Abstract
Parkinson's disease (PD) is a common disorder of middle-aged and elderly people in which degeneration of the extrapyramidal motor system causes significant movement problems. In some patients, however, there are additional disturbances in sensory systems including loss of the sense of smell and auditory and/or visual problems. This paper is a general overview of the visual problems likely to be encountered in PD. Changes in vision in PD may result from alterations in visual acuity, contrast sensitivity, colour discrimination, pupil reactivity, eye movements, motion perception, visual field sensitivity, and visual processing speeds. Slower visual processing speeds can also lead to a decline in visual perception especially for rapidly changing visual stimuli. In addition, there may be disturbances of visuospatial orientation, facial recognition problems, and chronic visual hallucinations. Some of the treatments used in PD may also have adverse ocular reactions. The pattern electroretinogram (PERG) is useful in evaluating retinal dopamine mechanisms and in monitoring dopamine therapies in PD. If visual problems are present, they can have an important effect on the quality of life of the patient, which can be improved by accurate diagnosis and where possible, correction of such defects.
1. Introduction
Parkinson's disease (PD) is a common neurodegenerative disorder affecting middle aged and elderly people. It is a disease characterised by deficiency of dopamine in areas of the midbrain causing a variety of movement problems such as akinesia, rigidity, and tremor. Despite the emphasis on motor function in PD, nonmotor symptoms may also play a significant role in determining the general quality of life of the patient. Hence, the symptoms of PD can include depression, apathy, sleep problems, cognitive impairment, dementia, and autonomic, gastrointestinal, and sensory problems [1]. Sensory problems may include visual loss, loss of smell, auditory problems, and “restless legs” syndrome (RLS). Visual signs and symptoms of PD may include defects in eye movement, pupillary function, and in more complex visual tasks involving the ability to judge distance or the shape of an object [2, 3]. The symptoms of PD can be treated successfully using drug therapy or surgery, and these treatments may also have visual side effects. Hence, this paper provides a general overview of (1) the visual signs and symptoms of PD, (2) the areas of the eye and brain which may be affected by the pathology of PD, and (3) the adverse ocular reactions to treatment.
2. Visual Symptoms in Parkinson's Disease
PD is associated with a variety of visual problems and these are summarised in Table 1.
Table 1.
Visual signs and symptoms of Parkinson's disease (PD).
| Ocular aspect | Change in PD | References |
|---|---|---|
| Visual acuity | Poor, especially at low contrast | [6] |
| [4] | ||
| Colour vision | Vision blurred for coloured stimuli | [8] |
| Shortened colour fusion time | [9] | |
| Progressive deterioration | [10] | |
| Visual fields | Increase in glaucomatous visual field defects | [13] |
| Side effects of surgery | [14] | |
| Saccadic eye movement | Reaction time and max. velocity of horizontal gaze slower | [15] |
| Hypometria | [16] | |
| Amplitude increased after cued saccades | [17] | |
| Smooth pursuit movement | Affected early in disease process | [20] |
| Superimposed saccades | [15] | |
| Reduction in response magnitude | [15] | |
| Optokinetic Nystagmus | Abnormal in some patients | [15] |
| Convergence | Impaired, associated with large exophoria, diplopia | [21] |
| [22] | ||
| Blink frequency | Reduced, causing abnormal tear film, dry eye and reduced vision | [23] |
| Blink reflex | Habituation not observed | [24] |
| Pupil diameter | Larger after light adaptation with anisocoria | [26] |
| Light reflex | Longer latency | |
| Constriction time | Increased | [26] |
| Contraction amplitude | Reduced | [23] |
| Contrast sensitivity (CS) | Abnormal in some cases, intermediate to high frequencies | [28] |
| Temporal processing | Impaired ability to track rapid fluctuations | [32] |
| Duration perception affected | [33] | |
| Flash ERG | Reduced amplitude of “b” wave | [35] |
| PERG | Reduced amplitudes. | [35] |
| Specific defect at medium SF | ||
| Delayed P50 | [36] | |
| Cortical VEP | Delayed P100 | [45] |
| [46] | ||
| Chromatic VEP | Increased latency and reduced | [44] |
| Amplitude (esp. blue-yellow) | ||
| ERP | Abnormal. Delayed reaction times | [42] |
| [41] | ||
| Visuo-spatial | Difficulty in judging verticals, | [48] |
| position of body parts, and in route-walking tasks | [49] | |
| Orientation and motion discrimination | Impaired | [50] |
| Facial perception | Impaired ability to perceive and imagine emotional faces | [51] |
| Visual hallucinations | Chronic in 30–60% of treated cases | [54] |
Abbreviations: ERG: Electroretinogram, ERP: event-related potentials, PERG: Pattern electroretinogram, SF: Spatial frequency, VEP: Visual evoked potentials.
2.1. Visual Acuity
PD patients often complain of poor vision especially as the disease progresses resulting, in part, from poor visual acuity [4], low contrast acuity being especially affected [5, 6]. Impaired visual acuity also appears to be a risk factor for the development of chronic hallucinations in PD [7]. Poor visual acuity may be caused by lack of dopamine in the retina, abnormal eye movements, or poor blinking and is only marginally improved by drug therapy [6].
2.2. Colour Vision
Vision has been reported to be blurred in PD to coloured stimuli [8] with reduced colour fusion times [9] which indicate the accuracy of perception of monochromatic contours. A progressive deterioration of colour discrimination is also evident and is often associated with impairments of higher motor function [10]. Using the Farnsworth-Munsell 100-hue test, however, colour visual discrimination does not appear to be consistently impaired in the early stages of PD [11].
2.3. Visual Fields
There have been few studies of visual field defects in patients with PD [12]. Retrospective analysis of ophthalmic charts from PD patients, however, using a cup-to-disc ratio of 0.8 or greater to define glaucoma, revealed glaucomatous visual field defects in approximately 24% of patients suggesting there may be an increased rate of glaucoma in PD [13]. In addition, intraocular pressure (IOP) was slightly higher in PD patients with glaucoma compared with glaucoma patients without PD (mean 18.9 compared with 16.0). Of eight PD patients with glaucoma, five were considered to have low tension glaucoma. In one study, visual fields were investigated in patients undergoing posterior pallidotomy, a procedure which risks damaging structures such as the optic tract [14]. Of 40 such patients, three had visual field defects likely to be attributable to the surgery, namely, contralateral superior quadrantanopia, associated in two patients with small paracentral scotomas.
2.4. Saccadic and Smooth Pursuit Eye Movements
Assessment of oculomotor function in PD can be made clinically or by using electro-oculography (EOG). EOG responses are often normal in PD patients when the eyes are in the primary position or when resting. Abnormal saccadic and smooth pursuit eye movements, however, have been reported in about 75% of patients [15]. Both reaction times and the maximum saccadic velocity of horizontal gaze are slower in PD [15]. Saccadic eye movements may exhibit hypometria, that is, “under reaching of task” [16] while smooth pursuit movements may be interrupted by small saccades [15]. In addition, the amplitude of saccadic eye movements are increased in normal subjects when there is a change from externally cued saccades to self-paced saccades and this effect is often greater in PD [17]. In a study in which the delay of remembered (imagined) saccades was gradually increased in untreated PD patients, there was a marked hypometria of saccadic gain at all delays suggesting a dysfunction of the striatocollicular inhibitory pathways in PD attributable to dopamine deficiency in the basal ganglia [18]. In a further experiment, spatial working memory was studied in relation to eye movements [19]. A sequence of four targets was memorised by the patient and then the eyes were moved to fixate the targets in their correct order. In PD, several discrete saccadic eye movements of reduced amplitude were necessary before reaching the final eye position, and the patients also exhibited an increasing proportion of errors in remembering the target sequence. The results suggested that memory representation was disrupted early in the development of PD.
EOG recordings have been made before and after apomorphine treatment in patients with early-stage disease and have confirmed that smooth pursuit movements are affected during the initial stages of the disease [20]. In addition, patients with PD often have difficulty in sustaining repetitive actions and hence, smooth pursuit movements exhibit a reduction in response magnitude and a progressive decline of response with stimulus repetition.
2.5. Nystagmus and Convergence
Abnormal optokinetic nystagmus “train nystagmus” [15] and convergence [21] have been reported in PD patients. Further abnormalities that have been observed include “jerkiness”, “cogwheeling”, and limitation of eye movement. Vertical eye movements are often more impaired than horizontal movements. Convergence can be associated with relatively large exophoria (outward deviation of the eye), and the result is often diplopia (double vision) [22].
2.6. Blink Reflex
Patients with PD exhibit a reduced frequency of blinking leading to a staring appearance [23]. Reduced blink rate can cause an abnormal tear film, dry eye, and reduced vision. A characteristic ocular sign may be the blink reflex, elicited by a light tap above the bridge of the nose, successive taps in normal individuals producing less and less response as the reflex habituates [24]. In PD, the blink reflex may not disappear on repeated tapping. In addition, blink duration may be increased in PD reflecting the loss of dopamine neurons [25].
2.7. Pupil Reactivity
Significantly larger pupil diameters, with anisocoria (unequal pupil sizes) after light adaptation, have been reported in PD [26], no differences being observed after dark adaptation. In addition, longer light reflex latencies and constriction times have been observed while contraction amplitudes may be reduced [23]. These results suggest that there is an autonomic imbalance in PD patients involving the parasympathetic system.
2.8. Psychophysics
Contrast sensitivity (CS) is affected in a proportion of PD patients especially at the high or intermediate frequencies [27–29]. In some individuals, a substantial decrease in CS can be demonstrated as the disease progresses and could be a contributory cause of poor vision in PD. Abnormalities in CS are likely to be related to dopamine dysfunction and are often orientation specific suggesting cortical involvement [30]. L-dopa therapy generally improves CS performance close to that of healthy control patients without any neurological dysfunction. In addition, apomorphine significantly improves achromatic spatial CS at all spatial frequencies but appears to have minimal effects on colour vision [31].
There may be decreased sensitivity to temporally changing stimuli in PD which have been well demonstrated by studies of the auditory system. Hence, in psychophysical tests assessing auditory processing, bilateral subthalamic nucleus stimulation caused dysfunction in ability to track rapid fluctuations in sound intensity [32]. In addition, in motor tasks involving finger tapping, a PD group were impaired both in the motor task itself and in assessing duration implicating the basal ganglia and thalamocortical connections in timing [33]. Subsequently, the substantia nigra, an important site of PD pathology, was shown to be involved in temporal processing involving motor and perceptual tasks [34]. Problems in the visual perception of rapidly moving stimuli are likely to cause problems in tracking fast moving targets.
2.9. Electrophysiology
Significant changes in the electroretinogram (ERG) have been found in PD. Studies show that the amplitude of the ERG “b” wave may be reduced in PD patients under a variety of light conditions [35]. Since the amplitude of the “b” wave may be a diagnostic indicator of the functioning of the inner nuclear layer, the reduction may reflect defects in visual processing involving dopamine neurons. In addition, the amplitude of the pattern ERG (PERG) to a checkerboard stimulus is decreased [35] and the latency of the P50 component delayed [36] in PD patients. Subsequent studies have suggested that retinal dopamine depletion may result in attenuated ERG responses to peak stimuli [37]. Two dopamine sensitive pathways have been postulated: (1) involving the D1 receptors which primarily affect the “surround” organisation of ganglion cells with large centres and (2) the D2 postsynaptic receptors which contribute to the “centre” response amplification of ganglion cells with smaller centres. In addition, steady-state pattern PERG to sinusoidal gratings was studied over a range of spatial frequencies [38]. Aging affected responses at all spatial frequencies but the pattern of age-related loss was different in PD. In PD, there was a specific deficit at medium spatial frequencies accompanied by a distorted PERG spatial frequency response function. PERG is also sensitive to dopamine manipulation in the monkey retina [39]. In a further experiment involving the use of the selective D2 blocker l-sulpiride, treatment affected the PERG to a sinusoidal vertical grating presented at four spatial frequencies [39]. The data suggested that dopamine is involved in retinal processing in primates and that the D2 receptor is necessary for spatial-temporal tuning of pattern vision. Subsequently it was shown that the two dopamine receptors play different roles in retinal function and therefore in the different visual alterations in PD [40]. Hence, PERG is useful in evaluating retinal dopamine mechanisms and in monitoring dopamine therapies in PD.
Event-related potentials (ERP) employing various “oddball” tasks have been used to study the sensory and cognitive processing in PD. Abnormal ERP responses in PD often correlate with worsening Wechsler and motor dysfunctional scale scores [41]. In a study of the P300 response, believed to reflect orientation, attention, stimulus evaluation, and memory, reductions in reaction time were actually less in PD than in other “parkinsonian syndromes” such as progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) [42].
Evoked responses to coloured stimuli are also affected in PD [43] supporting the hypothesis that dopamine modulates the retinal colour system. In idiopathic PD, for example, amplitude is decreased and latency increased for all chromatic stimuli and especially for those using blue-yellow (B-Y) horizontal gratings [44], and this test may be a simple tool for distinguishing different parkinsonian syndromes. Increased latency of the visually evoked potential (VEP) P100 peak to a checkerboard stimulus has been reported in a proportion of patients suggesting a delay in visual processing at one or more stages of the visual system [45–47].
2.10. Complex Visual Functions
There are prominent deficits in PD involving neuropsychological tests requiring self-motivation and a demanding response from the patient [1]. PD patients may exhibit a variety of deficits in visuo-spatial orientation [48, 49] including difficulty in judging verticals and the position of body parts, and in carrying out a route-walking task. Visuo-spatial working memory appears to be selectively impaired early in PD which probably reflects degeneration of the basal ganglia, the dorsal visual stream, and the frontal-prefrontal cortex [1]. Patients may also have problems with memory tasks involving spatial orientation. PD patients often show an impairment of orientation and motion discrimination [50] suggesting that the visual pathway beyond the retina may be affected since these tasks are most likely to involve the visual cortex. In addition, impairments in the ability to perceive and imagine faces have been reported in PD [51]. Medicated and unmedicated patients exhibit facial recognition problems but these deficits are most frequently present in the untreated group [52]. Normal subjects contract their facial muscles while imaging faces, a process which is often impaired in PD patients. In a problem solving task involving arranging coloured balls in pockets on a computer screen, PD patients made more errors on the task than controls and also did not show any dissociation in the amount of time fixating the two halves of the display [53]. The results suggested difficulties in encoding and/or maintaining current goals during problem solving in PD.
2.11. Visual Hallucinations
Visual hallucinations are a chronic complication of PD [54, 55] and especially in patients treated with L-dopa and dopamine agonists. In a large study of PD patients, hallucinations occurred in the previous three months in 40% of patients examined. Hallucinations were visual in 22% and auditory in approximately 10% of patients [55]. Patients with minor hallucinations had higher depression scores than those without. Three factors were the best predictors of hallucinations, namely, severe cognitive defects, daytime somnolence, and longer duration of disease. Hallucinations in PD are often complex with flickering lights, and illusionary misconceptions often preceding the most common manifestation, namely, stereotypical colourful images. Visual hallucinations may involve a disturbance in the regulation of the gating and filtering of external perception and internally generated visual images. Risk factors for hallucinations in PD patients include poor primary vision and reduced activity of the primary visual cortex (area V1).
3. Pathological Changes Affecting the Visual System
3.1. Eye Pathology
Few pathological changes have been reported in the eye in PD with the exception of the retina [56]. However, the maximum contraction ability of the iris muscle measured in vitro is greater in PD than in controls suggesting that the muscle may have acquired adaptive sensitivity changes [57].
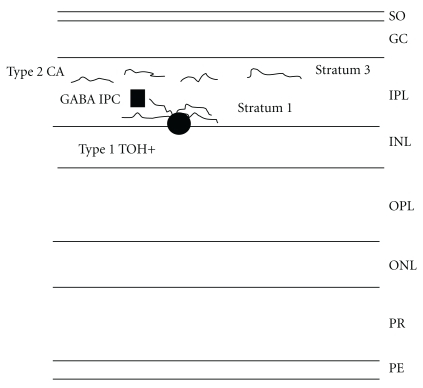
Dopamine is an important neurotransmitter in the retina and is present in amacrine cells and along the inner border of the inner nuclear layer [58] (Figure 1). In addition, dopamine may be accumulated by interplexiform cells [59]. Two types of amacrine cells appear to be involved. Type 1 cells send ascending processes to the outer plexiform layer where they synapse with γ-aminobutyric acid (GABA) interplexiform cells in stratum 1, whereas type 2 cells have their dendrites stratifying above those of the type 1 cells of the inner plexiform layer (Figure 1). Dopamine may be involved in the organisation of the ganglion cell and bipolar cell receptive fields and appears to modulate the physical activity of the photoreceptors [60]. In addition, dopamine is involved in the coupling of the horizontal and amacrine lateral system [61].
Figure 1.
The layers of the retina (PE: pigment epithelium, PR: visual receptors, ONL: outer nuclear layer, OPL: outer plexiform layer, INL: internal nuclear layer, IPL: internal plexiform layer, GC: ganglion cell layer, and SO: Stratum opticum). Dopamine neurons (TOH+: Tyrosine hydroxylase positive neurons, Type 1 cells and Type 2 amacrine cells) are primarily concentrated in the INL and dopamine positive neurites (Type 1 in stratum 1 and type 2 ramify above stratum 1) in the IPL. Type 1 cells may synapse onto GABA interpelexiform cells (IPCs). Some dopamine activities may also be observed in the ganglion cell layer.
Pathological changes which have been observed in the PD retina include cell losses, which often affect the peripheral segments of the retina more severely and reductions in retinal dopamine [62]. In addition, the thickness of the circumpapillary retinal nerve fibre layer was studied using optical coherence tomography (OCT) [63]. The inferior quadrant layer, and especially the inferior temporal region, was significantly thinner in PD than in controls. In normal subjects, the foveola contains no dopamine neurons, innervation being achieved by processes originating in the avascular zone. In PD, swelling and loss of these processes has been observed. These observations are consistent with the ERG data and support the hypothesis that at least some of the cortical VEP changes could be retinal in origin.
3.2. Brain Pathology
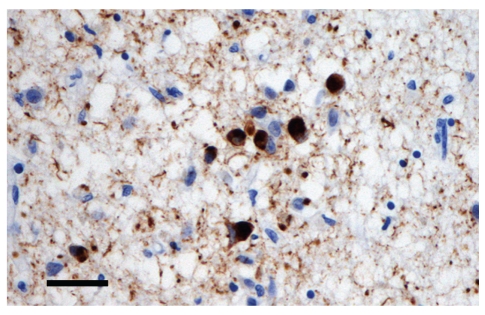
The surviving neurons of the substantia nigra and cerebral cortex often contain inclusions called Lewy bodies (LB) (Figure 2). LB are found in the cytoplasm of the cell and may be derived from cytoskeletal filaments. Recent research suggests that LB differ significantly from other neurofibrillary pathologies in neurodegenerative disease, for example, the neurofibrillary tangles (NFT) found in AD [64], in that they contain abnormal aggregates of the protein α-synuclein [65]. α-Synuclein is a small presynaptic protein and the entire molecule undergoes a conformational change to result in the insoluble protein that forms a major component of the LB.
Figure 2.
Cells from the cerebral cortex showing the presence of Lewy bodies (α-synuclein immunohistochemistry, haematoxylin stain, bar = 25 μm).
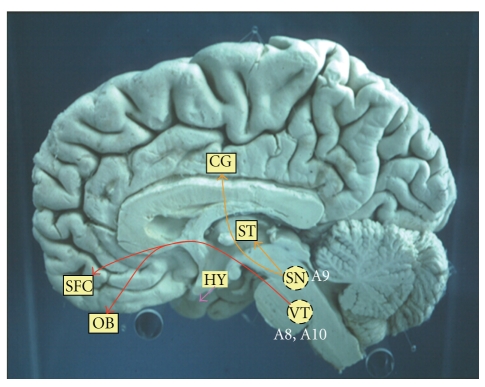
There are two major dopamine pathways in the brain (Figure 3). First, there is the striatonigral pathway from the substantia nigra (cell group A9) to the cortex and striatum. Second, a major pathway originates in the ventral tegmentum (cell groups A8, A10) and projects to the amygdala, septum, nucleus accumbens, olfactory tubercle, and frontal cortex. There are also dopamine pathways within the hypothalamus. Hence, within the brain significant dopamine activity is limited to the frontal and limbic areas of the cerebral cortex with significantly less activity in the visual cortex [62]. Cerebral metabolic rates for glucose, however, are reduced by up to 23% in the primary visual cortex of PD patients [66]. Reductions in dopamine levels in the basal ganglia and frontal cortex may also deplete levels in the superior colliculus and thus could be a factor in the production of defective saccades [16].
Figure 3.
The dopamine projections of the central nervous system (OB: Olfactory bulb, SFC: superior frontal cortex, CG: Cingulate gyrus, ST: Striatum, HY: Hypothalamus, VT: Ventral tegmentum, SN: Substantia nigra).
Within the cerebral cortex, functional MRI (fMRI) and EEG studies have both revealed the essential role of the occipital cortex in producing saccadic eye movements while PET studies have revealed occipital hypometabolism in these areas in PD. In addition, in a study using “event-related” fMRI and which utilised a visual attention/motor inhibition task, during motor inhibition there was activation of the prefrontal cortex and basal ganglia [67]. In addition, there was a reduced and less coherent haemodynamic response in the occipital cortex. Hence, specific functional changes involving the frontostriatal network and temporal-occipital cortex were present in the early stages of PD. PD patients with damage to the medial temporal lobe perform the poorest in all explicit memory tasks and memory problems are often apparent in this group in early stage disease [68].
In subcortical regions, areas of the basal ganglia appear to be the most affected by the developing pathology. Within the basal ganglia, the substantia nigra pars reticulata, the subthalamic nucleus, and the caudate nucleus are all involved in saccadic eye movements [69]. There is, however, an overlap in the anatomical pathways involved in saccadic and smooth pursuit movements which may explain why both are affected in PD. Dopamine also has a peripheral role in sympathetic ganglia, visceral ganglia, and in all artery walls. Hence, reductions in dopamine in some of these areas could be a factor contributing to eye movement problems and defects in pupil reactivity.
4. Adverse Ocular Reactions to Treatment
There are several drugs given alone or in combination used to treat PD (Table 2). Most act on the brain either by reducing cholinergic activity or by encouraging dopamine activity in the basal ganglia [70].
Table 2.
Adverse ocular reactions to treatment for Parkinson's disease.
| Treatment | Examples | Ocular side effects |
|---|---|---|
| Anticholinergi | Benzhexol, Diphenydramine | Mydriasis, photophobia, dry eyes, decreased accommodation, anisocoria, blurred vision, anterior angle closure |
| Dopamine agonists | Bromocriptine | May exacerbate visual hallucinations |
| Pramipexole | May exacerbate visual hallucinations | |
| Ropinirole | May exacerbate visual hallucinations | |
| L-dopa | L-dopa/carbidopa | Mydriasis, miosis, blepharospasm eyelid ptosis, may prolong latency of saccades |
| MAO inhibitors | Selegiline | May cause loss of visual acuity and blurred vision |
| Antiviral | Amantadine | Mydriasis, superficial keratitis, reduced accommodation, hallucinations |
| Antidepressant | Imipramine | Mydriasis, cycloplegia, dry eyes, ocular muscle paresis, nystagmus |
Anticholinergic drugs such as benzhexol and diphenydramine act to decrease acetylcholine levels, the effect of which is enhanced by the lack of dopamine. Benzhexol may have a significant mydriatic effect and therefore, should not be given to patients with anterior angle closure and should be used with caution in those with a narrow anterior chamber angle. Prolonged exposure to this drug in a few patients may cause an angle closure of gradual onset but without acute symptoms. Optometrists may be the only health practitioners aware of this risk, that is, it is always important to assess anterior chamber depth in PD patients. In addition, photophobia and decreased accommodation can occur resulting in blurred vision [71].
Dopamine agonists such as bromocriptine, pramipexole, and ropinirole enhance the effect of dopamine by directly stimulating dopamine receptors. Pramipexole and ropinirole are often indicated as a treatment for early stage PD and RLS. These drugs may cause less motor complications and dyskinesia than L-dopa but are often given in combination with the latter. Use of dopamine agonists may exacerbate visual hallucinations in PD.
L-dopa is a precursor of dopamine and can penetrate the blood-brain barrier more successfully than dopamine itself. It is often given with a peripheral decarboxylase inhibitor, for example, carbidopa, to reduce the breakdown of L-dopa outside the brain. Mydriasis may occur at first, and this may be followed by miosis. Lid ptosis and blepharospasm have been reported in a few patients [72]. In addition, L-dopa may prolong the latency of saccades [73].
Monoamine oxidase B (MAO-B) inhibitors, such as seliginine, slow down the breakdown of dopamine at the synapse. Patients treated with MAO-B inhibitors and multiple ergotamine-derived dopamine agonists may exhibit blurring of vision [74].
The antiviral drug amantadine appears to have a beneficial effect on many of the symptoms of the disease. A few adverse reactions have been reported including a superficial keratitis, mydriasis and reduced accommodation while in some patients visual hallucinations may occur [75]. By contrast, imipramine has antidepressant and anticholinergic properties and acts by inhibiting the reuptake of dopamine. Ocular side effects include mydriasis, cycloplegia, dry eyes, nystagmus, and the paresis of ocular muscles.
5. Discussion and Conclusions
Patients who have been diagnosed as having PD may develop a range of visual problems during the course of the disease. Hence, changes in vision in PD may result from alterations in visual acuity, contrast sensitivity, colour discrimination, pupil reactivity, eye movements, motion perception, visual field sensitivity, and visual processing speeds. Slower visual processing speeds can also lead to a decline in visual perception especially for rapidly changing visual stimuli. In addition, there may be disturbances of visuo-spatial orientation, facial recognition problems, and chronic visual hallucinations. Some of the treatments used in PD may also have adverse ocular reactions. Visual deficits in PD are important in influencing overall motor function [10], are a risk factor for developing hallucinations [7] and are important in influencing general quality of life [7]. Hence, identifying and correcting the visual problems as far as possible can significantly benefit a PD patient.
Clinical examination of the patient by eye practitioners requires sensitivity to both the physical disability and mental state of the patient and the problems involved have been described in detail by Naylor [70]. Some of the visual problems may be adverse reactions to treatment. Side effects may occur relatively rapidly at the beginning of, or after a change, in drug treatment, but can also occur after a long latent period. It is important that those symptoms due to adverse reactions are distinguished from those due to the disease process itself. If ocular side effects are identified and become severe, then it is essential that these are monitored and the patient referred back to their physician for further clinical assessment.
References
- 1.Antal A, Bandini F, Kéri S, Bodis-Wollner I. Visuo-cognitive dysfunctions in Parkinson’s disease. Clinical Neuroscience. 1998;5(supplement 2):147–152. [PubMed] [Google Scholar]
- 2.Armstrong RA. Parkinson’s disease and the eye. Ophthalmic and Physiological Optics. 1997;17(2):S9–S16. doi: 10.1016/s0275-5408(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong RA. Visual signs and symptoms of Parkinson’s disease. Clinical and Experimental Optometry. 2008;91(2):129–138. doi: 10.1111/j.1444-0938.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 4.Repka MX, Claro MC, Loupe DN, Reich SG. Ocular motility in Parkinson’s disease. Journal of Pediatric Ophthalmology and Strabismus. 1996;33(3):144–147. doi: 10.3928/0191-3913-19960501-04. [DOI] [PubMed] [Google Scholar]
- 5.Jones RD, Donaldson IM. Fractionation of visuoperceptual dysfunction in Parkinson’s disease. Journal of the Neurological Sciences. 1995;131(1):43–50. doi: 10.1016/0022-510x(95)00043-2. [DOI] [PubMed] [Google Scholar]
- 6.Jones RD, Donaldson IM, Timmings PL. Impairment of high-contrast visual acuity in Parkinson’s disease. Movement Disorders. 1992;7(3):232–238. doi: 10.1002/mds.870070308. [DOI] [PubMed] [Google Scholar]
- 7.Matsui H, Udaka F, Tamura A, et al. Impaired visual acuity as a risk factor for visual hallucinations in Parkinson’s disease. Journal of Geriatric Psychiatry and Neurology. 2006;19(1):36–40. doi: 10.1177/0891988705284739. [DOI] [PubMed] [Google Scholar]
- 8.Price MJ, Feldman RG, Adelberg D, Kayne H. Abnormalities in color vision and contrast sensitivity in Parkinson’s disease. Neurology. 1992;42(4):887–890. doi: 10.1212/wnl.42.4.887. [DOI] [PubMed] [Google Scholar]
- 9.Buttner T, Kuhn W, Klotz P, et al. Disturbance of colour perception in Parkinson’s disease. Journal of Neural Transmission. 1993;6(1):11–15. doi: 10.1007/BF02252618. [DOI] [PubMed] [Google Scholar]
- 10.Diederich NJ, Raman R, Leurgans S, Goetz CG. Progressive worsening of spatial and chromatic processing deficits in Parkinson disease. Archives of Neurology. 2002;59(8):1249–1252. doi: 10.1001/archneur.59.8.1249. [DOI] [PubMed] [Google Scholar]
- 11.Veselá O, Růžička E, Jech R, et al. Colour discrimination impairment is not a reliable early marker of Parkinson’s disease. Journal of Neurology. 2001;248(11):975–978. doi: 10.1007/s004150170051. [DOI] [PubMed] [Google Scholar]
- 12.Ture S, Inci I, Gedizlioglu M. Abnormalities of contrast sensitivity, visual fields and visual evoked potentials in Parkinson’s disease and effect of dopaminergic treatment. Journal of Neurology. 2007;254(supplement 3):p. 93. [Google Scholar]
- 13.Bayer AU, Keller ON, Ferrari F, Maag KP. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. American Journal of Ophthalmology. 2002;133(1):135–137. doi: 10.1016/s0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- 14.Biousse V, Newman NJ, Carroll C, et al. Visual fields in patients with posterior GPi pallidotomy. Neurology. 1998;50(1):258–265. doi: 10.1212/wnl.50.1.258. [DOI] [PubMed] [Google Scholar]
- 15.Shibasaki H, Tsuji S, Kuroiwa Y. Oculomotor abnormalities in Parkinson’s disease. Archives of Neurology. 1979;36(6):360–364. doi: 10.1001/archneur.1979.00500420070009. [DOI] [PubMed] [Google Scholar]
- 16.Crawford T, Goodrich S, Henderson L, Kennard C. Predictive responses in Parkinson’s disease: manual keypresses and saccadic eye movements to regular stimulus events. Journal of Neurology Neurosurgery and Psychiatry. 1989;52(9):1033–1042. doi: 10.1136/jnnp.52.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winograd-Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB. Self-paced saccades and saccades to oddball targets in Parkinson’s disease. Brain Research. 2006;1106(1):134–141. doi: 10.1016/j.brainres.2006.05.103. [DOI] [PubMed] [Google Scholar]
- 18.Shaunak S, O’Sullivan E, Blunt S, et al. Remembered saccades with variable delay in Parkinson’s disease. Movement Disorders. 1999;14(1):80–86. doi: 10.1002/1531-8257(199901)14:1<80::aid-mds1014>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson TL, Dittrich WH, Henderson L, Kennard C. Eye movements and spatial working memory in Parkinson’s disease. Neuropsychologia. 1999;37(8):927–938. doi: 10.1016/s0028-3932(98)00151-1. [DOI] [PubMed] [Google Scholar]
- 20.Bares M, Brazdil M, Kanovsky P, et al. The effect of apomorphine administration on smooth pursuit ocular movements in early Parkinson’s disease. Parkinsonism & Related Disorders. 2003;9:139–144. doi: 10.1016/s1353-8020(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 21.Corin MS, Elizan TS, Bender MB. Oculomotor function in patients with Parkinson’s disease. Journal of the Neurological Sciences. 1972;15(3):251–265. doi: 10.1016/0022-510x(72)90068-8. [DOI] [PubMed] [Google Scholar]
- 22.Lepore FE. Parkinson’s disease and diplopia. Neuro-Ophthalmology. 2006;30(2-3):37–40. [Google Scholar]
- 23.Biousse V, Skibell BC, Watts RL, Loupe DN, Drews-Botsch C, Newman NJ. Ophthalmologic features of Parkinson’s disease. Neurology. 2004;62(2):177–180. doi: 10.1212/01.wnl.0000103444.45882.d8. [DOI] [PubMed] [Google Scholar]
- 24.Garland HG. Refresher course for general practitioners. Parkinsonism. British Medical Journal. 1952;1:153–155. [Google Scholar]
- 25.Peshori KR, Schicatano EJ, Gopalaswamy R, Sahay E, Evinger C. Aging of the trigeminal blink system. Experimental Brain Research. 2001;136(1):351–363. doi: 10.1007/s002210000585. [DOI] [PubMed] [Google Scholar]
- 26.Micieli G, Tassorelli C, Martignoni E, et al. Disordered pupil reactivity in Parkinson’s disease. Clinical Autonomic Research. 1991;1(1):55–58. doi: 10.1007/BF01826058. [DOI] [PubMed] [Google Scholar]
- 27.Bulens C, Meerwaldt JD, Van Der Wildt GJ, Van Deursen JBP. Effect of levodopa treatment on contrast sensitivity in Parkinson’s disease. Annals of Neurology. 1987;22(3):365–369. doi: 10.1002/ana.410220313. [DOI] [PubMed] [Google Scholar]
- 28.Hutton JT, Morris JL, Elias JW. Levodopa improves spatial contrast sensitivity in Parkinson’s disease. Archives of Neurology. 1993;50(7):721–724. doi: 10.1001/archneur.1993.00540070041012. [DOI] [PubMed] [Google Scholar]
- 29.Bodis-Wollner I, Marx MS, Mitra S, Bobak P, Mylin L, Yahr M. Visual dysfunction in Parkinson’s disease. Loss in spatiotemporal contrast sensitivity. Brain. 1987;110(6):1675–1698. doi: 10.1093/brain/110.6.1675. [DOI] [PubMed] [Google Scholar]
- 30.Rodnitzky RL. Visual dysfunction in Parkinson’s disease. Clinical Neuroscience. 1998;5(2):102–106. [PubMed] [Google Scholar]
- 31.Büttner TH, Müller TH, Kuhn W. Effects of apomorphine on visual functions in Parkinson’s disease. Journal of Neural Transmission. 2000;107(1):87–94. doi: 10.1007/s007020050007. [DOI] [PubMed] [Google Scholar]
- 32.Guehl D, Burbaud P, Lorenzi C, et al. Auditory temporal processing in Parkinson’s disease. Neuropsychologia. 2008;46(9):2326–2335. doi: 10.1016/j.neuropsychologia.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12(1):3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- 34.Jones CR, Jahanshahi M. The substantia nigra, the basal ganglia, dopamine and temporal processing. Journal of Neural Transmission. 2009;(73):161–171. doi: 10.1007/978-3-211-92660-4_13. [DOI] [PubMed] [Google Scholar]
- 35.Gottlob I, Schneider E, Heider W, Skrandies W. Alteration of visual evoked potentials and electroretinograms in Parkinson's disease. Electroencephalography & Clinical Neurophysiology. 1987;66:349–357. doi: 10.1016/0013-4694(87)90032-0. [DOI] [PubMed] [Google Scholar]
- 36.Peppe A, Stanzione P, Pierelli F, De Angelis D, Pierantozzi M, Bernardi G. Visual alterations in de novo Parkinson's disease: pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials. Neurology. 1995;45:1144–1148. doi: 10.1212/wnl.45.6.1144. [DOI] [PubMed] [Google Scholar]
- 37.Bodis-Wollner I, Tzelepi A. The push-pull action of dopamine on spatial tuning of the monkey retina: the effects of dopaminergic deficiency and selective D and D receptor ligands on the pattern electroretinogram. Vision Research. 1998;38(10):1479–1487. doi: 10.1016/s0042-6989(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 38.Tagliati M, Bodis-Wollner I, Yahr MD. The pattern electroretinogram in Parkinson’s disease reveals lack of retinal spatial tuning. Electroencephalography and Clinical Neurophysiology. 1996;100(1):1–11. doi: 10.1016/0168-5597(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 39.Tagliati M, Bodis-Wollner I, Kovanecz I, Stanzione P. Spatial frequency tuning of the monkey pattern ERG depends on D2 receptor-linked action of dopamine. Vision Research. 1994;34(16):2051–2057. doi: 10.1016/0042-6989(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 40.Stanzione P, Bodis-Wollner I, Pierantozzi M, et al. A mixed D1 and D2 antagonist does not replay pattern electroretinogram alterations observed with a selective D2 antagonist in normal humans: relationship with Parkinson’s disease pattern electroretinogram alterations. Clinical Neurophysiology. 1999;110(1):82–85. doi: 10.1016/s0168-5597(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Kuroiwa Y, Wang L, et al. Early sensory information processes are enhanced on visual oddball and S1-S2 tasks in Parkinson’s disease: a visual event-related potentials study. Parkinsonism and Related Disorders. 2003;9(6):329–340. doi: 10.1016/s1353-8020(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Kuroiwa Y, Kamitani T, et al. Visual event-related potentials in progressive supranuclear palsy, corticobasal degeneration, striatonigral degeneration, and Parkinson’s disease. Journal of Neurology. 2000;247(5):356–363. doi: 10.1007/s004150050602. [DOI] [PubMed] [Google Scholar]
- 43.Barbato L, Rinalduzzi S, Laurenti M, Ruggieri S, Accornero N. Color VEPs in Parkinson’s disease. Electroencephalography and Clinical Neurophysiology. 1994;92(2):169–172. doi: 10.1016/0168-5597(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 44.Sartucci F, Orlandi G, Bonuccelli U, et al. Chromatic pattern-reversal electroretinograms (ChPERGs) are spared in multiple system atrophy compared with Parkinson’s disease. Neurological Sciences. 2006;26(6):395–401. doi: 10.1007/s10072-006-0522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodis-Wollner I, Yahr MD. Measurements of visual evoked potentials in Parkinson’s disease. Brain. 1978;101(4):661–671. doi: 10.1093/brain/101.4.661. [DOI] [PubMed] [Google Scholar]
- 46.Onofrj M, Bosid-Wollner I. Dopaminergic deficiency causes delayed visual evoked potentials in rats. Annals of Neurology. 1982;11(5):484–490. doi: 10.1002/ana.410110508. [DOI] [PubMed] [Google Scholar]
- 47.Bodis-Wollner I, Yahr MD, Mylin L, Thornton J. Dopaminergic deficiency and delayed visual evoked potentials in humans. Annals of Neurology. 1982;11(5):478–483. doi: 10.1002/ana.410110507. [DOI] [PubMed] [Google Scholar]
- 48.Levin BE, Llabre MM, Reisman S, et al. Visuospatial impairment in Parkinson’s disease. Neurology. 1991;41(3):365–369. doi: 10.1212/wnl.41.3.365. [DOI] [PubMed] [Google Scholar]
- 49.Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson’s disease. Vision Research. 2005;45(10):1285–1296. doi: 10.1016/j.visres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Trick GL, Kaskie B, Steinman SB. Visual impairment in Parkinson’s disease: deficits in orientation and motion discrimination. Optometry and Vision Science. 1994;71(4):242–245. doi: 10.1097/00006324-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Lang PJ. Presidential address, 1978. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16(6):495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 52.Sprengelmeyer R, Young AW, Mahn K, et al. Facial expression recognition in people with medicated and unmedicated Parkinson’s disease. Neuropsychologia. 2003;41(8):1047–1057. doi: 10.1016/s0028-3932(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 53.Hodgson TL, Tiesman B, Owen AM, Kennard C. Abnormal gaze strategies during problem solving in Parkinson’s disease. Neuropsychologia. 2002;40(4):411–422. doi: 10.1016/s0028-3932(01)00099-9. [DOI] [PubMed] [Google Scholar]
- 54.Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Movement Disorders. 2005;20(2):130–140. doi: 10.1002/mds.20308. [DOI] [PubMed] [Google Scholar]
- 55.Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease. Prevalence, phenomenology and risk factors. Brain. 2000;123(4):733–745. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 56.Hunt LA, Sadun AA, Bassi CJ. Review of the visual system in Parkinson’s disease. Optometry and Vision Science. 1995;72(2):92–99. doi: 10.1097/00006324-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Patil PN, Mauger TF. Cholinergic sensitivity of irides from donors with various pathological conditions and lens implants. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1992;346(6):620–628. doi: 10.1007/BF00168734. [DOI] [PubMed] [Google Scholar]
- 58.Dowling JE, Ehinger B, Hedden WL. The interplexiform cell: a new type of retinal neuron. Investigative Ophthalmology. 1976;15(11):916–926. [Google Scholar]
- 59.Frederick JM, Rayborn ME, Laties AM. Dopaminergic neurons in the human retina. Journal of Comparative Neurology. 1982;210(1):65–79. doi: 10.1002/cne.902100108. [DOI] [PubMed] [Google Scholar]
- 60.Masson G, Mestre D, Blin O. Dopaminergic modulation of visual sensitivity in man. Fundamental and Clinical Pharmacology. 1993;7(8):449–463. doi: 10.1111/j.1472-8206.1993.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 61.Djamgoz MBA, Hankins MW, Hirano J, Archer SN. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Research. 1997;37(24):3509–3529. doi: 10.1016/S0042-6989(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen-Legros J, Harnois C, Di Paolo T, Simon A. The retinal dopamine system in Parkinson’s disease. Clinical Vision Sciences. 1993;8(1):1–12. [Google Scholar]
- 63.Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Research. 2004;44(24):2793–2797. doi: 10.1016/j.visres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Armstrong RA, Syed AB. Alzheimer’s disease and the eye. Ophthalmic and Physiological Optics. 1996;16(1):S2–S8. doi: 10.1016/0275-5408(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 65.Spillantini MG, Anthony Crowther R, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neuroscience Letters. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 66.Eberling JL, Richardson BC, Reed BC, Wolfe N, Jagust WJ. Cortical glucose metabolism in Parkinson’s disease without dementia. Neurobiology of Aging. 1994;15(3):329–335. doi: 10.1016/0197-4580(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 67.Baglio F, Blasi V, Falini A, et al. Functional brain changes in early Parkinson’s disease during motor response and motor inhibition. Neurobiology of Aging. 2009;32(1):115–124. doi: 10.1016/j.neurobiolaging.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 68.Rieger B, Markowitsch HJ. Implicit and explicit mnestic performance of patients with prefrontal, medial temporal, and basal ganglia damage. Neurology Psychiatry and Brain Research. 1996;4(2):53–74. [Google Scholar]
- 69.Basso MA, Pokorny JJ, Liu P. Activity of substantia nigra pars reticulata neurons during smooth pursuit eye movements in monkeys. European Journal of Neuroscience. 2005;22(2):448–464. doi: 10.1111/j.1460-9568.2005.04215.x. [DOI] [PubMed] [Google Scholar]
- 70.Naylor RJ. The ocular effects of parkinsonism and its treatment. Optometry in Practice. 2005;6:19–31. [Google Scholar]
- 71.Friedman Z, Neumann E. Benzhexol-induced blindness in Parkinson’s disease. British Medical Journal. 1972;1(800):p. 605. doi: 10.1136/bmj.1.5800.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spiers AS, Calne DB, Fayers PM. Miosis during L-dopa therapy. British Medical Journal. 1970;2(7):639–640. doi: 10.1136/bmj.2.5710.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michell AW, Xu Z, Fritz D, et al. Saccadic latency distributions in Parkinson’s disease and the effects of L-dopa. Experimental Brain Research. 2006;174(1):7–18. doi: 10.1007/s00221-006-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peters S, Schweibold G, Przuntek H, Muller T. Loss of visual acuity under dopamine substitution therapy. Neuro-Ophthalmology. 2000;24(1):273–277. [Google Scholar]
- 75.Pearlman JT, Kadish AH, Ramseyer JC. Vision loss associated with amantadine hydrochloride use. Journal of the American Medical Association. 1977;237(12):p. 1200. doi: 10.1001/jama.237.12.1200a. [DOI] [PubMed] [Google Scholar]