Abstract
GSK3 has diverse functions, including an important role in brain pathology. In this paper, we address the primary functions of GSK3 in development and neuroplasticity, which appear to be interrelated and to mediate age-associated neurological diseases. Specifically, GSK3 plays a pivotal role in controlling neuronal progenitor proliferation and establishment of neuronal polarity during development, and the upstream and downstream signals modulating neuronal GSK3 function affect cytoskeletal reorganization and neuroplasticity throughout the lifespan. Modulation of GSK3 in brain areas subserving cognitive function has become a major focus for treating neuropsychiatric and neurodegenerative diseases. As a crucial node that mediates a variety of neuronal processes, GSK3 is proposed to be a therapeutic target for restoration of synaptic functioning and cognition, particularly in Alzheimer's disease.
1. GSK3 Signaling Pathway
Many diseases of the central nervous system are characterized by changes in the structural organization of neuronal networks, developmental abnormalities, or dysregulation of signaling pathways, leading to altered brain plasticity and, ultimately, neurodegeneration. The proline-directed serine/threonine kinase, glycogen synthase kinase 3 (GSK3), has been suspected to be a contributing factor in psychiatric illness and age-associated neurodegenerative diseases for some time [1]. The involvement of GSK3 misregulation in a variety of brain abnormalities strongly supports its pivotal role as a metabolic crossroads for controlling basic mechanisms of neuronal function from brain bioenergetics to establishment of neuronal circuits, modulation of neuronal polarity, migration, neuronal proliferation, and survival [2]. In particular, the role of GSK3 in phosphorylation of cytoskeletal proteins impacts neuronal plasticity, as cytoskeletal constituents are involved in the development and maintenance of neurites, and changes in the rate of stabilization/destabilization of microtubules (MT) could influence major cellular compartments of neurons, such as dendrites, spines, axons, and synapses.
The metabolic function of GSK3 was first described in glycogen metabolism, as GSK3 phosphorylates glycogen synthase in response to insulin [3]. Since then, research has identified a multitude of substrates and functions for this enzyme. GSK3 exists in cells as two distinct gene products, α and β, which exhibit high homology in the catalytic domain but differ in the N- and C-terminal sequences [4]. GSK3 is ubiquitous throughout the animal kingdom [5] and is widely expressed in all tissues with particularly abundant levels in the brain [4], where the neuron-specific isoform GSK3β2 is found [6].
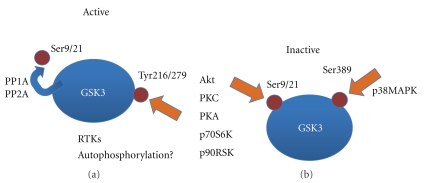
GSK3 is unique because it is constitutively active, and upstream signals downregulate its activity by phosphorylation at specific residues. The most important phospho-residues are serine (Ser) 21 for GSK3α and Ser9 for GSK3β, which inhibit its kinase activity [2, 7–10], while phosphorylation on tyrosine (Tyr) residues (Tyr 216/279 for GSK3β and GSK3α, resp.), is required for its activation [11–13]. The latter kind of phosphorylation is mediated by diverse tyrosine kinases [14] or by autophosphorylation [15] (Figure 1).
Figure 1.
Modulation of GSK3 activity by phosphorylation. Protein phosphatases 1 and 2A activate GSK3 by removing Ser9/21 phosphorylation. It has also been reported that phosphorylation in tyrosine residues by members of the receptor tyrosine kinase family of cell surface receptors (RTKs) or by autophosphorylation may activate GSK3. On the other hand, signaling networks activate several protein kinases, which may bring about phosphorylation of different residues and inhibition of GSK3.
Multiple kinases can phosphorylate Ser21/9, including Akt, protein kinases A and C, p70S6K, and p90RSK [16]. In contrast, protein phosphatases 1 (PP1) and 2A (PP2A) dephosphorylate the inhibitory site of GSK3, resulting in activation of the enzyme. In addition to the inhibitory phosphorylation of GSK3β described above, an additional inhibitory site at Ser389 has been detected in the brain, which is phosphorylated by p38 mitogen-activated protein kinase (MAPK) [17].
In addition to its phosphorylation state, GSK3 activity may be regulated by proteolysis through disruption of the axin-β-catenin complex [18] or N-terminal cleavage by the calcium-dependent protease calpain [19]. GSK3 activity also depends on its cellular localization. Although GSK3 is predominantly located in the cytosol, it is also present in nuclei and mitochondria, where it is highly activated compared with the cytosolic pool [20]. Nuclear GSK3 regulates the expression of diverse genes via various transcription factors, such as Ap-1, β-catenin, c-myc, and p53 [16]. Subtle control of GSK3-mediated activation and inhibition is required to ensure a proper balance among cell morphoregulation, proliferation, and growth. Thus, prolonged inhibition of GSK3 is associated with hypertrophic cell growth [21], while sustained activation is associated with neurodegeneration [22]. Unlike other kinases, the majority of GSK3 substrates require a “priming” phosphorylation on Ser/Thr residues, which is catalyzed by a protein kinase other than GSK3 [2, 10, 16].
2. Implications of GSK3 Activity in Brain
In adulthood, both GSK3α and GSK3β are expressed in mice adult brain and are particularly enriched in hippocampus, neocortex, and cerebellum [23]. In rodent adult hippocampus GSK3β is more abundant than GSK3α [24], and in aged hippocampus GSK3β is elevated, but not GSK3α [25]. Two splice variants of the GSK3β gene are found in neurons from mouse, rat, and human: GSK3β1 and GSK3β2, the latter being highly expressed during brain development and specific to neurons [6, 26–28]. The two isoforms are differentially involved in phosphorylation of different substrates [29] and establishment of neuronal polarity and axon guidance [2, 30–32].
The importance of GSK3 in brain function has been established by several studies in transgenic mice, which have shown different neurological defects depending of the specific GSK3 isoform involved. While deletion of GSK3β is lethal, heterozygote mice survive and present increased anxiety and reduced exploration [33–35]. Conversely, knockout GSK3α mice are quite normal [36], although neuron-specific knockout of GSK3α results in reduced anxiety, locomotor activity, and aggression [37]. Overexpression of an inhibitory phosphorylation-resistant form of GSK3 results in increased locomotor activity and has been proposed as a model of manic illness [38]. Moreover, overexpressed GSK3β in dentate gyrus results in tau-dependent neurodegeneration of this region [39]. In the brain, GSK3 regulates developmental processes, including neurogenesis, migration, axon growth and guidance, and synaptic plasticity [40], and its activity is controlled through several signaling pathways activated by growth factors, wingless (Wnt) proteins, G-protein-coupled receptors (GPCR), β-arrestin, among other proteins [41].
Abnormal activation of GSK3 has been associated with several neurological and psychiatric disorders that share developmental abnormalities and altered neurocircuitry maintenance, such as schizophrenia, bipolar disorder, autism, and Alzheimer's disease (AD) [42–46]. GSK3 is indeed a common therapeutic target for neuropsychiatric drugs [41, 47].
3. Signaling Pathways Involved in GSK3 Activity in Brain
GSK3 is a downstream component of several signaling pathways in the brain. One of the most studied is the phosphoinositide-3-OH kinase (PI3K)/Akt pathway, which plays a crucial role in differentiation and survival of neuronal and glial cells [48]. Growth signals, Ras proteins [49], or diminished phosphatase and tensin homolog (PTEN) all activate the catalytic subunit of PI3K, which phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3) and activates phosphoinositide-dependent protein kinase-1 (PDK-1). Meanwhile, signaling proteins with pleckstrin homology (PH) domains accumulate at sites of PI3K activation on the inner surface of the plasma membrane through interactions between their PH domains and the phospholipid products of PI3K. Next, the serine-threonine kinase Akt/protein kinase B is recruited and phosphorylated by PDK-1, which stimulates the catalytic activity of Akt, in turn phosphorylating GSK3 to downregulate its activity.
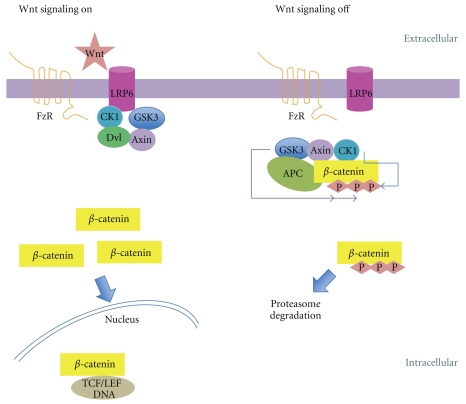
The canonical Wnt pathway is also classically involved in negative regulation of GSK3. Although the role of Wnt proteins in mature neurons remains largely unexplored, recent data indicate that Wnts are important mediators of neuronal function, neuronal morphology, neurogenesis, and synaptic plasticity [50–52]. Interestingly, Wnt signaling has also been implicated in neurological disorders associated with developmental abnormalities, such as schizophrenia [53], as well as in chronic neurodegenerative diseases, such as AD [54]. Extracellular secreted Wnt proteins activate Frizzled receptor and/or the low-density lipoprotein-related protein 5 and 6 (LRP5/6) receptors, leading to the characteristic activation of the Wnt canonical pathway [55]. Due to Frizzled activation, the Dishevelled mammalian homolog Dvl1 is recruited, inducing destabilization of the protein complex composed of axin, adenomatous polyposis coli (APC) protein, β-catenin, and GSK3β, which results in GSK3β inactivation [56]. Inhibition of GSK3β favors an increase in unphosphorylated β-catenin levels, allowing interaction with members of the lymphoid enhancer factor/ T-cell factor (LEF/TCF) family of transcription factors and, as a consequence, promoting the expression of cell survival genes [57]. Although the molecular mechanism of GSK3 inhibition is not completely understood, Wnt signaling has recently been reported to trigger the sequestration of GSK3 from the cytosol to multivesicular organelles, preventing its interaction with cytoplasmic substrates [58] (Figure 2).
Figure 2.
Canonical Wnt signaling and GSK3 regulation. Wnt activation trough Frizzled receptor (FzR) induces destabilization of the protein complex composed of axin, adenomatous polyposis coli (APC) protein, β-catenin, casein kinase (Ck1), and GSK3, which results in GSK3 inhibition leading to the induction of β-catenin/TCF target gene expression. When Wnt signalling is off the GSK3/axin complex is not inhibited and β-catenin phosphorylated and is degraded by the proteasome machinery.
The outcome is different in the absence of the Wnt stimulation, which may occur due to lack of Wnt ligands or the presence of Wnt negative modulators, such as the extracellular protein Dickkopf-1 (DKK1), which regulates the canonical Wnt signaling, or the secreted Frizzled-related protein, which modulates both canonical and noncanonical Wnt signaling [59]. Under these circumstances, GSK3β is activated and able to phosphorylate its target proteins. Several regulators also target β-catenin/GSK3β signaling. For example, the product of disrupted in schizophrenia 1 (DISC1) gene inhibits GSK3β activity through a direct physical interaction, causing stabilization of β-catenins. DISC1 loss-of-function in the dentate gyrus has been shown to result in reduced neural progenitor proliferation and to elicit hyperactive and depressive behaviors in mice [60], suggesting the involvement of GSK3β overactivation in mental illnesses, such as depression and schizophrenia. Moreover, DISC1 function seems to be essential for neural progenitor proliferation in embryonic brains and in the dentate gyrus of adult brains through its ability to control GSK3 activity and to maintain β-catenin levels, which ultimately impacts the neural circuitry [60].
GSK3β is also a downstream mediator of dopamine signaling via the dopamine D2 receptor/β-arrestin 2/PP2A complex. In this signaling pathway, Akt activates neuregulin-1 signaling leading to inhibition of GSK3β activity [61]. Interestingly, neuregulin-1 has been also implicated as schizophrenia risk factor [62].
In addition to the described role of GSK3β in neurodevelopment, it has been recently found the potentiation of Notch signalling by PI3K through GSK3β inhibition [63]. The Notch pathway has been implicated in controlling cell fate, differentiation, development as well as synaptic plasticity, learning and memory [64].
4. GSK3: A Switch for Cytoskeletal Reorganization and Synaptic Plasticity
Changes in neuronal morphology and plasticity are affected by GSK3-induced phosphorylation of proteins involved in the modulation of MT and neurofilament stabilization, which affect the cytoskeleton [65]. Among these proteins are tau, microtubule-associated protein 2 (MAP2), microtubule-associated protein 1B (MAP1B), collapsin response mediator protein 2, APC, axin, neurofilaments, kinesin light chain, and cytoplasmic linker protein [9, 16, 30, 31, 40, 53, 66–70].
The induction of polarity during neuronal development is essential for the establishment of circuits that support complex functioning [71, 72]. Subcellular location of the inactive form of GSK3β varies depending on the state of neuronal polarization, as it moves from nonpolarized neurites to the neurite tip that will form the axon at the beginning of the differentiation process. Local inactivation of GSK3 is important to allow axonal growth concurrent with its activation in dendrites [73–76]. These mechanisms support the establishment of neuronal polarity, which is dependent on the stability and dynamism of the MT in each neuronal compartment [40, 53]. The relationship between GSK3β and the microtubule stabilizing protein complex APC-mPar3, which are both present at the tip of the actively growing nascent axon, is important for the establishment of neuronal polarity. Shi and colleagues [74] have demonstrated that spatially regulated GSK3 activity in hippocampal neurons during development leads to axonal generation [74]. The inactivation of GSK3 at the nascent axon is required for mPar3 targeting through APC and kinesin-mediated transport at the plus end of the axonal MT [74].
Two further studies showed that GSK3β inhibition in hippocampal neurons induces formation of multiple axons [75, 76]. However, the role of GSK3 in neurodevelopment remains only partially understood due to contradictory data; other studies have found that GSK3 inhibition induces axonal spreading, reduces axonal elongation, and increases growth cone size, but it does not induce the formation of multiple axons [66, 68, 77–79].
One mechanism related to both synaptic reorganization and MT dynamics is Wnt signaling [80–82], which directs the growing axon towards the synaptic terminal. This process involves the reduction of axonal growth speed and the extension of axonal distal portions at the growth cone [83] until arborization forms functional synaptic endings where the presynaptic apparatus can be assembled. Transmembrane proteins, such as neuroligin/neurexin and cadherins, are also involved in this process and serve to regulate assembly on both sides of the synapse [52, 84]. Wnt proteins have a fundamental role in synapse formation, acting as retrograde signals that regulate assembly of the presynaptic apparatus [84]. Specifically, Wnt7a has a dual function in synaptic differentiation, promoting axon remodeling and increasing incorporation of synaptic proteins [66, 84]. These effects are linked to changes in the reorganization and dynamics (stabilization-destabilization) of MT, which are achieved through the canonical Wnt signaling, independent of the transcription pathway, in which GSK3β activity is inhibited, and, consequently, the phosphorylation state of the axonal MAP1B is reduced [84–86]. The addition of Wnt7a to neuronal cultures reduces MAP1B phosphorylation and induces MT depolymerization from growing areas of the axon, promoting axonal growth cone enlargement and axonal spread [51, 66, 87]. The classical inhibition of GSK3β by lithium chloride (LiCl) reproduces the effects of Wnt7a, inducing axonal arborization and widening and enlargement of the growth cone through remodeling of axonal MT during postnatal development of cerebellar cells [52, 87, 88]. On the other hand, it has been shown that Wnt7a increases the level of Synapsin I (SynI), which is known to be involved in synapse formation, as well as in the maturation and transport of synaptic vesicles in areas of growth [87, 89, 90]. Accumulation of SynI promotes both axonal remodeling and synaptogenesis during cerebellar development [87] and is mimicked by LiCl treatment [66, 88, 91].
GSK3 is also present in mature synapses [92], where its activity, along with that of cyclin-dependent kinase (Cdk5), participates in the recovery of synaptic vesicles during high neuronal activity. During this process, Cdk5 phosphorylates the GTPase dynamin I, and then GSK3β phosphorylates the same dynamin I [93]. Both phosphorylation events are necessary and sufficient to trigger and maintain activity-dependent bulk endocytosis of vesicles [94].
As a result of controlling different morphofunctional aspects of adult brain plasticity, GSK3 also plays a role in long-term potentiation (LTP) [95, 96] and long-term depression (LTD). LTP might be considered the electrophysiological correlate of learning based on its synaptic mechanisms and long-lasting experience-dependent cortical circuits [97–99]. On the other hand, LTD has been suggested as a mechanism to enhance the signal-to-noise ratio of sensory input from stored memories [97]. Some studies have shown that GSK3β inhibition upregulates and maintains LTP [24, 50, 91, 100–102], while GSK3β remains active during LTD [101]. In rat hippocampus, GSK3β overactivation has been shown to impede LTP and affect synapses by decreasing both synaptic transmission and release of the presynaptic neurotransmitter glutamate [91]. This is regulated by proteins associated with synaptic vesicles, such as SynI [103–108], which is considered to be a synaptic plasticity marker [109, 110]. GSK3β activation inhibits SynI expression after LTP induction and simultaneously disrupts SynI clustering, which results from elevated neuronal activity [91].
An other evidence that underscores the importance of GSK3 in brain plasticity is derived from experiments conducted in rat hippocampus by Gómez de Barreda and colleagues. The authors found that inhibitory phosphorylation of GSK3 at Ser9 increased at the time of LTP induction was maintained for up to one hour in vivo and was significantly higher in the hippocampal CA1 and dentate gyrus subregions, which are involved in learning and memory acquisition [39]. Transgenic mice overexpressing GSK3 showed reduced LTP induction [100]. These data confirm the significant participation of GSK3 in LTP regulation by enabling LTP when its catalytic activity is inhibited and preventing LTP when it is overactive. The inhibition of the two main signaling pathways (insulin/PI3K and Wnt) which induced an activation of GSK3 also prevents the induction of LTP [50, 64, 111–113].
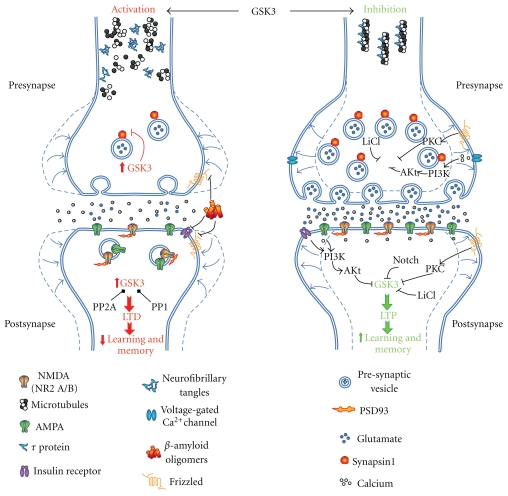
GSK3 has been shown to induce LTD through presynaptic and postsynaptic mechanisms. In the presynaptic neuron, upregulation of GSK3 decreases the expression of SynI [91], which has been linked to a decrease in glutamate release [103]. In the postsynaptic neuron, GSK3 activation causes changes in levels of synapse-associated proteins [114, 115], evident as downregulation of the NR2A/B subunits of NMDA receptors and of the scaffolding protein postsynaptic density 93 (PSD93) [24, 91]. In addition, a transient activation of NMDA receptors and endocytosis of AMPA receptors occurs [116, 117], leading to the loss of GSK3 inhibition due to insufficient Ca2+ entry. This GSK3 inhibition is mediated by NMDA-PI3K-Akt signaling [112, 118]. Over-activity of GSK3 may also induce MT destabilization in dendrites and axons [80, 86, 119] (Figure 3).
Figure 3.
Schematic representation of pre- and postsynaptic mechanisms involved in neuronal plasticity and the role of GSK3. In the presynapse GSK3 activity decreases the expression of SynI reducing the release of glutamate while in postsynapses GSK3 transiently activates NMDA receptors leading to endocytosis of AMPA receptors and reduces the levels of PSD93 protein, favoring LTD. In contrast, Wnt and PI3K signaling pathways or pharmacological inhibition of GSK3 by LiCl supports the induction of LTP, facilitating learning and memory. GSK3 inhibition is also involved in axon and dendritic widening in both pre- and postsynaptic sites. Serine/threonine phosphatases PP1 and PP2A can activate GSK3 regulating phosphor-GSK3 levels through its dephosphorylation. GSK3 is important in the modulation of multiple signaling pathways including Notch pathway that plays an important role in different developmental processes. In AD, amyloid-β oligomers inhibit Wnt and insulin signaling pathways leading to activation of GSK3. In addition, GSK3 overactivation mediates τ hyperphosphorylation and microtubule destabilization.
Overexpression of GSK3β in mice prevents the induction of LTP [100] and causes spatial memory deficits [120]. These data suggest that GSK3β plays an essential role in memory formation through three general processes: (i) phosphorylation of substrates involved in synaptic remodeling, necessary for the establishment of new connections, (ii) turnover of cytoskeletal proteins such as MAPs, actin, and tubulin, promoting disassembly, a condition required for a proper synaptic reorganization, and (iii) involvement in the two major forms of synaptic plasticity in the brain, LTP, and LTD [121].
In summary, the functional consequence of GSK3 overactivation in mature neurons is inhibition of LTP and induction of LTD [101, 121], which could be linked to deficiencies of memory and learning characteristic of some neurological diseases, such as AD.
5. GSK3 and Alzheimer's Disease
AD represents a serious epidemiological problem, as it is now recognized as the most common age-related neurodegenerative disease. Evidence supports a role for GSK3 in producing some of the characteristic hallmarks of AD: extracellular accumulation of amyloid-β protein (Aβ) and intraneuronal neurofibrillary tangles (NFTs) composed of hyperphosphorylated forms of tau and inflammatory markers [122]. All of these effects contribute to synaptic and neuronal loss and memory decline [123, 124].
It has been proposed that overactivation of GSK3 in AD leads to inhibition of LTP and may partially explain the learning and memory deficits present early in this neurodegenerative disorder. On the other hand, changes in GSK3 activity may be a molecular link between the two main histopathological markers: Aβ overproduction and tau hyperphosphorylation [39, 46, 125, 126].
The NFTs that accumulate in AD are anomalous filamentous structures composed mainly of abnormal, hyperphosphorylated forms of tau protein [127]. Hence, numerous studies have focused on identification of the protein kinases and phosphatases regulating tau phosphorylation in vivo. GSK3β was recognized as a primary kinase involved in tau phosphorylation, as was apparent from the first studies that termed it tau protein kinase-I [128]. Thus, GSK3β has been identified as one of the major enzymes mediating tau hyperphosphorylation at the residues implicated in neurodegenerative tauopathies, including AD [129].
Normally, tau protein contains a total of 85 phosphorylable sites: 45 Ser, 35 Thr, and 5 Tyr. Of these, 40 have been identified as phosphorylated in insoluble tau in AD brain: 28 Ser, 10 Thr, and 2 Tyr, and GSK3β can phosphorylate 23 of these sites [130]. Although GSK3β commonly needs priming phosphorylation of tau, three sites were recently found that can be phosphorylated by GSK3β alone, without priming: Ser396, Ser400, and Ser404 [131]. Furthermore, initial phosphorylation of the Ser214 by cAMP-dependent protein kinase was shown to lead to the rapid modification of four additional sites by GSK3β [131]. Studies in transgenic mouse models have shown that overexpression of GSK3β results in neurodegeneration and have unequivocally demonstrated that GSK3β phosphorylates tau in AD-related phospho-epitopes in vivo [93, 132, 133]. Moreover, co-overexpression of tau and GSK3β synergistically increased tau phosphorylation and induced neuronal death in a transgenic model in Drosophila [134] while GSK3 inhibition reduces the phosphorylation and aggregation of tau [135, 136]. Similarly, tau hyperphosphorylation and neurodegeneration after GSK3β overexpression are exacerbated by co-overexpression of tau with mutations characteristic of frontotemporal dementia with parkinsonism, associated with chromosome 17 (FDTP-17). This study also showed that tauopathy progression could be prevented by administration of a GSK3β inhibitor at the first signs of pathology [133]. Tau knockout mice overexpressing GSK3β show reduced hippocampal degeneration, indicating that tau partially contributes to the pathology observed in mouse brain [39]. Finally, GSK3β inhibitors decrease tau phosphorylation and amyloid deposition in a double transgenic mouse model coexpressing human mutant amyloid precursor protein (APP) and tau [137]. In brains of AD patients, GSK3β colocalizes with NFT [138], and active GSK3β is present in neuronal cytoplasm of neurons with tangle-like inclusions when abnormal tau hyperphosphorylation begins [139]. In fact, polymorphisms in GSK3 were recently reported to be risk factors for late-onset AD [140, 141].
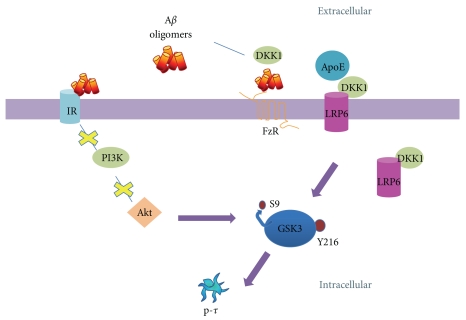
Evidence suggests that GSK3β regulates APP processing [126, 142], leading to increased production of Aβ. Neuronal exposure to Aβ increases GSK3β activity through PI3K inhibition [143], causing a positive feedback loop. Aβ peptide can regulate GSK3 activity, acting as an insulin receptor antagonist and preventing activation of PI3K and Akt. In turn, the absence of activated Akt prevents the inhibitory phosphorylation of GSK3, increasing its activity [144]. Aβ seems to interfere with the Wnt canonical pathway as well, leading to increased GSK3 activity [145]. Thus, deregulation of GSK3 in AD might be due, in part, to alterations in insulin and Wnt signaling. In the canonical Wnt signaling pathway, the gene for LRP6 coreceptor has been identified as a risk factor for late-onset AD in ApoE4-negative individuals [146]. Interestingly, it has been suggested that the Wnt pathway might be inhibited by ApoE protein, which likely binds to the coreceptor LRP5/6 [147]. Moreover, the ApoE4, implicated in sporadic AD [148], may activate GSK3 [46, 149].
Wnt dysregulation has also been implicated in AD. For example, protein Dickkopf-1 negatively modulates the canonical Wnt signaling pathway and thus activates GSK3. DKK1 colocalizes with NFT and dystrophic neurites in degenerating neurons of AD brains [150]. Moreover, using Wnt and PI3K signaling inhibitors, cultured cortical neurons have shown increased tau phosphorylation and morphological changes mediated by GSK3β [151]. Taken together, this evidence suggests an important role for GSK3 in AD and supports the notion that GSK3 could be the link between amyloid and tau pathology [46] (Figure 4).
Figure 4.
Proposed model of GSK3 activation by amyloid-β protein in AD. Amyloid-β oligomers bind to the insulin receptor and inhibit PI3K/Akt pathway, and Akt is unable to phosphorylate and inactivate GSK3. Aβ also induces the expression of DKK1, which internalizes LRP6 receptor and inhibits Wnt signaling leading to GSK3 activation. Aβ can bind to Frizzled receptor (FzR) and inactivate Wnt signaling as well. ApoE also inhibits this signaling pathway and activates GSK3. Tau hyperphosphorylation and NFT formation may result from GSK3 overactivation.
6. Concluding Remarks
GSK3 has attracted a great deal of interest due to the myriad of processes it controls. GSK3 is implicated in many fundamental functions, ranging from bioenergetics to developmental and plasticity events, particularly in the brain. Altered GSK3 activity in the brain negatively influences neuronal structure, which in turn may affect maintenance of neuronal circuits that support cognitive function. The use of therapeutic drugs to control GSK3 activity has been hampered by the variety of substrates targeted by this enzyme and the long-term ramifications of its downstream signaling. Future studies could focus on identifying spatiotemporal expression patterns of specific GSK3 isoforms in the brain with the goal of developing specific inhibitors for clinical use in devastating neurological diseases, such as AD.
Acknowledgments
This work was supported by PAPIIT IN219509-3. P. Salcedo-Tello was supported by CONACYT 220709.
References
- 1.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Current Biology. 1996;6(12):1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 2.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochemical Journal. 2001;359(1):1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. European Journal of Biochemistry. 1980;107(2):519–527. [PubMed] [Google Scholar]
- 4.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/Factor A. EMBO Journal. 1990;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochimica et Biophysica Acta. 1992;1114(2-3):147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 6.Mukai F, Ishiguro K, Sano Y, Fujita SC. Aternative splicing isoform of tau protein kinase I/glycogen synthase kinase 3β. Journal of Neurochemistry. 2002;81(5):1073–1083. doi: 10.1046/j.1471-4159.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 7.Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions, and regulation. Chemical Reviews. 2001;101(8):2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P, Frame S. The renaissance of GSK3. Nature Reviews Molecular Cell Biology. 2001;2(10):769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 9.Woodgett JR. Judging a protein by more than its name: GSK-3. Science"s STKE. 2001;2001(100):p. RE12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- 10.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nature Reviews Drug Discovery. 2004;3(6):479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 11.Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO Journal. 1993;12(2):803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. Glycogen synthase kinase-3β is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. Journal of Biological Chemistry. 1994;269(20):14566–14574. [PubMed] [Google Scholar]
- 13.Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, Cleghone V. A chaperone-dependent GSK3β transitional intermediate mediates activation-loop autophosphorylation. Molecular Cell. 2006;24(4):627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Hartigan JA, Xiong WC, Johnson GVW. Glycogen synthase kinase 3β is tyrosine phosphorylated by PYK2. Biochemical and Biophysical Research Communications. 2001;284(2):485–489. doi: 10.1006/bbrc.2001.4986. [DOI] [PubMed] [Google Scholar]
- 15.Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochemical Journal. 2004;377(1):249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jope RS, Johnson GVW. The glamour and gloom of glycogen synthase kinase-3. Trends in Biochemical Sciences. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Thornton TM, Pedraza-Alva G, Deng B, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3β inactivation. Science. 2008;320(5876):667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. Journal of Cell Science. 2006;119(3):395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 19.Goñi-Oliver P, Lucas JJ, Avila J, Hernández F. N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation. Journal of Biological Chemistry. 2007;282(31):22406–22413. doi: 10.1074/jbc.M702793200. [DOI] [PubMed] [Google Scholar]
- 20.Bijur GN, Jope RS. Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport. 2003;14(18):2415–2419. doi: 10.1097/00001756-200312190-00025. [DOI] [PubMed] [Google Scholar]
- 21.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. British Journal of Pharmacology. 2008;153(1):S137–S153. doi: 10.1038/sj.bjp.0707659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández F, de Barreda EG, Fuster-Matanzo A, Goñi-Oliver P, Lucas JJ, Avila J. The role of GSK3 in Alzheimer disease. Brain Research Bulletin. 2009;80(4-5):248–250. doi: 10.1016/j.brainresbull.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Yao HB, Shaw PC, Wong CC, Wan DCC. Expression of glycogen synthase kinase-3 isoforms in mouse tissues and their transcription in the brain. Journal of Chemical Neuroanatomy. 2002;23(4):291–297. doi: 10.1016/s0891-0618(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 24.Giese KP. GSK-3: a key player in neurodegeneration and memory. IUBMB Life. 2009;61(5):516–521. doi: 10.1002/iub.187. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Chung YH, Joo KM, et al. Age-related changes in glycogen synthase kinase 3β (GSK3β) immunoreactivity in the central nervous system of rats. Neuroscience Letters. 2006;409(2):134–139. doi: 10.1016/j.neulet.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Tomizawa K, Kato R, et al. Localization and developmental changes of τ protein kinase I/glycogen synthase kinase-3β in rat brain. Journal of Neurochemistry. 1994;63(1):245–255. doi: 10.1046/j.1471-4159.1994.63010245.x. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M, Tomizawa K, Ishiguro K. Distribution of tau protein kinase I/glycogen synthase kinase-3β, phosphatases 2A and 2B, and phosphorylated tau in the developing rat brain. Brain Research. 2000;857(1-2):193–206. doi: 10.1016/s0006-8993(99)02424-5. [DOI] [PubMed] [Google Scholar]
- 28.Leroy K, Brion JP. Developmental expression and localization of glycogen synthase kinase-3β in rat brain. Journal of Chemical Neuroanatomy. 1999;16(4):279–293. doi: 10.1016/s0891-0618(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 29.Soutar MPM, Kim W-Y, Williamson R, et al. Evidence that glycogen synthase kinase-3 isoforms have distinct substrate preference in the brain. Journal of Neurochemistry. 2010;115(4):974–983. doi: 10.1111/j.1471-4159.2010.06988.x. [DOI] [PubMed] [Google Scholar]
- 30.Goold RG, Gordon-Weeks PR. Glycogen synthase kinase 3β and the regulation of axon growth. Biochemical Society Transactions. 2004;32(5):809–811. doi: 10.1042/BST0320809. [DOI] [PubMed] [Google Scholar]
- 31.Trivedi N, Marsh P, Goold RG, Wood-Kaczmar A, Gordon-Weeks PR. Glycogen synthase kinase-3β phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. Journal of Cell Science. 2005;118(5):993–1005. doi: 10.1242/jcs.01697. [DOI] [PubMed] [Google Scholar]
- 32.Castaño Z, Gordon-Weeks PR, Kypta RM. The neuron-specific isoform of glycogen synthase kinase-3β is required for axon growth. Journal of Neurochemistry. 2010;113(1):117–130. doi: 10.1111/j.1471-4159.2010.06581.x. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien WT, Harper AD, Jové F, et al. Glycogen synthase kinase-3β haploinsufficiency mimics the behavioral and molecular effects of lithium. Journal of Neuroscience. 2004;24(30):6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaulieu JM, Zhang X, Rodriguiz RM, et al. Role of GSK3β in behavioral abnormalities induced by serotonin deficiency. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(4):1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura T, Yamashita S, Nakao S, et al. GSK-3β is required for memory reconsolidation in adult brain. PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003540. Article ID e3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacAulay K, Doble BW, Patel S, et al. Glycogen synthase kinase 3α-specific regulation of murine hepatic glycogen metabolism. Cell Metabolism. 2007;6(4):329–337. doi: 10.1016/j.cmet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Kaidanovich-Beilin O, Lipina TV, Takao K, et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Molecular Brain. 2009;2(1, article no. 35) doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prickaerts J, Moechars D, Cryns K, et al. Transgenic mice overexpressing glycogen synthase kinase 3β: a putative model of hyperactivity and mania. Journal of Neuroscience. 2006;26(35):9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Barreda EG, Pérez M, Gómez-Ramos P, et al. Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiology of Disease. 2010;37(3):622–629. doi: 10.1016/j.nbd.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Zhou FQ, Snider WD. GSK-3β and microtubule assembly in axons. Science. 2005;308(5719):211–214. doi: 10.1126/science.1110301. [DOI] [PubMed] [Google Scholar]
- 41.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annual Review of Pharmacology and Toxicology. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 42.Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric disease and therapeutic interventions. Current Drug Targets. 2006;7(11):1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nature Reviews Drug Discovery. 2007;6(6):464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 44.Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends in Neurosciences. 2007;30(4):142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. British Journal of Pharmacology. 2009;156(6):885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernández F, Gómez de Barreda E, Fuster-Matanzo A, Lucas JJ, Avila J. GSK3: a possible link between beta amyloid peptide and tau protein. Experimental Neurology. 2010;223(2):322–325. doi: 10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Avila J, Wandosell F, Hernández F. Role of glycogen synthase kinase-3 in Alzheimer’s disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Review of Neurotherapeutics. 2010;10(5):703–710. doi: 10.1586/ern.10.40. [DOI] [PubMed] [Google Scholar]
- 48.Rodgers EE, Theibert AB. Functions of PI 3-kinase in development of the nervous system. International Journal of Developmental Neuroscience. 2002;20(3–5):187–197. doi: 10.1016/s0736-5748(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 49.Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Current Topics in Microbiology and Immunology. 2010;346:143–169. doi: 10.1007/82_2010_56. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Chang SP, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. Journal of Biological Chemistry. 2006;281(17):11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 51.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends in Neurosciences. 2007;30(6):268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farías GG, Godoy JA, Cerpa W, Varela-Nallar L, Inestrosa NC. Wnt signaling modulates pre- and postsynaptic maturation: therapeutic considerations. Developmental Dynamics. 2010;239(1):94–101. doi: 10.1002/dvdy.22065. [DOI] [PubMed] [Google Scholar]
- 53.Hur E-M, Zhou F-Q. GSK3 signalling in neural development. Nature Reviews Neuroscience. 2010;11(8):539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Ferrari and GV, Inestrosa NC. Wnt signaling function in Alzheimer’s disease. Brain Research Reviews. 2000;33(1):1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 55.Wehrli M, Dougan ST, Caldwell K, et al. Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407(6803):527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 56.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO Journal. 1998;17(5):1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Noort M, Clevers H. TCF transcription factors, mediators of Wnt-signaling in development and cancer. Developmental Biology. 2002;244(1):1–8. doi: 10.1006/dbio.2001.0566. [DOI] [PubMed] [Google Scholar]
- 58.Taelman VF, Dobrowolski R, Plouhinec J-L, et al. Wnt signaling requires sequestration of Glycogen Synthase Kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. Journal of Cell Science. 2003;116(13):2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 60.Mao Y, Ge X, Frank CL, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nature Reviews Neuroscience. 2008;9(6):437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mckenzie G, Ward G, Stallwood Y, et al. Cellular notch responsiveness is defined by phosphoinositide 3-kinase-dependent-signals. BMC Cell Biology. 2006;7, article no. 10 doi: 10.1186/1471-2121-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Chan SL, Miele L, et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(25):9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon-Weeks PR. Microtubules and growth cone function. Journal of Neurobiology. 2004;58(1):70–83. doi: 10.1002/neu.10266. [DOI] [PubMed] [Google Scholar]
- 66.Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3β leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. Journal of Cell Science. 1998;111(10):1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 67.Goold RG, Owen R, Gordon-Weeks PR. Glycogen synthase kinase 3β phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. Journal of Cell Science. 1999;112(19):3373–3384. doi: 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- 68.Goold RG, Gordon-Weeks PR. The MAP kinase pathway is upstream of the activation of GSK3β that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Molecular and Cellular Neuroscience. 2005;28(3):524–534. doi: 10.1016/j.mcn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 69.González-Billault C, Del Río JA, Ureña JM, et al. A role of MAP1B in reelin-dependent neuronal migration. Cerebral Cortex. 2005;15(8):1134–1145. doi: 10.1093/cercor/bhh213. [DOI] [PubMed] [Google Scholar]
- 70.Kim WY, Zhou FQ, Zhou J, et al. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron. 2006;52(6):981–996. doi: 10.1016/j.neuron.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nature Reviews Neuroscience. 2009;10(5):319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 72.Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. Journal of Cell Science. 2009;122(20):3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eickholt BJ, Walsh FS, Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. Journal of Cell Biology. 2002;157(2):211–217. doi: 10.1083/jcb.200201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3β are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Current Biology. 2004;14(22):2025–2032. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Jiang H, Guo W, Liang X, Rao YI. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3β and its upstream regulators. Cell. 2005;120(1):123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 76.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120(1):137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Krylova O, Herreros J, Cleverley KE, et al. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35(6):1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 78.Lyuksyutova AI, Lu CC, Milanesio N, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302(5652):1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 79.Owen R, Gordon-Weeks PR. Inhibition of glycogen synthase kinase 3β in sensory neurons in culture alters filopodia dynamics and microtubule distribution in growth cones. Molecular and Cellular Neuroscience. 2003;23(4):626–637. doi: 10.1016/s1044-7431(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 80.Krylova O, Messenger MJ, Salinas PC. Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3β. Journal of Cell Biology. 2000;151(1):83–93. doi: 10.1083/jcb.151.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmad-Annuar A, Ciani L, Simeonidis I, et al. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. Journal of Cell Biology. 2006;174(1):127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Endo Y, Rubin JS. Wnt signaling and neurite outgrowth: insights and questions. Cancer Science. 2007;98(9):1311–1317. doi: 10.1111/j.1349-7006.2007.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cellular Signalling. 2010;22(5):717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 84.Salinas PC. Retrograde signalling at the synapse: a role for Wnt proteins. Biochemical Society Transactions. 2005;33(6):1295–1298. doi: 10.1042/BST0331295. [DOI] [PubMed] [Google Scholar]
- 85.Salinas PC. Wnt factors in axonal remodelling and synaptogenesis. Biochemical Society Symposium. 1999;65:101–109. [PubMed] [Google Scholar]
- 86.Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. Journal of Cell Biology. 2004;164(2):243–253. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100(5):525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 88.Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Developmental Biology. 1997;192(1):31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- 89.Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(20):9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosahl TW, Spillane D, Missler M, et al. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375(6531):488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- 91.Zhu L-Q, Wang S-H, Liu D, et al. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. Journal of Neuroscience. 2007;27(45):12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis EK, Zou Y, Ghosh A. Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Development. 2008;3(1, article no. 32) doi: 10.1186/1749-8104-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. Journal of Biological Chemistry. 2006;281(35):25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- 94.Clayton EL, Sue N, Smillie KJ, et al. Dynamin i phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nature Neuroscience. 2010;13(7):845–851. doi: 10.1038/nn.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pittenger C, Kandel E. A genetic switch for long-term memory. Comptes Rendus de l’Academie des Sciences - Serie III. 1998;321(2-3):91–96. doi: 10.1016/s0764-4469(97)89807-1. [DOI] [PubMed] [Google Scholar]
- 96.Huang EP. Synaptic plasticity: going through phases with LTP. Current Biology. 1998;8(10):R350–R352. doi: 10.1016/s0960-9822(98)70219-2. [DOI] [PubMed] [Google Scholar]
- 97.Stanton PK. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6(1):35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 98.Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philosophical Transactions of the Royal Society B. 2003;358(1432):829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 100.Hooper C, Markevich V, Plattner F, et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. European Journal of Neuroscience. 2007;25(1):81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 101.Peineau S, Taghibiglou C, Bradley C, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53(5):703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 102.Cai F, Wang F, Lin FK, et al. Redox modulation of long-term potentiation in the hippocampus via regulation of the glycogen synthase kinase-3β pathway. Free Radical Biology and Medicine. 2008;45(7):964–970. doi: 10.1016/j.freeradbiomed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 103.Nichols RA, Chilcote TJ, Czernik AJ, Greengard P. Synapsin I regulates glutamate release from rat brain synaptosomes. Journal of Neurochemistry. 1992;58(2):783–785. doi: 10.1111/j.1471-4159.1992.tb09788.x. [DOI] [PubMed] [Google Scholar]
- 104.Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259(5096):780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- 105.Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 1995;375(6531):493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- 106.Hilfiker S, Pieribone VA, Czernik AJ, Kao H-T, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philosophical Transactions of the Royal Society B. 1999;354(1381):269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nature Neuroscience. 2001;4(12):1187–1193. doi: 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- 108.Bloom O, Evergren E, Tomilin N, et al. Colocalization of synapsin and actin during synaptic vesicle recycling. Journal of Cell Biology. 2003;161(4):737–747. doi: 10.1083/jcb.200212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Melloni RH, Jr., Hemmendinger LM, Hamos JE, DeGennaro LJ. Synapsin I gene expression in the adult rat brain with comparative analysis of mRNA and protein in the hippocampus. Journal of Comparative Neurology. 1993;327(4):507–520. doi: 10.1002/cne.903270404. [DOI] [PubMed] [Google Scholar]
- 110.Sato K, Morimoto K, Suemaru S, Sato T, Yamada N. Increased synapsin I immunoreactivity during long-term potentiation in rat hippocampus. Brain Research. 2000;872(1-2):219–222. doi: 10.1016/s0006-8993(00)02460-4. [DOI] [PubMed] [Google Scholar]
- 111.Bortolotto ZA, Collingridge GL. A role for protein kinase C in a form of metaplasticity that regulates the induction of long-term potentiation at CA1 synapses of the adult rat hippocampus. European Journal of Neuroscience. 2000;12(11):4055–4062. doi: 10.1046/j.1460-9568.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 112.Sanna PP, Cammalleri M, Berton F, et al. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of longterm potentiation in the hippocampal CA1 region. Journal of Neuroscience. 2002;22(9):3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Opazo P, Watabe AM, Grant SGN, O'Dell TJ. Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. Journal of Neuroscience. 2003;23(9):3679–3688. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gardoni F, Caputi A, Cimino M, Pastorino L, Cattabeni F, Di Luca M. Calcium/calmodulin-dependent protein kinase II is associated with NR2A/B subunits of NMDA receptor in postsynaptic densities. Journal of Neurochemistry. 1998;71(4):1733–1741. doi: 10.1046/j.1471-4159.1998.71041733.x. [DOI] [PubMed] [Google Scholar]
- 115.Teter B, Ashford JW. Neuroplasticity in Alzheimer’s disease. Journal of Neuroscience Research. 2002;70(3):402–437. doi: 10.1002/jnr.10441. [DOI] [PubMed] [Google Scholar]
- 116.Beattie EC, Carroll RC, Yu X, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature Neuroscience. 2000;3(12):1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 117.Collingridge GL, Isaac JTR, Yu TW. Receptor trafficking and synaptic plasticity. Nature Reviews Neuroscience. 2004;5(12):952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 118.Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(23):9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Purro SA, Ciani L, Hoyos-Flight M, Stamatakou E, Siomou E, Salinas PC. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. Journal of Neuroscience. 2008;28(34):8644–8654. doi: 10.1523/JNEUROSCI.2320-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hernández F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3β in the brain but do not form tau filaments. Journal of Neurochemistry. 2002;83(6):1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- 121.Peineau S, Bradley C, Taghibiglou C, et al. The role of GSK-3 in synaptic plasticity. British Journal of Pharmacology. 2008;153(supplement 1):S428–S437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. Journal of Neurochemistry. 2008;104(6):1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diaz A, Mendieta L, Zenteno E, Guevara J, Limon ID. The role of NOS in the impairment of spatial memory and damaged neurons in rats injected with amyloid beta 25–35 into the temporal cortex. Pharmacology Biochemistry and Behavior. 2011;98(1):67–75. doi: 10.1016/j.pbb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 124.Sydow A, Van Der Jeugd A, Zheng F, et al. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic tau mutant. Journal of Neuroscience. 2011;31(7):2511–2525. doi: 10.1523/JNEUROSCI.5245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun X, Sato S, Murayama O, et al. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neuroscience Letters. 2002;321(1-2):61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- 126.Phiel CJ, Wilson CA, Lee VMY, Klein PS. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature. 2003;423(6938):435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 127.Iqbal K, Grundke-Iqbal I. Discoveries of Tau, abnormally hyperphosphorylated tau and others of neurofibrillary degeneration: a personal historical perspective. Journal of Alzheimer’s Disease. 2006;9(3):219–242. doi: 10.3233/jad-2006-9s325. [DOI] [PubMed] [Google Scholar]
- 128.Ishiguro K, Shiratsuchi A, Sato S, et al. Glycogen synthase kinase 3β is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Letters. 1993;325(3):167–172. doi: 10.1016/0014-5793(93)81066-9. [DOI] [PubMed] [Google Scholar]
- 129.Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. Journal of Neuropathology and Experimental Neurology. 1997;56(1):70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 130.Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends in Molecular Medicine. 2009;15(3):112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 131.Leroy A, Landrieu I, Huvent I. Spectroscopic studies of GSK3β phosphorylation of the neuronal Tau protein and its interaction with the N-terminal domain of apolipoprotein E. Journal of Biological Chemistry. 2010;285(43):33435–33444. doi: 10.1074/jbc.M110.149419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lucas JJ, Hernández F, Gómez-Ramos P, Morán MA, Hen R, Avila J. Decreased nuclear β-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3β conditional transgenic mice. EMBO Journal. 2001;20(1-2):27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Engel T, Goñi-Oliver P, Lucas JJ, Avila J, Hernández F. Chronic lithium administration to FTDP-17 tau and GSK-3β overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. Journal of Neurochemistry. 2006;99(6):1445–1455. doi: 10.1111/j.1471-4159.2006.04139.x. [DOI] [PubMed] [Google Scholar]
- 134.Jackson GR, Wiedau-Pazos M, Sang TK, et al. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34(4):509–519. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- 135.Pérez M, Hernández F, Lim F, Díaz-Nido J, Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. Journal of Alzheimer’s Disease. 2003;5(4):301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- 136.Noble W, Planel E, Zehr C, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(19):6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serenó L, Coma M, Rodríguez M, et al. A novel GSK-3β inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiology of Disease. 2009;35(3):359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 138.Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere CA, Imahori K. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3β and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathologica. 1996;92(3):232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 139.Pei JJ, Braak E, Braak H, et al. Distribution of active glycogen synthase kinase 3β (GSK-3β) in brains staged for Alzheimer disease neurofibrillary changes. Journal of Neuropathology and Experimental Neurology. 1999;58(9):1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 140.Mateo I, Infante J, Llorca J, Rodríguez E, Berciano J, Combarros O. Association between glycogen synthase kinase-3β genetic polymorphism and late-onset Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2006;21(4):228–232. doi: 10.1159/000091044. [DOI] [PubMed] [Google Scholar]
- 141.Schaffer BAJ, Bertram L, Miller BL, et al. Association of GSK3B with Alzheimer disease and frontotemporal dementia. Archives of Neurology. 2008;65(10):1368–1374. doi: 10.1001/archneur.65.10.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Su Y, Ryder J, Li B, et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-β precursor protein processing. Biochemistry. 2004;43(22):6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- 143.Takashima A, Noguchi K, Michel G, et al. Exposure of rat hippocampal neurons to amyloid β peptide (25-35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3β. Neuroscience Letters. 1996;203(1):33–36. doi: 10.1016/0304-3940(95)12257-5. [DOI] [PubMed] [Google Scholar]
- 144.Townsend M, Mehta T, Selkoe DJ. Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. Journal of Biological Chemistry. 2007;282(46):33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 145.Magdesian MH, Carvalho MMVF, Mendes FA, et al. Amyloid-β binds to the extracellular cysteine-rich domain of frizzled and inhibits Wnt/β-catenin signaling. Journal of Biological Chemistry. 2008;283(14):9359–9368. doi: 10.1074/jbc.M707108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De Ferrari GV, Papassotiropoulos A, Biechele T, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(22):9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Caruso A, Motolese M, Iacovelli L, et al. Inhibition of the canonical Wnt signaling pathway by apolipoprotein E4 in PC12 cells. Journal of Neurochemistry. 2006;98(2):364–371. doi: 10.1111/j.1471-4159.2006.03867.x. [DOI] [PubMed] [Google Scholar]
- 148.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cedazo-Mínguez A, Popescu BO, Blanco-Millán JM, et al. Apolipoprotein E and β-amyloid (1–42) regulation of glycogen synthase kinase-3β. Journal of Neurochemistry. 2003;87(5):1152–1164. doi: 10.1046/j.1471-4159.2003.02088.x. [DOI] [PubMed] [Google Scholar]
- 150.Caricasole A, Copani A, Caraci F, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. Journal of Neuroscience. 2004;24(26):6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mercado-Gómez O, Hernández-Fonseca K, Villavicencio-Queijeiro A, Massieu L, Chimal-Monroy J, Arias C. Inhibition of Wnt and PI3K signaling modulates GSK-3β activity and induces morphological changes in cortical neurons: role of tau phosphorylation. Neurochemical Research. 2008;33(8):1599–1609. doi: 10.1007/s11064-008-9714-9. [DOI] [PubMed] [Google Scholar]