant) in the occipital region. Brain MRI at 11 months was normal. Spine MRI revealed mildly flattened posterior medulla and upper cord, mild reduction in 4th ventricle size with sharply angled roof, and moderate cerebellar tonsilar herniation, which was progressive (downward herniation measuring 7mm, 12 mm, and 20–22 mm at 11 mo, 18 mo and 5 yrs). At age 5 years, MRA evaluation revealed a left posterior cerebral artery coming off the internal carotid, consistent with a persistent trigeminal fetal artery on the left, as well an absent left vertebral artery. Oligo aCGH revealed an 8.77 Mb deletion of 1q23.3-q25.1, which included AT3.
Patient 3
This patient was born to a 36-year-old G3P3 mother via emergency cesarean at 36 weeks with Apgar scores of 1, 3, and 7 at 1, 5, and 10 minutes. He had prenatal-onset growth deficiency that included length, weight (1600 g, −4 .0 SD) and head circumference (28 cm, −2.3 SD), and he remained in the neonatal intensive care unit for 4 weeks because of necrotizing enterocolitis and failure to thrive, which required gastrostomy tube feedings for his first 3.5 years of life. Shortly after birth, he was evaluated by genetics for cleft lip and palate, ventriculo-septal defect, cryptorchidism, and microcephaly, and a karyotype was interpreted as normal, 46,XY, with normal FISH for 22q11.2 deletion. At age 1 and 3/12 years, the patient's bone age was markedly delayed to age 6 months, and he had low IGFBP-3. At 2 and 9/12 years, his bone age was 1and 3/12 years. A brain MRI at 1 and 3/12 years showed enlarged sulci, thin corpus callosum and small frontal lobes.
At age 4 years he had developmental delay, dysmorphic features and persistent growth deficiency. He had recurrent otitis media requiring bilateral myringotomy and insertion of ventilation tubes, with sensorineural hearing loss and inconsistent responses to hearing tests. He had significant astigmatism but was non-compliant with wearing glasses. He had Meckel’s diverticulum leading to bowel obstruction and gastroesophageal reflux. At age 11 years, he ate mostly soft foods by mouth and was still on growth hormone with a persistently delayed bone age of 8 years. Long-term treatment with growth hormone had no impact on his short stature, with height over 6 SD below mean for age at age 11 years.
Language was severely delayed at age 11 and 4/12 years, , with only babbling and no words or signs. His receptive language skills were much better than his expressive skills. His academic skills remained at the kindergarten or pre-kindergarten level through age 10 years and he used a Picture Exchange Communication System (PECS) in his school program for children with autism, where he received physical, occupational and speech therapy, as well as bladder and bowel training. A brain MRI was interpreted as showing decreased myelination. A genetic evaluation at age 11 and 4/12 years, revealed height 96.7 cm (−6.8 SD), weight 15 kg (−7.0 SD), and head circumference 46.4 cm (−5.0 SD). He had microbrachycephaly, fullness of his upper eyelids, inverse epicanthal folds, broad nasal bridge, bulbous nasal tip, and a distorted columella from his cleft lip repair (Figure 1G, Figure 2G). His ears were small, with deficient superior crura, hypoplastic ear lobes and over-folding of the superior helices, which made them appear low-set and posteriorly angulated (Figure 3D). He also had bilateral preauricular pits, a short holosystolic murmur at the upper left sternal border, limited elbow extension and supination suggestive of radio-ulnar synostosis, distal brachydactyly of fingers and toes, proximally placed thumbs with hypoplastic distal phalanges, partial 2–3 toe syndactyly, and brisk deep tendon reflexes with slightly delayed relaxation. Array CGH revealed a 26.7 Mb deletion of 1q24.1q31.1, which included AT3.
Patient 4
This patient was born to a 40-year-old primagravida mother and her 40-year-old partner following an uncomplicated pregnancy with normal ultrasounds. The patient was delivered by caesarean for breech presentation weighing 2495 g (−1.5 SD) and measuring 49.53 cm (−1.2 SD) in length. Her neonatal course was complicated by respiratory distress. The patient had feeding difficulties, taking no more than 3–4 oz in one sitting. At 9 months of age, she was evaluated for poor growth of length, weight and head circumference (61.6 cm - 4.3 SD, 5273 g −5.2 SD, and 38.5 cm −4.0 SD respectively). There were no gastrointestinal problems or other significant medical problems.
When evaluated at 1 and 5/12 yearsof age, her length was 66 cm (−5.0 SD), head circumference was 42 cm (−2.9 SD), and she was developmentally delayed. Physical exam (Fig 1A, Fig 2A) revealed fullness of the upper eyelids, bulbous nasal tip, nevus flammeus on the back of the neck, mild pectus excavatum, prominent umbilicus, and a blind sacral dimple. She had flexible fingers with bilateral clinodactyly. At 1 and 5/12 years, bone age was delayed by 2.5 SD, and karyotype was 46,XX, with normal subtelomere FISH studies. Hand X-ray at 1 and 10/12 years showed small 2nd and 5th middle phalanges, normal distal phalanges, normal thumbs, markedly delayed carpal and epiphyseal ossification. Hip radiographs at 3 and 3/12 years showed slight coxa valga and slightly delayed femoral epiphyses.
At 3 and 3/12 years, she was proportionately short, with height 79.9 cm (−4.8 SD), weight 19.1 kg (−2.0 SD), and head circumference of 44 cm (−3.4 SD). Her inner canthal distance was 2.5 cm (25th centile), outer canthal distance 6.5 cm (3rd centile), interpupillary distance was 4.4 cm (10th centile), and there was a pointed configuration of the superior helix of the left ear. Her inter-nipple distance was 10.5 cm, chest circumference was 44.5 cm, and the ratio of these measurements was 0.235 (50th centile). Her palm creases were normal with fifth finger clinodactyly on the left. H19 methylation studies were normal, and she had bi-parental inheritance of chromosome 7. Array CGH revealed a 6.91 Mb deletion of 1q24.2q24.3, which did not include AT3.
Patient 5
This patient was born to a 26-year-old primigravida and 27-year-old father. Labor was induced at 38 weeks due to poor fetal growth. He was delivered vaginally with birth weight 2835 g (−1.1 SD), length 44.5 cm (−2.9 SD), and head circumference 32.5 cm (−1.5 SD). Newborn examination was remarkable for a cleft of the hard and soft palate, low-set posteriorly angulated ears, and bilateral single palmar creases. Renal and brain ultrasounds were normal. Echocardiogram identified a small patent ductus arteriosus and small patent foramen ovale with left to right shunting. He had a normal karyotype, 46,XY. Family history was non-contributory.
He was referred to genetics at age 2 years. His weight, height, and OFC were 10.1 kg (−1.9 SD), 78.5 cm (−2.5 SD), and 45 cm (−1.9 SD) respectively. Total hand length, middle finger length, and total foot length were 9.2 cm, 3.4 cm, and 11 cm respectively, each less than 3rd centile for age. Facial findings included prominent forehead, bilateral epicanthal folds, left esotropia, wide nasal bridge with bulbous nasal tip, low-set posteriorly angulated ears, overfolded anterior superior helices, pointed posterior superior helix, tented upper lip, down-turned corners of the mouth, everted lower lip, and micrognathia (Fig 1C, Fig 2 C, Fig 3B). Other findings included: brachydactyly without segmental shortening, fifth finger clinodactyly, short wide thumbs, hypermobile digits, wide feet, umbilical hernia, and diffuse hypotonia.
Developmental history was significant for speech delay, delayed tooth eruption, fine motor delays, and lack of purposeful play. He sat unassisted at 6 months, crawled at 10 months, and walked at 1 and 2/12 years. His behavioral phenotype included head banging, hand waving when excited, frequent laughter, and mouthing of objects. He had significant feeding difficulties and only ate pureed foods, and upper GI, barium swallow study, and intestinal biopsies were all normal. He experienced frequent ear and upper respiratory tract infections, and he took longer to recover from illnesses than other children. A hand x-ray at 2 and 6/12 years showed short middle and distal phalanges with no significant metacarpal or proximal phalangeal shortening, and normal thumbs (Fig 4B and E). There was severe delay in his bone age with disharmonic osseous maturation. Growth hormone stimulation testing was normal, as were serum IGF-1 and TSH. Skeletal survey at 2 and 5/12 years showed rounded iliac wings and coxa valga. Brain MRI at 2 and 2/12 years was normal.
At 3 and 10/12 years, of age, the patient’s weight, height, and OFC were 12.7 kg (−1.7 SD), 89.4 cm (−2.5 SD), and 46.4 cm (−2.5 SD), respectively. The patient continued to have feeding difficulties and could not eat solid foods. He had no speech, did not comprehend simple commands, and did not point to objects. He exhibited hyperactivity and inattention, as well as self-hitting. Auditory evoked responses were normal. Oligonucleotide-based microarray detected a 7.3 Mb deletion of 1q24.1q25.1, resulting in a deletion of AT3 as well as MYOC. A thrombophilia workup revealed only decreased antithrombin 3 activity (60%, normal 93–153%). The patient is followed by ophthalmology for hypertropia and glaucoma because mutations in MYOC cause autosomal dominant juvenile-onset open angle glaucoma. No signs of glaucoma have been noted in early childhood.
Patient 6
This male was born to a 29-year-old G2P2 mother after a pregnancy complicated by maternal hypertension. He was delivered at term by cesarean due to breech presentation. Birth weight was 2550 grams (−1.7 SD), birth length 43.5 cm (−3.5 SD), and OFC 31.5 cm (−2.3 SD). He had bilateral single transverse palmar flexion creases, bilateral clinodactyly, mild micrognathia, high arched palate and low set ears. After delivery he was hospitalized for suspected seizure activity and treated with phenobarbital. An EEG was inconclusive, and a head CT showed some hypodensity in both hemispheres. A repeat EEG done at 8 days of life was within normal limits. He had delayed developmental milestones and began ambulating with a spastic gait. A brain MRI at age 9 months was read as showing mild delay in myelination, consistent with age 6–7 months. At 1 and 7/12 yearsof age, the patient was evaluated for growth delay, and hand radiograph showed mildly delayed bone age of 1 and 3/12 years.
When evaluated for developmental delay at age 6 and 4/12 years, his motor and cognitive functioning were at the level of a 4–5 year old with associated delays in language and social skills. Ophthalmologic exam revealed anisocoria, slight myopia and mildly hypoplastic optic nerves. At age 7 years, cortisol, T4, TSH, IGF-1, and IGF-BP3 were normal. Hand X-ray at 7 years showed short 2nd and 5th middle phalanges with 5th finger clinodactyly. His metacarpals and distal phalanges did not show significant shortening. The 2nd middle phalanx showed a coned epiphysis, and his thumbs were normal. Bone age was severely delayed to 4 and 9/12 years (Fig 4C and F). Brain MRI showed mild hippocampal and parahippocampal atrophy, and MRI of the spine at age 7 showed syringohydromelia in the lower cervical spine (C6-C7) measuring 1.2 cm, with mild prominence of the central canal but no narrowing of the bony spinal canal. Orthopedic exam revealed equinovarus feet with significant hamstring tightness. At 7 and 10/12 years, this patient had bilateral lengthening of the medial hamstring muscles with transfer of the rectus femoris muscle, gastrocsoleus resection and tibial rotation osteotomies.
At age 8 and 7/12 years, the patient (Fig 1E, Fig 2E, 3C) remained severely growth deficient with height (108 cm, −4.0 SD), weight (18.9 kg, −2.9 SD) and head circumference (47.7 cm, −3.7 SD). The patient had anisocoria with both pupils reactive. Inner canthal distance was 2.5 cm (10th centile), outer canthal distance 7.8 cm (25th centile), and interpupillary distance was 5.5 cm (75th centile). His lips were thin, philtral length was 1.4 cm (25th centile) and nasal length was 3.5 cm (<3rd centile). His teeth were small and crowded, and he had only lost 2 of his primary teeth. His palate was intact and genitalia were normal. Hands were small with bilateral single transverse palmar flexion creases and bilateral clinodactyly of the 5th digits. The length of the right hand was 11.5 cm and left hand 11.2 cm (both ≪ 3rd centile). The width of each palm was 6.4 cm (25th centile), length of the right middle digit was 5.1 cm, and length of the left middle digit was 4.9 cm (both <3rd centile). There was increased space between the first and second toes bilaterally. On the left foot, there was partial syndactyly of the 2nd and 3rd digits. Foot length was 15.4 cm bilaterally (≪3rd centile). The patient had joint laxity and a spastic gait. He could answer in sentences and follow directions, read at the beginning level and do simple math. Parents noted that he had a short attention span but no real behavioral problems. Oligo-array CGH revealed a 9.81 Mb deletion of 1q24.3q25.3 that included AT3. A thrombophilia evaluation revealed decreased antithrombin 3 activity (53%, normal 89–123%), MTHFR A1298C heterozygosity, and normal Prothrombin G20210A, Factor V Leiden, Protein C activity, and Proteins S activity.
Patient 7
This patient was born to a 26-year-old primagravida woman and her 28-year-old partner after an uncomplicated pregnancy, labor and delivery. She was born vaginally at term and was small for gestational age with weight 2438 g (−2.1 SD), length 45.7 cm (−2.2 SD) and head circumference 31.1 cm (−2.3 SD). During an evaluation for short stature at age 2 years, she was noted to have short fingers, decreased tone and delayed dentition. Lymphocyte and skin fibroblast karyotypes were normal, and thyroid panel, urine organic acids, serum amino acids, and pediatric ophthalmologic evaluation were all normal. A genetic skeletal survey noted shortened metacarpals, with no signs of a specific skeletal dysplasia. Around 2 years of age she began experiencing febrile seizures, although EEG was normal. At age 6–7 years she developed bruxism and began pulling out her scalp hair, and these behaviors seemed to decrease on valproic acid. A repeat EEG at age 9 years was normal, so anticonvulsants were discontinued. She walked on her toes and developed hand-wringing and repetitive speech. Her gait abnormality was treated initially with casting to stretch her heel cords, followed by AFOs. Psychiatry thought her repetitive behaviors were due to autism. She was treated with growth hormone from age 8 to 9 years without any demonstrable benefit, so it was discontinued. Hand X-ray at age 12 years showed shortening of the 3rd, 4th and 5th metacarpals with milder shortening of the 2nd metacarpal (Figure 4H and K). Proximal phalanges appeared normal, but there was shortening of the 2nd, 4th and 5th middle phalanges and shortening of the 2nd, 3rd, 4th and 5th distal phalanges. There was disharmonic osseous maturation with small carpal centers and short thumbs. Feet revealed shortening of the 2nd, 3rd and 4th metatarsals, which resulted in hallux valgus.
Menses started at age 16 but became progressively irregular. The patient had increased appetite resulting in her being overweight for height. She had frequent urinary tract infections and passed a kidney stone at age 17. A pelvic ultrasound revealed clinically insignificant calcification in the superior pole of her kidney. Persistent abdominal pain and bladder infections were treated with oral antibiotics. She had intermittent rashes that seemed to improve following treatment with oral antibiotics. Seizure activity recurred at age 17, and EEG revealed frontal lobe seizures that lasted about 10 minutes a day, about four times a week. Immunologic evaluation for swollen, painful joints, revealed normal CBC, normal sedimentation rate and CRP, and normal levels of IgE, IgM, and IgG, with low IgA levels at 21 (normal 82–453 mg/dL). Though she demonstrated antibodies for Influenza B, lack of tetanus toxoid antibodies suggested an underlying immunodeficiency, and she was revaccinated. Thyroid evaluation was normal, as was her brain MRI.
At age 18 years, her height was 141 cm (−4.7 SD), with weight 46.4 kg (−3.0 SD), and head circumference 49 cm (−4.3 SD). She had distinctive dysmorphic features (Figure 1H, Figure 2H, Figure 3E, Figure 4H and K) with proportionate short stature and small hands and feet with brachydactyly due to short middle phalanges of digits 2–5 (middle finger length 4 cm at 18 years). She had microbrachycephaly, short palpebral fissures, broad nasal tip, small ears (5 cm at 18 years), incompletely folded helices, attached earlobes, downturned mouth, supernumerary teeth, short, wide neck, thin, sparse hair, seizures, severe intellectual disability, autism, and associated behavioral issues. Oligo array CGH revealed a 12.9 Mb deletion at 1q24.3q25.3 that included the AT3 gene. A thrombophilia evaluation revealed decreased antithrombin 3 activity (69%, normal 80–120%), heterozygous MTHFR C677T nucleotide change, and negative for Prothrombin mutation G20210A or Factor V Leiden, with normal Protein C activity and Proteins S activity.
Patient 8
The patient was born to a G2P2 26-year-old mother and her 31-year-old partner with no known consanguinity. The mother noted decreased fetal movements, but no ultrasounds were performed. He was born at term and was small for gestational age with weight 1673 g (−3.7 SD), length 35.56 cm (−7.6 SD), microcephaly and unilateral cleft lip and cleft palate. He was significantly delayed in all developmental milestones, most notably speech. Family history was non-contributory except for cleft lip and cleft palate on the paternal side.
At age 17 years, the patient was evaluated for severe developmental disabilities and short stature, with weight 26.3 kg (−9.0 SD), height 131 cm (−5.6 SD) and head circumference 47 cm (−5.8 SD). Physical exam revealed microcephaly and multiple dysmorphic facial features: ocular hypertelorism, upslanting palpebral fissures, small, low-set, posteriorly-angulated ears with hypoplastic earlobes, nasal deformity secondary to a repaired cleft lip and palate, and midface deficiency with a pointed chin (Fig 1I, Fig 2I and Fig 3F). Additional findings included hypoplastic genitalia, pectus excavatum, severe scoliosis which necessitated rod placement, bilateral brachydactyly with single transverse palmar flexion creases, and flexed wrists at rest. Hand X-ray at age 24 years showed shortening of the 2nd and 5th middle phalanges with normal proximal and distal phalanges and normal metacarpals. The distal phalanx of the thumb was also short. His gait was ataxic, and he had stereotypical, repetitive movements. He had severe cognitive disability and was nonverbal with inability use signs effectively, but he had a happy demeanor with occasional outbursts of laughter. He had a history of chronic constipation and reflux. Standard karyotype was interpreted as normal, but oligo-array CGH revealed the patient had a 5.65 Mb deletion at 1q24.2q25.2 that included the AT3 gene. A thrombophilia evaluation revealed decreased antithrombin 3 activity (41%, normal 80–130%) and normal Prothrombin G20210A, Factor V Leiden, Protein C activity, and Proteins S activity.
Patient 9
At 34 weeks gestation, prenatal ultrasonography in a 31 year old G2P1 woman revealed suspected schizencephaly with cleft lip and palate. Paternal age was 31, and family history was negative with no consanguinity or exposure to known teratogens. Amniocentesis results were normal 46,XY. After spontaneous onset of labor at 38 weeks, delivery was by urgent Cesarean section due to fetal distress, and Apgar scores were 7 and 9 at one and five minutes. Examination revealed proportionate growth deficiency with birth length of 39.5 cm (−5.6 SD), weight 2025 gm (−3.0 SD), and head circumference 32 cm (−2.1 SD). He had small ears with hypoplastic ear lobes, bilateral cleft lip and palate with a floating premaxilla, downturned corners of the mouth, micrognathia, loose nuchal skin, diastasis recti, right-sided cryptorchidism, inguinal hernia, short limbs, small hands and feet, 5th finger clinodactyly, wide gap between first and second toes, and hypotonia.
Radiographs revealed short tubular bones with normal metaphyses; there were 11 pairs of ribs. Brain MRIs at birth and age 1 month revealed stable marked left ventriculomegaly, with a thin mantle of cortex and subcortical white matter peripheral to the enlarged left ventricle in the left frontal and parietal regions, which was thought to be an area of porencephaly. There was a subtle zone of diminished T2 intensity in the inferior left lateral ventricle, possibly due to hemosiderin or calcium deposition. A neurogenetics consultant thought these brain abnormalities were consistent with a destructive fetal cerebrovascular accident. His cleft lip repair took place at one year of age, and his cleft palate was repaired at age 2 years. Myringotomies have been repeatedly performed in both ears.
At 5 and 1/12 years, a Peabody Developmental Motor Scales yielded a total motor quotient of 44. At 80 months he was crawling, but not walking, with no expressive language, limited receptive skills, and severe hypotonia with right-sided hemiparesis. He has severe growth deficiency despite being fed through a gastrostomy tube. At 3 and 9/12 years bone age was 1 and 6/12 to 2 years. On endocrine evaluation, somatomedin, prolactin, cortisol and thyroid levels were normal. At age 8 and 8/12 years (Fig 1F, Fig 2F), his height was 99.1 cm (−5.4 SD), weight was 15 kg (−3.5 SD) with head circumference 48.3 cm (−3.6 SD), and his bone age was markedly delayed to 5–6 years (−3.6 SD; Fig 4G and J). He continued to manifest severe cognitive and language deficits.
A high-resolution lymphocyte karyotype suggested a 1q23q31 deletion, and SNP array confirmed a 22.3 Mb 1q24.3q31.3 deletion that included AT3. At 64 months of age, serum level of anti-thrombin III was 59% (normal range 80–120%), with antithrombin III antigen level 16 (normal range 19–30), so he was started on daily low dose aspirin to decrease the risk for thomboembolic events. Full thrombophila evaluation at age 8 years, 8 months revealed normal factor V Leiden, normal prothrombin 20210, normal protein C and S activities, and confirmed decreased antithrombin III activity (67% versus normal 83–122%).
MATERIALS AND METHODS
Microarray analysis
Samples from Patients 1–6 and 8 were analyzed using a 105K–feature oligonucleotide whole-genome array (SignatureChip® OS version 1.1, custom designed by Signature Genomics, manufactured by Agilent Technologies, Santa Clara, CA), according to previously described methods [Ballif et al., 2008]. The sample from patient 7 and DNA from a previously reported individual with a 1q23q25 deletion [Franco et al., 1991] was analyzed using a 135K–feature oligonucleotide whole-genome microarray (SignatureChip OS version 2.0, custom designed by Signature Genomics, manufactured by Roche Nimblegen, Madison, WI), according to previously described methods [Duker et al., 2010]. The sample from patient 9 was analyzed using a 250K–feature SNP whole-genome microarray (Affymetrix, Santa Clara, CA), according to manufacturer’s instructions.
Fluorescent in situ hybridization (FISH)
Metaphase FISH studies were performed on samples from all patients using BAC probes from the regions determined to have copy number changes by aCGH as previously described [Traylor et al., 2009]. When available, samples from the patients’ parents were also studied by metaphase FISH.
RESULTS
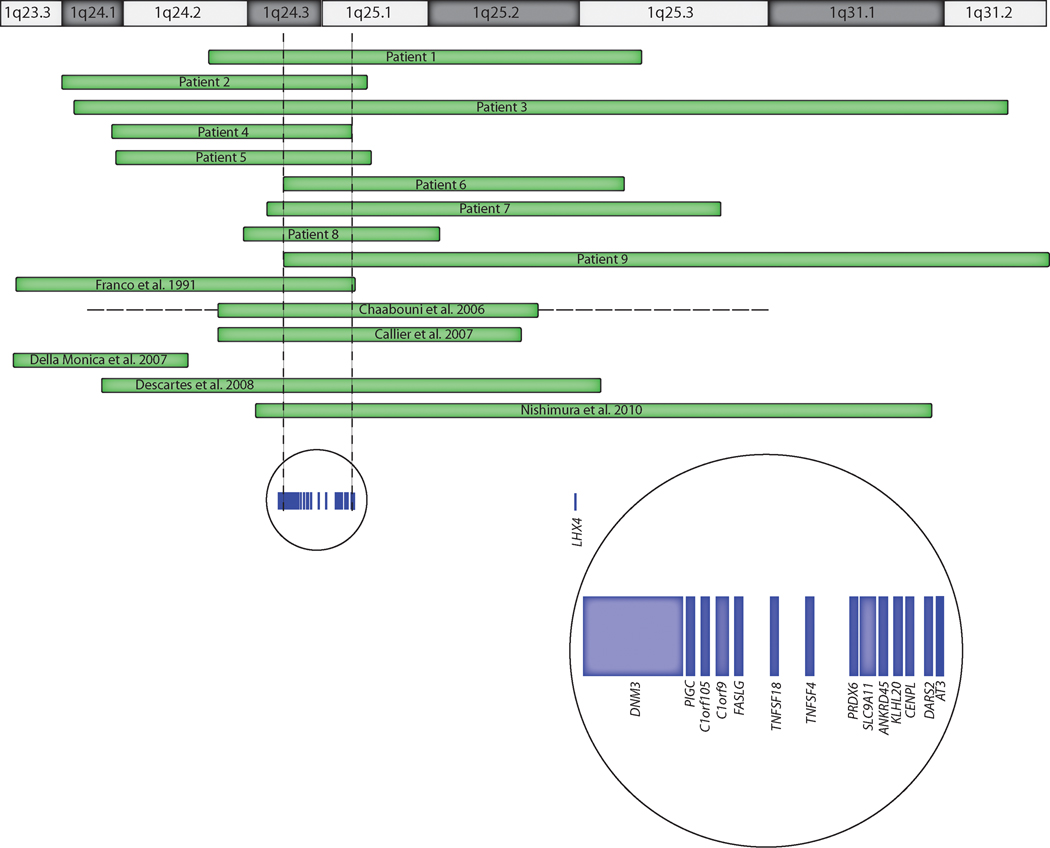
We identified nine individuals with overlapping deletions within 1q24q31, varying in size from 5.65 Mb to 26.77 Mb (Table III, Fig 6). The smallest region of overlap (SRO) of the nine deletions was 1.9 Mb at 1q24.3q25.1 (chr1: 170135865–172099327, hg18 coordinates). No other significant copy number changes were detected in any these individuals. Oligo-aCGH on DNA from a previously described individual [Franco et al., 1991] more precisely defined the deletion to be an 11.4 Mb 1q23.3q25.1 deletion (chr1:161,447,293–172,806,135, hg18 coordinates). Metaphase FISH confirmed the deletion in all individuals studied. Parental FISH studies were performed for Patients 1 and 4, confirming apparent de novo deletions. Additionally, the mother of Patient 7 did not carry the deletion nor rearrangement of the region deleted in her daughter, but her father was not studied.
Table III.
Summary of results of oligo-aCGH or SNP array
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Breakpoints | q24.2 q25.3 |
q23.3 q25.1 |
q24.1 q31.2 |
q24.1 q25.1 |
q24.1 q25.1 |
q24.3 q25.3 |
q24.3 q25.3 |
q24.2 q25.2 |
q24.3 q31.3 |
| Coordinates of minimal deletion (hg18 UCSC March 2006 build) |
chr1: 167,948,097– 180,429,484 |
chr1: 163,760,542– 172,532,315 |
chr1: 164,116,498– 190,885,553 |
chr1: 165,188,238– 172,099,327 |
chr1: 165,344,243– 172,662,904 |
chr1: 170,099,984– 179,914,925 |
chr1: 169,425,149– 182,293,176 |
chr1: 168,991,860– 174,645,488 |
chr1: 170,135,865– 192,469,924 |
| Size | 12.48 Mb | 8.77 Mb | 26.77 Mb | 6.91 Mb | 7.32 Mb | 9.81 Mb | 12.87 Mb | 5.65 Mb | 22.33 Mb |
Figure 6.
Schematic showing deletions in individuals with molecularly defined deletions in the 1q24q25 region and genes in the common region of overlap. Green bars represent minimum deletion sizes, and horizontal dashed lines extend through gaps in coverage to show maximum deletion sizes.
DISCUSSION
The nine cases presented here plus five previously reported cases characterized by aCGH [Franco et al., 1991; Chaabouni et al., 2006; Callier et al., 2007; Descartes et al., 2008; Nishimura et al., 2010] provide a clear picture of the phenotype associated with 1q24q25 deletion and help to clarify the involvement of specific genetic contributions to this phenotype (Fig 6, Table III). These patients presented with prenatal onset proportionate short stature, microcephaly, brachydactyly with clinodactyly of the 5th digits. Distinctive dysmorphic facial features include: fullness of the upper eyelids, broad nasal bridge, short nose with bulbous tip and flared nares, tented upper lip with downturned corners of the mouth, micrognathia, small ears with hypoplastic superior (posterior) crus of the antihelix and hypoplastic ear lobes (Figs 1–3). Regarding their neurobehavioral phenotype, older patients had communicative problems with severe delay in expressive language in comparison with receptive language skills. Some had inability to speak or use signs necessitating a combined approach toward using pictures to communicate. Parents also reported stereotypic movements suggestive of autistic spectrum disorder (ASD), and many patients were receiving therapy for ASD.
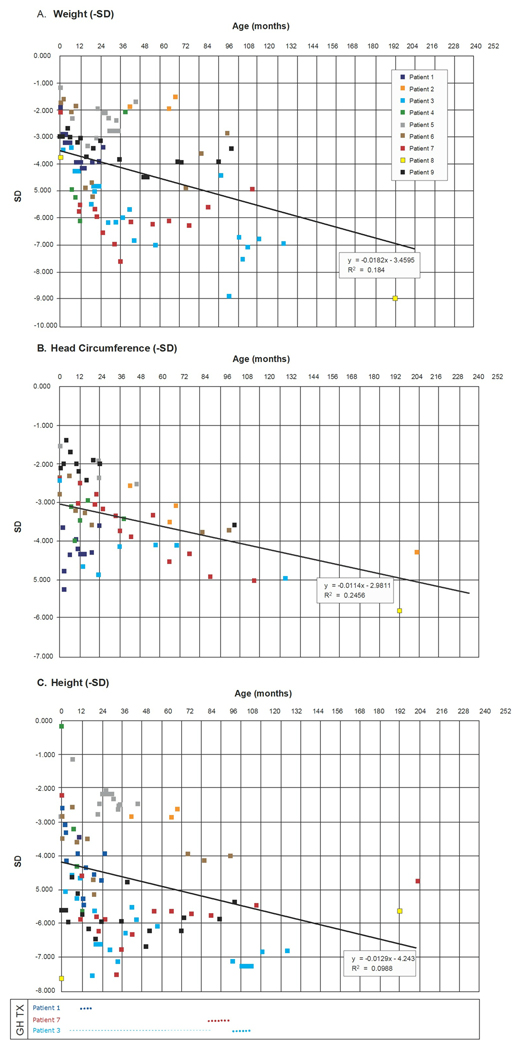
The growth deficiency seen in patients with deletions of the 1q24q25 region is distinctive in that it arises prenatally with postnatal persistence. A previously reported fetal case by Chaabouni et al. [2006] demonstrated intrauterine linear growth restriction that was evident at 28 weeks gestational age. In most cases, the birth length was at or less than 3rd centile, and the patients failed to show catch-up growth after birth, even after treatment with growth hormone (Fig 5). Some patients had feeding problems severe enough to necessitate a gastrostomy tube, while others had milder problems with sucking and swallowing that only required nasogastric tube feedings during the neonatal period. Some older patients had persistent problems with solid foods and chronic constipation. Stature and head circumference usually fall from −3 SD below mean at birth to −5 SD below mean for age at age 10 years despite treatment with growth hormone (Fig 5). No patient remained underweight for length, and their proportionate short stature and microcephaly tended to fall away from the normal growth curves during childhood.
Figure 5.
These figures compare standard deviations (SD) below mean for age for weight (A) head circumference (B) and height (C) to demonstrate the characteristic growth deficiency for height and head circumference for these 9 patients with 1q24q25 deletion syndrome. Stature and head circumference usually fall from −3 SD below mean at birth to −5 SD below mean for age by age 10 years despite treatment with growth hormone (as shown for patients 1, 3, and 7 with dotted lines to indicate timing and length of treatment). The black line shows a significant downward trend in the linear regression for growth measurements with advancing age. No patient remained underweight for length, and their proportionate short stature and microcephaly tended to fall away from the normal growth curves during childhood.
Occasional features in our nine patients include cleft lip and/or palate, postaxial polydactyly, acquired tonsilar herniation, seizures, hernias, and scoliosis. Other patients with cleft lip and/or cleft palate/bifid uvula were mentioned among previously reported cases with deletions that overlapped the area of interest [de Pablo et al., 1980; Schinzel and Schmid, 1980; Taysi et al., 1982; Silengo et al., 1984; Zaletaev et al., 1987; Lo et al., 1993; Okamoto et al., 2005]. In these cases, cleft lip was accompanied by cleft palate in five cases [de Pablo et al., 1980; Schinzel and Schmid, 1980; Taysi et al., 1982, Patient 2; Silengo et al., 1984; Lo et al., 1993]. Strabismus was reported in several cases [Beemer et al., 1985; Franco et al., 1991], and other features in previously reported cases are tabulated in Table I.
Table I.
Common reported findings in patients with del(1)(q21/22-q25)
| Characteristic | Previously reported cases with cytogenetically visible deletionsa |
Individuals with molecularly confirmed deletion of 1q24q25 SROb |
|---|---|---|
| Birth Weight (≤2.5 SD) |
10/11 | 9/13 |
| Birth Length (≤2.5 SD) |
7/11 | 10/12 |
| Birth OFC (≤2.5 SD) |
5/8 | 5/8 |
| Proportional growth deficiency |
10/11 | 14/14 |
| Delayed bone age | 4/10 | 13/13 |
| Microbrachycephaly | 11/11 | 12/12 |
| Sparse/fine hair | 9/11 | 7/7 |
| Facial Features | ||
| Fullness of the upper eyelid |
8/8 | 9/9 |
| Broad nasal bridge | 7/7 | 9/9 |
| Bulbous nasal tip | 5/5 | 9/11 |
| Cleft palate alone bifid uvula |
3/11 | 2/13 |
| Cleft lip/palate | 7/11 | 4/13 |
| Micrognathia | 11/11 | 9/9 |
| Small ears with deficient superior crus and hypoplastic ear helices |
11/11 | 10/10 |
| Extremities | ||
| Small hands/feet | 11/11 | 12/12 |
| Hypoplastic/Dysplastic nails |
6/9 | 3/4 |
| Transverse palmar creases | 9/10 | 10/11 |
| 5th finger clinodactyly | 11/11 | 13/13 |
| Developmental disability | 11/11 | 13/13 |
| Speech Language Delay > motor development |
4/4 | 10/11 |
| Hearing Loss | 2/2 | 4/12 |
| Stereotypic Movements | 1/1 | 3/3 |
| Hypotonia | 9/10 | 7/10 |
| Seizures | 3/11 | 2/13 |
| CNS Anomalies | 7/9 Chiari I malformation 1/9 hypoplastic corpus callosum-2/9; Cortical thining-1/9 meningeomyelocele-1/9 spina bifida-1/9 dilated ventricles/ hydropcephalus-3/9 |
3/11 Chiari I malformation- 1/11 porencephaly-1/11 syringohydromelia- 1/11 |
| Ophthalmologic | 5/11 aniscoria-1/11 strabismus-2/11 exophthalmos-1/11 glaucoma-1/11 microophthalmia-2/11 |
4/13 aniscoria-1/13 strabismus-3/13 hypoplastic optic nerves-1/13 |
| Occasional Features | ||
| Cardiac anomalies | 7/11 | 4/6 |
| Umbilical/Inguinal Hernia | 3/11 | 7/14 |
| Feeding Difficulties | 3/11 | 7/14 |
| Cryptorchidism | 5/5 Hypoplastic External Genitalia/Hypoplastic lower vagina- 2/6 |
6/9 Hypoplastic External Genitalia/Hypoplastic lower vagina- 2/5 |
| Renal Abnormalities | 6/11 | 0/12 |
| Skeletal Anomalies | Scoliosis −2/11 11 ribs 2/11 pectus carinatum 1/11 |
Scoliosis-2/14 11 ribs-2/14 pectus excavatum 2/14 Unequally short upper ribs 1/14 |
Patients reported by [de Pablo et al., 1980; Schinzel and Schmid, 1980; Taysi et al., 1982; Silengo et al., 1984; Beemer et al., 1985; Zaletaev et al., 1987; Lo et al., 1993; Takano et al., 1997; Pallotta et al., 2001; Okamoto et al., 2005]
Patients 1–9 in this report and those reported by [Franco et al., 1991; Chaabouni et al., 2006; Callier et al., 2007; Descartes et al., 2008; Nishimura et al., 2010]. The patient reported by Callier et al. [2007] also had a 2.8 Mb 12q24.31 deletion that could cause additional phenotypic features.
On clinical exam, the small hands and feet in this syndrome have a distinctive brachydactyly with clinodactyly of the fifth digits, single transverse palmar flexion creases, proximally placed thumbs, and small nails. Because of the severe growth deficiency, hand and wrist radiographs for bone age are commonly done, and these radiographs often demonstrate shortened middle phalanges with delayed and disharmonic bone age. Hands and feet remain strikingly small, with brachydactyly and severely delayed carpal ossification followed by less severely delayed epiphyseal ossification. The brachydactyly begins with shortening of the 2nd and 5th middle phalanges in early childhood, followed by shortening of other middle phalanges, followed by shortening of the lateral three metacarpals and distal phalanges by late childhood (Fig 4). There may be associated similar changes in the feet, but these changes are seen less consistently. The disharmonic delays in ossification usually affect carpal bones more severely than epiphyseal centers, and developmental delay and congenital hypotonia usually result in some degree of coxa valga in the hips. Although endocrine evaluations were normal, bone age was consistently delayed in most patients, usually 2–4 SDs below what was expected for their chronological ages.
Microbrachycephaly seems to be a common trait, seen in our cases and in previous reports [Schwanitz et al., 1977; de Pablo et al., 1980; Schinzel and Schmid, 1980; Martin and Simpson, 1982; Taysi et al., 1982; Silengo et al., 1984; Beemer et al., 1985; Zaletaev et al., 1987; Franco et al., 1991; Lo et al., 1993; Pallotta et al., 2001; Okamoto et al., 2005; Callier et al., 2007; Della Monica et al., 2007; Descartes et al., 2008; Nishimura et al., 2010]. Because these patients had microcephaly and severe developmental delay, a brain MRI was usually done, but there was no consistent brain imaging finding.
Patient 2 had progressive acquired cerebellar tonsilar herniation, as well as vascular variants detected by MRA: an absent left vertebral artery and a left posterior cerebral artery coming off the internal carotid—consistent with a persistent trigeminal fetal artery on the left. Acquired tonsilar herniation was also reported by Pallotta et al. [2001], but the deleted region in this patient was considerably larger (1q23q31.2). Patient 7 had a history of seizures with a normal MRI. Patient 6 was hospitalized after birth for suspected seizures, and a head CT showed hypodensity in both hemispheres around the temporal-occipital junction. An EEG was normal, and no other seizures have been noted. Patient 9 had brain abnormalities detected on prenatal ultrasound, and MRI after birth showed asymmetric ventriculomegaly with a dysplastic corpus callosum, thalamus and cerebellum as well decreased gyri on the left, which suggested a destructive fetal cerebrovascular event. A meningomyelocele was reported by Takano et al. [1997], and in Patient 6, brain MRI showed mild hippocampal and parahippocampal atrophy, and MRI of the spine showed syringohydromelia in the lower cervical spine (C6-C7), with mild prominence of the central canal but no narrowing of the bony spinal canal. Underdevelopment of the frontal and parietal lobes was reported by Lo et al. [1993], and Patient 3 had enlarged sulci, thin corpus callosum and small frontal lobes.
A destructive prenatal cerebrovascular event was suspected in patient 9, and Taysi et al. [1982] attributed seizures at 28 months in their patient to a previous stroke. Recently, Kibe et al. [2011] reported a patient who had a 1q24q25 microdeletion that included AT3, as well as a more complex deplication and deletion of 10q26 on aCGH, who had a sudden stroke at 35 months with severe growth deficiency, developmental delay, microcephaly, and brachydactyly. The gene encoding antithrombin III (SERPINC1 or AT3, 1q25.1) is deleted in most of our patients, and six of our patients showed decreased activity of antithrombin III. This decreased activity must be kept in mind when these patients have to undergo surgical procedures and should be considered in the pre and post-operative work up.
There are 13 genes in the 1.9 Mb SRO (Fig 6) at 1q24.3q25.1 common to all our nine patients as well as the case reports published by Franco et al. [1991], Chaabouni et al. [2006], Callier et al. [2007], Descartes et al. [2008], and Nishimura et al. [2010]. These genes and their functions are summarized in Table II. Of the genes in the deleted SRO, two major candidate genes may play a role in the primordial short stature, microcephaly, and neurodevelopmental impairments, CENPL and DNM3. A more proximal 1q23.3q24.2 deletion in a patient without severe growth deficiency was reported by Della Monica et al. [2007], as shown in Figure 6.
Table II.
Genes in the 1q24.3q25.1 SRO
| Gene | OMIM | Protein, reported mutations, and function, if known |
|---|---|---|
| DNM3 | 611445 | DYNAMIN 3 - involved in actin-membrane processes, interacts with Homer-Shank scaffold complex in post- synaptic hippocampal neurons [Lu et al., 2007] |
| PIGC | 601730 | PHOSPHATIDYLINOSITOL GLYCAN, CLASS C – part of a complex with GPI-GlcNAc transferase (GPI-GnT) activity |
| C1orf105 | Chromosome 1 open reading frame 105 (C1orf105) | |
| C1orf9 | Chromosome 1 open reading frame 9 (C1orf9) | |
| FASLG/TNFSF6 | 134638 | TUMOR NECROSIS FACTOR LIGAND SUPERFAMILY, MEMBER 6 – heterozygous mutation causes autoimmune lymphoproliferative syndrome [Wu et al., 1996] |
| TNFSF18 | 603898 | TUMOR NECROSIS FACTOR LIGAND SUPERFAMILY, MEMBER 18 |
| TNFSF4 | 603594 | TUMOR NECROSIS FACTOR LIGAND SUPERFAMILY, MEMBER 4 |
| PRDX6 | 602316 | PEROXIREDOXIN 6 – hydrolyzes the fatty acyl or alkyl bonds of membrane phospholipids |
| SLC9A11 | Solute carrier family 9, member 11 (SLC9A11) | |
| ANKRD45 | Ankyrin repeat domain 45 (ANKRD45) | |
| KLHL20 | Kelch-like 20 (Drosophila) (KLHL20) | |
| CENPL | 611503 | CENTROMERIC PROTEIN L – a subunit of centromeric complex that targets CENPA to centromeres and is required for proper kinetochore function and mitotic progression |
| DARS2 | 610956 | ASPARTYL-tRNA SYNTHETASE 2 – autosomal recessive mutations cause leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation |
Primordial dwarfism is a rare, clinically heterogeneous condition characterized by severe prenatal and postnatal growth restriction. Two similar microcephalic primordial dwarfism conditions, Seckel syndrome and Majewski osteodysplastic primordial dwarfism type 2 (MOPD2), can be caused by recessive mutations in pericentrin (PCNT) [Griffith et al., 2008; Rauch et al., 2008]. Seckel syndrome can also be caused by recessive mutations in CENPJ [Al-Dosari et al., 2010]. PCNT is an integral component of the centrosome that organizes the mitotic spindle for segregation of chromosomes during mitosis, and CENPJ is involved in the regulation of cell cycle through its action on the centrosome and is essential for normal mitosis. Depletion of PCNT or CENPJ arrests cell growth and mitosis. Furthermore, other mutations in CENPJ and CEP152 with functions similar to PCNT are associated with primary microcephaly without primordial short stature [Guernsey et al., 2010]. Defects in this process decrease cellular divisions, which replenish the neuronal stem cells that are necessary for normal brain growth. CENPL is within the 1q24q25 SRO and encodes centromeric protein L, a subunit of the CENPA-CAD complex [Foltz et al., 2006], which targets the CENP-A chromatin domain to centromeres and is essential for proper kinetochore function and mitotic progression [Okada et al., 2006]. Thus deletion or mutation of genes encoding centrosomal proteins such as CENPL may explain the association between primordial growth deficiency and severe microcephaly in the 1q24q25 deletion syndrome. Furthermore, the radiographic hand changes in the 1q24q25 deletion syndrome are quite similar to those seen in MOPD2 (Fig 4, I and L).
Nishimura et al. [2010] described a case of 1q24.3q31.2 deletion with growth hormone (GH) deficiency and suggested that the severe growth deficiency and microcephaly in their case (length −8.5 SD and head circumference −5,5 SD at age 3 years) was due to deletion of LHX4 at 1q25.2. LHX4 is a homeobox gene important in pituitary development and GH secretion, and it is located distal to the commonly deleted region in our nine cases (Fig 6). Previously reported individuals with deletions including LHX4 had had GH deficiency [Callier et al., 2007; Descartes et al., 2008; Nishimura et al., 2010], but only one of three of our patients with LHX4 deletion and endocrine studies had GH deficiency. Furthermore, treatment with GH in our cohort did not result in catch-up growth, though the patient reported by Descartes et al. [2008] demonstrated partial GH deficiency and was treated with GH from age 5 to 8 years with height at the 10th centile for age at age 14 years. The degree of growth deficiency is slightly worse in our patients with deletions of LHX4 (Patients 1, 3, 6, 7 and 9; Fig. 5), but numbers were too small for statistical analysis to determine if there was an additive effect. However, deletion of LHX4 alone is insufficient to result in the growth deficiency characteristic of 1q24q25 deletion syndrome, as a 10-year-old male with a 16p11.2 deletion also had a maternally inherited 450 kb deletion of LHX4 that was interpreted as likely benign, and he had normal growth [Rosenfeld et al., 2010, Fig. 2J]. Therefore, CENPL remains the primary candidate for growth deficiency in this disorder.
A second candidate gene for some of the features seen in this syndrome is DNM3 (dynamin 3). DNM3 has mechanicochemical properties and is involved in actin-membrane processes, where it interacts with the Homer-Shank scaffold complex in post-synaptic hippocampal neurons [Lu et al., 2007]. Of the three vertebrate dynamins, only DNM3 is expressed in the brain, where it is the only known isoform to bind the post-synaptic Homer protein. The Shank-Homer complex promotes maturation of glutamatergic synapses and increases synaptic strength [Sala et al., 2001]. Disruption of dynamin-3 in rat hippocampal neurons uncouples the post-synaptic density scaffold from the endocytic zones, resulting in synapses lacking postsynaptic clathrin [Lu et al., 2007]. On another level, DNM3 is also a constituent of myelin and cell trafficking plays a crucial role in the process of myelin formation and the myelination of axons [Jahn et al., 2009]. Additionally, three microRNAs are present on an antisense transcript from a DNM3 intron, and mice lacking both copies of this gene (termed Dnm3os) exhibited skeletal and craniofacial anomalies [Loebel et al., 2005; Watanabe et al., 2008]. Thus, haploinsufficiency of DNM3 in the 1q24q25 microdeletion syndrome may contribute to delayed myelination and other neurodevelopmental impairments, and haploinsufficiency for the microRNAs within it may contribute to the skeletal and dysmorphic features. It is possible that other genes proximal to the SRO in our patients contribute to the severe neurodevelopmental phenotype since the patient reported by Della Monica and others [2007] had autism and cognitive disability but was not deleted for our SRO.
Submicroscopic deletions of the 1q24q25 region result in a clinically recognizable syndrome with prenatal proportionate growth deficiency with microbrachycephaly. There is associated severe growth deficiency, which does not respond to treatment with growth hormone, possibly because there is deletion of CENPL, which may disrupt the centrosome and interfere with cellular mitosis. In some cases there is deletion of LHX4 at 1q25.3, a homeobox gene that plays an important role in pituitary development and growth hormone secretion, which may further diminish growth. Deletion of DNM3 in the 1q24q25 microdeletion syndrome may contribute to the severe neurodevelopmental impairments in this disorder. Although there is some variation in phenotype, early recognition of this syndrome and proactive intervention can improve the quality of life and prevent possible complications from thrombophilia during procedures and surgeries. For patients with deletion of MYOC, surveillance for glaucoma may be indicated. Molecular cytogenetic techniques can confirm the suspected diagnosis, and this condition could be diagnosed prenatally in some cases of IUGR if appropriate studies are pursued.
ACKNOWLEDGMENTS
We appreciate support from SHARE's Childhood Disability Center, the Steven Spielberg Pediatric Research Center, the NIH/NICHD Program Project Grant (HD36657), the Medical Genetics NIH/NIGMS Training Program Grant (5-T32-GM08243), and the Cedars-Sinai General Clinical Research Center Grant (M01-RR00425) for samples collected under CSMC IRB Protocols 0463 and 4232. We appreciate the efforts of medical students Brennan Shutt, who assisted in assembling data regarding Patient 9, and Aaron Theisen, who created figure 6. We are also indebted to Dr. James R. Lupski of Baylor College of Medicine for allowing us to confirm the deletion interval in the patient he reported [Franco et al., 1991] with oligo-array CGH.
REFERENCES
- Al-Dosari MS, Shaheen R, Colak D, Alkuraya FS. Novel CENPJ mutation causes Seckel syndrome. J Med Genet. 2010;47:411–414. doi: 10.1136/jmg.2009.076646. [DOI] [PubMed] [Google Scholar]
- Ballif BC, Theisen A, McDonald-McGinn DM, Zackai EH, Hersh JH, Bejjani BA, Shaffer LG. Identification of a previously unrecognized microdeletion syndrome of 16q11.2q12.2. Clin Genet. 2008;74:469–475. doi: 10.1111/j.1399-0004.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- Beemer FA, Klep-de Pater JM, Sepers GJ, Janssen B. Two cases of interstitial deletion of the long arm of chromosome 1: del(1)(q21----q25) and del(1)(q41----q43) Clin Genet. 1985;27:515–519. doi: 10.1111/j.1399-0004.1985.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Callier P, Faivre L, Marle N, Thauvin-Robinet C, Mosca AL, Masurel-Paulet A, Borgnon J, Falcon-Eicher S, Danino A, Malka G, Le Merrer M, Huet F, Mugneret F. Untreated growth hormone deficiency with extremely short stature, bone dysplasia, cleft lip--palate and severe mental retardation in a 26-year-old man with a de novo unbalanced translocation t(1;12)(q24;q24) Eur J Med Genet. 2007;50:455–464. doi: 10.1016/j.ejmg.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Chaabouni M, Martinovic J, Sanlaville D, Attie-Bittach T, Caillat S, Turleau C, Vekemans M, Morichon N. Prenatal diagnosis and molecular characterization of an interstitial 1q24.2q25.2 deletion. Eur J Med Genet. 2006;49:487–493. doi: 10.1016/j.ejmg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- de Pablo CE, Garcia Sagredo JM, Ferro MT, Ferrando P, San Roman C. Interstitial deletion in the long arms of chromosome 1: 46,XY,del(1)(pter leads to q22∷q25 leads to qter) J Med Genet. 1980;17:483–486. doi: 10.1136/jmg.17.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Monica M, Lonardo F, Faravelli F, Pierluigi M, Luquetti DV, De Gregori M, Zuffardi O, Scarano G. A case of autism with an interstitial 1q deletion (1q23.3–24.2) and a de novo translocation of chromosomes 1q and 5q. Am J Med Genet A. 2007;143A:2733–2737. doi: 10.1002/ajmg.a.32006. [DOI] [PubMed] [Google Scholar]
- Descartes M, Hain JZ, Conklin M, Franklin J, Mikhail FM, Lachman RS, Nolet S, Messiaen LM. Molecular characterization of a patient with an interstitial 1q deletion [del(1)(q24.1q25.3)] and distinctive skeletal abnormalities. Am J Med Genet A. 2008;146A:2937–2943. doi: 10.1002/ajmg.a.32550. [DOI] [PubMed] [Google Scholar]
- Duker AL, Ballif BC, Bawle EV, Person RE, Mahadevan S, Alliman S, Thompson R, Traylor R, Bejjani BA, Shaffer LG, Rosenfeld JA, Lamb AN, Sahoo T. Paternally inherited microdeletion at 15q11.2 confirms a significant role for the SNORD116 C/D box snoRNA cluster in Prader-Willi syndrome. Eur J Hum Genet. 2010 doi: 10.1038/ejhg.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz DR, Jansen LE, Black BE, Bailey AO, Yates JR, 3rd, Cleveland DW. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8:458–469. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- Franco B, Lai LW, Patterson D, Ledbetter DH, Trask BJ, van den Engh G, Iannaccone S, Frances S, Patel PI, Lupski JR. Molecular characterization of a patient with del(1)(q23-q25) Hum Genet. 1991;87:269–277. doi: 10.1007/BF00200903. [DOI] [PubMed] [Google Scholar]
- Griffith E, Walker S, Martin CA, Vagnarelli P, Stiff T, Vernay B, Al Sanna N, Saggar A, Hamel B, Earnshaw WC, Jeggo PA, Jackson AP, O'Driscoll M. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet. 2008;40:232–236. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guernsey DL, Jiang H, Hussin J, Arnold M, Bouyakdan K, Perry S, Babineau-Sturk T, Beis J, Dumas N, Evans SC, Ferguson M, Matsuoka M, Macgillivray C, Nightingale M, Patry L, Rideout AL, Thomas A, Orr A, Hoffmann I, Michaud JL, Awadalla P, Meek DC, Ludman M, Samuels ME. Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. Am J Hum Genet. 2010;87:40–51. doi: 10.1016/j.ajhg.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40:55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibe T, Mori Y, Okanishi T, Shimojima K, Yokochi K, Yamamoto T. Two concurrent chromosomal aberrations involving interstitial deletion in 1q24.2q25.2 and inverted duplication and deletion in 10q26 in a patient with stroke associated with antithrombin deficiency and a patent foramen ovale. Am J Med Genet A. 2011;155:215–220. doi: 10.1002/ajmg.a.33786. [DOI] [PubMed] [Google Scholar]
- Lo LJ, Noordhoff MS, Huang CS, Chen KT, Chen YR. Proximal deletion of the long arm of chromosome 1: [del(1)(q23-q25)] Cleft Palate Craniofac J. 1993;30:586–589. doi: 10.1597/1545-1569_1993_030_0586_pdotla_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Loebel DA, Tsoi B, Wong N, Tam PP. A conserved noncoding intronic transcript at the mouse Dnm3 locus. Genomics. 2005;85:782–789. doi: 10.1016/j.ygeno.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, Ehlers MD. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO, Simpson JL. Interstitial deletion 46,XY, del(1)(q23q25) Hum Genet. 1982;61:277. doi: 10.1007/BF00296461. [DOI] [PubMed] [Google Scholar]
- Melis D, Perone L, Sperandeo MP, Sabbatino MS, Tuzzi MR, Romano A, Parenti G, Andria G. Mild phenotype associated with an interstitial deletion of the long arm of chromosome 1. J Med Genet. 1998;35:1047–1049. doi: 10.1136/jmg.35.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghe M, Patel ZM, Peter JJ, Ambani LM. Cytogenetic studies in a selected group of mentally retarded children. Hum Genet. 1981;58:184–187. doi: 10.1007/BF00278708. [DOI] [PubMed] [Google Scholar]
- Nishimura A, Hiraki Y, Shimoda H, Nishimura G, Tadaki H, Tsurusaki Y, Miyake N, Saitsu H, Matsumoto N. De novo deletion of 1q24.3-q31.2 in a patient with severe growth retardation. Am J Med Genet A. 2010;152A:1322–1325. doi: 10.1002/ajmg.a.33371. [DOI] [PubMed] [Google Scholar]
- Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates JR, 3rd, Desai A, Fukagawa T. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol. 2006;8:446–457. doi: 10.1038/ncb1396. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Hatsukawa Y, Shiraishi J, Harada N, Matsumoto N. Chromosome 1q deletion and congenital glaucoma. Pediatr Int. 2005;47:477–479. doi: 10.1111/j.1442-200x.2005.02097.x. [DOI] [PubMed] [Google Scholar]
- Pallotta R, Dalpra L, Miozzo M, Ehresmann T, Fusilli P. A patient defines the interstitial 1q deletion syndrome characterized by antithrombin III deficiency. Am J Med Genet. 2001;104:282–286. doi: 10.1002/ajmg.10068. [DOI] [PubMed] [Google Scholar]
- Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, van Essen AJ, Goecke TO, Al-Gazali L, Chrzanowska KH, Zweier C, Brunner HG, Becker K, Curry CJ, Dallapiccola B, Devriendt K, Dorfler A, Kinning E, Megarbane A, Meinecke P, Semple RK, Spranger S, Toutain A, Trembath RC, Voss E, Wilson L, Hennekam R, de Zegher F, Dorr HG, Reis A. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 2008;319:816–819. doi: 10.1126/science.1151174. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JA, Coppinger J, Bejjani BA, Girirajan S, Eichler EE, Shaffer LG, Ballif BC. Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. J Neurodevelop Disord. 2010;2:26–38. doi: 10.1007/s11689-009-9037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Schinzel A, Schmid W. Interstitial deletion of the long arm of chromosome 1, del(1)(q21 leads to q25) in a profoundly retarded 8-year-old girl with multiple anomalies. Clin Genet. 1980;18:305–313. doi: 10.1111/j.1399-0004.1980.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Schwanitz G, Schmid P, Hagele C, Daffner HW, Grosse KP. Partial deletion of 1q, following a pericentric inversion, in a boy with multiple minor morphologic anomalies and mental retardation. Acta Genet Med Gemellol (Roma) 1977;26:173–175. doi: 10.1017/s0001566000009971. [DOI] [PubMed] [Google Scholar]
- Silengo MC, Davi GF, Bianco R, Biagioli M, Guala A, Franceschini P, Novelli G. Interstitial deletion of chromosome 1 (q23-q25). Report of a case. Clin Genet. 1984;25:549–552. doi: 10.1111/j.1399-0004.1984.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Takano T, Yamanouchi Y, Mori Y, Kudo S, Nakayama T, Sugiura M, Hashira S, Abe T. Interstitial deletion of chromosome 1q [del(1)(q24q25.3)] identified by fluorescence in situ hybridization and gene dosage analysis of apolipoprotein A-II, coagulation factor V, and antithrombin III. Am J Med Genet. 1997;68:207–210. [PubMed] [Google Scholar]
- Taysi K, Sekhon GS, Hillman RE. A new syndrome of proximal deletion of the long arm of chromosome 1: 1q21-23 leads to 1q25. Am J Med Genet. 1982;13:423–430. doi: 10.1002/ajmg.1320130411. [DOI] [PubMed] [Google Scholar]
- Traylor RN, Fan Z, Hudson B, Rosenfeld JA, Shaffer LG, Torchia BS, Ballif BC. Microdeletion of 6q16.1 encompassing EPHA7 in a child with mild neurological abnormalities and dysmorphic features: case report. Mol Cytogenet. 2009;2:17. doi: 10.1186/1755-8166-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sato T, Amano T, Kawamura Y, Kawamura N, Kawaguchi H, Yamashita N, Kurihara H, Nakaoka T. Dnm3os, a non-coding RNA, is required for normal growth and skeletal development in mice. Dev Dyn. 2008;237:3738–3748. doi: 10.1002/dvdy.21787. [DOI] [PubMed] [Google Scholar]
- Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996;98:1107–1113. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletaev DV, Dadali EL, Kuleshov NP. [Del(1)(q22-q25) syndrome. Cytogenetics and phenotype] Tsitol Genet. 1987;21:213–216. [PubMed] [Google Scholar]