Summary
Background
Elderly and frail patients with cancer, although often treated with chemotherapy, are under-represented in clinical trials. We designed FOCUS2 to investigate reduced-dose chemotherapy options and to seek objective predictors of outcome in frail patients with advanced colorectal cancer.
Methods
We undertook an open, 2 × 2 factorial trial in 61 UK centres for patients with previously untreated advanced colorectal cancer who were considered unfit for full-dose chemotherapy. After comprehensive health assessment (CHA), patients were randomly assigned by minimisation to: 48-h intravenous fluorouracil with levofolinate (group A); oxaliplatin and fluorouracil (group B); capecitabine (group C); or oxaliplatin and capecitabine (group D). Treatment allocation was not masked. Starting doses were 80% of standard doses, with discretionary escalation to full dose after 6 weeks. The two primary outcome measures were: addition of oxaliplatin ([A vs B] + [C vs D]), assessed with progression-free survival (PFS); and substitution of fluorouracil with capecitabine ([A vs C] + [B vs D]), assessed by change from baseline to 12 weeks in global quality of life (QoL). Analysis was by intention to treat. Baseline clinical and CHA data were modelled against outcomes with a novel composite measure, overall treatment utility (OTU). This study is registered, number ISRCTN21221452.
Findings
459 patients were randomly assigned (115 to each of groups A–C, 114 to group D). Factorial comparison of addition of oxaliplatin versus no addition suggested some improvement in PFS, but the finding was not significant (median 5·8 months [IQR 3·3–7·5] vs 4·5 months [2·8–6·4]; hazard ratio 0·84, 95% CI 0·69–1·01, p=0·07). Replacement of fluorouracil with capecitabine did not improve global QoL: 69 of 124 (56%) patients receiving fluorouracil reported improvement in global QoL compared with 69 of 123 (56%) receiving capecitabine. The risk of having any grade 3 or worse toxic effect was not significantly increased with oxaliplatin (83/219 [38%] vs 70/221 [32%]; p=0·17), but was higher with capecitabine than with fluorouracil (88/222 [40%] vs 65/218 [30%]; p=0·03). In multivariable analysis, fewer baseline symptoms (odds ratio 1·32, 95% CI 1·14–1·52), less widespread disease (1·51, 1·05–2·19), and use of oxaliplatin (0·57, 0·39–0·82) were predictive of better OTU.
Interpretation
FOCUS2 shows that with an appropriate design, including reduced starting doses of chemotherapy, frail and elderly patients can participate in a randomised controlled trial. On balance, a combination including oxaliplatin was preferable to single-agent fluoropyrimidines, although the primary endpoint of PFS was not met. Capecitabine did not improve QoL compared with fluorouracil. Comprehensive baseline assessment holds promise as an objective predictor of treatment benefit.
Funding
Cancer Research UK and the Medical Research Council.
Introduction
Advanced colorectal cancer is the second most common cause of death from cancer in developed countries, after lung cancer.1,2 In the UK, the median age at death from advanced colorectal cancer is 77 years, with 60% of deaths occurring in patients older than 75 years and 42% in those older than 80 years.3 Frailty, whether or not related to the cancer diagnosis, is frequent in elderly patients.
Standard treatment for advanced colorectal cancer includes palliative chemotherapy, with an expanding range of treatment options. But the evidence supporting these treatments is from clinical trials that under-represented elderly, frail, and especially frail elderly patients.4 Several pivotal trials were restricted to patients younger than 75 years;5–7 however, even without a formal upper age limit there are several impediments to the recruitment of elderly participants.8 Reports of outcomes in older4 or frailer9 patient subsets within these trials, although interesting, are limited by the fact that the participants were by definition sufficiently robust to have been included in the trials in the first place, whereas many other patients were not.
In 2002, the UK Medical Research Council (MRC) noted that investigators of its first-line trial for advanced colorectal cancer, FOCUS (Fluorouracil, Oxaliplatin, CPT11 [irinotecan]: Use and Sequencing),10 despite permissive entry criteria and no upper age limit, were recruiting patients with a median age of only 64 years. A survey of investigators showed that the 59 trial oncologists who responded, while recruiting 422 patients into FOCUS, had treated a further 715 patients off-trial during the same period, frequently using reduced-dose or single-agent schedules. The most common reasons cited for non-inclusion of technically eligible patients were physicians' concerns about the adverse effects of standard-dose treatments, patients' wishes to avoid toxic effects, and an assumption that oral therapy would improve quality of life (QoL). We therefore designed FOCUS2 for patients with advanced colorectal cancer who were to receive chemotherapy, but for whom the treating oncologist considered standard full-dose regimens to be unsuitable.
Methods
Study design and patients
Three trial design innovations were used to make FOCUS2 suitable for the frail and elderly population to be studied. First, as is common in non-trial practice, cytotoxic drugs were started at below-standard doses; second, a comprehensive geriatric health assessment was used to identify factors that might aid future selection of patients or regimens; third, alongside standard outcome measures a composite measure of overall treatment utility (OTU) was devised, incorporating objective and subjective measures of benefit and harm.
FOCUS2 was undertaken in 61 UK centres, recruiting patients between January, 2004, and July, 2006. To enter FOCUS2, the oncologist had first to confirm, stating reasons, that the patient was in his or her opinion not a candidate for standard full-dose combination therapy. Patients had to have histologically confirmed colorectal adenocarcinoma, with unidimensionally measurable inoperable advanced or metastatic disease, and a WHO performance status of 2 or better. Patients had to have received no previous systemic chemotherapy for metastases. There was no upper or lower age limit. Previous adjuvant chemotherapy was allowed if completed more than 4 months before randomisation; previous rectal chemoradiotherapy was allowed if completed more than 1 month before randomisation. Patients were not excluded for medical comorbidity unless the condition was so severe as to preclude protocol treatment. However, the following criteria were required: white blood cell count 3×109 per L or greater, platelet count 100×109 per L or greater, serum bilirubin no more than three times the upper limit of normal (ULN), serum transaminases no more than 2·5 times ULN, and glomerular filtration rate (GFR) 30 mL per min or greater.
We obtained written consent after verbal explanation and a written information sheet had been given to the patient, with at least 24 h allowed for consideration. Thereafter, but before randomisation, a 117-item comprehensive health assessment (CHA) was done (webappendix pp 1–8). This assessment comprised four nurse-administered modules (physical parameters including timed 20-m walk,11 mini-nutritional assessment,12 mini-mental state examination,13 and medical comorbidity14) and four patient-completed modules (activities of daily living,15 symptoms,16 anxiety or depression,17 and global QoL or health resources18).
FOCUS-2 was approved by national and institutional research ethics committees and undertaken by the MRC Clinical Trials Unit (CTU), with MRC Good Clinical Research Practice,19 and was overseen by an independent Trial Steering Committee. Confidential interim analyses were reviewed every year by an independent Data Monitoring Committee.
Randomisation and masking
Patients were randomly assigned in a 1:1:1:1 ratio by telephone with a computerised algorithm developed and maintained centrally at the MRC CTU. Randomisation was done by use of the method of minimisation stratified by clinician, WHO performance status, status of primary tumour (resected or not), and age. Treatment allocation was not masked.
Procedures
Treatment was started with standard regimens but at 80% of standard cytotoxic drug doses. Group A received levofolinate 175 mg 2-h intravenous infusion, fluorouracil 320 mg/m2 5-min intravenous bolus, and fluorouracil 2240 mg/m2 46-h intravenous infusion. The cycle was repeated every 14 days (FU regimen). This regimen is 80% of the simplified LV5FU2 regimen used in FOCUS.10,20 Group B received levofolinate 175 mg/m2 and oxaliplatin 68 mg/m2 by concurrent 2-h intravenous infusion, fluorouracil 320 mg/m2 5-min intravenous bolus, and fluorouracil 1920 mg/m2 46-h intravenous infusion. The cycle was repeated every 14 days (OxFU regimen). This regimen is 80% of the simplified FOLFOX regimen in FOCUS.10,20 Group C received capecitabine 1000 mg/m2 orally twice per day on days 1–15. The cycle was repeated every 21 days (Cap regimen). This regimen is 80% of the standard licensed schedule. Group D received oxaliplatin 104 mg/m2 2-h intravenous infusion, and capecitabine 800mg/m2 orally twice per day on days 1–15. The cycle was repeated every 21 days (OxCap regimen). This regimen is 80% of the standard XELOX regimen.21 In patients with GFR 30–50 mL per min, oxaliplatin and capecitabine were further reduced by 25%.
Before each cycle, toxicity was scored with common terminology criteria for adverse events (version 3.0). Detailed management of side-effects was specified; briefly, grade 1 and worse effects were treated symptomatically; persisting grade 2 and worse toxicity at day 1 of the next treatment cycle incurred a 1-week delay. Cytotoxic doses were reduced by 20% after two delays, or one delay of 2 weeks or more. If grade 2 or worse transaminitis (>2·5 times ULN) developed during capecitabine therapy, treatment was held until recovery. For grade 3 hyperbilirubinaemia (>3 times ULN), all cytotoxic drugs were reduced by 50%. Oxaliplatin was omitted for persistent grade 2 and worse neurological toxic effects. Compliance with capecitabine was assessed with patient diary cards and tablet returns.
A senior clinician assessment was scheduled after 6 weeks, when doses could be escalated to 100% of standard doses (an increase of 25% of starting doses), provided that no grade 2 or worse non-haematological toxic effects had occurred and that the patient assented. After week 12, radiological response was assessed with Response Evaluation Criteria In Solid Tumors (RECIST) criteria;22 the clinician assessed whether there had been clinical deterioration in the patient; the CHA was repeated (omitting the comorbidity and mental-state modules) and the patient was asked two additional questions: whether their treatment had been worthwhile and how much it had interfered with activities (webappendix pp 9–13).
Thereafter, patients without radiological or clinical evidence of deterioration could continue the same regimen, immediately or after a planned break, with reassessment every 12 weeks. In groups A and C, when progression occurred on the FU or Cap regimens, second-line treatment was considered with the OxFU or OxCap regimens, respectively. Second-line therapy in groups B and D, and third-line therapy in all groups, was at the discretion of the physician.
Statistical analysis
The primary questions in the two factorial comparisons were: does oxaliplatin improve first-line progression-free survival (PFS; [A vs B] + [C vs D])?; and does substitution of fluorouracil with capecitabine improve global QoL ([A vs C] + [B vs D])?
For the first question, PFS was defined as time from randomisation to first progression or death from any cause, assessed by intention to treat. FOCUS2 was designed to detect a 3-month improvement in median PFS from 6 months to 9 months. For 90% power at the two-sided 5% significance level, 460 patients were needed.
For the second question, the primary outcome was QoL improvement. This outcome was defined as any increase between baseline and 12 weeks in the EORTC-QLQ-C30 global QoL subscale, reported as a percentage of patients with baseline and 12-week data. In a previous MRC trial,23 40% of 117 patients reported improved global QoL with this criterion. Paired data from 260 patients (57% of the total) would be sufficient to detect an increase from 40% to 60%, at the two-sided 5% significance level, with 90% power. PFS was a secondary outcome for this comparison.
Secondary outcome measures for both comparisons included response rate (RR), toxic effects, and overall survival (OS). For time-to-event endpoints, Kaplan-Meier curves were produced with patients alive and event-free being censored at the time last seen. Hazard ratios (HRs) and 95% CIs were calculated for each comparison and compared with stratified log-rank tests. RR and toxic effects are reported as percentage of assessable patients and compared with χ2 tests. We compared QoL improvement with the Mann-Whitney test, which allows for non-normality. Tests for heterogeneity were done for time-to-event outcomes and tests of interaction for all other outcomes.
The novel composite measure OTU was devised to reflect whether, from the viewpoint of both patient and clinician and with use of both objective and subjective measures, the treatment had been worthwhile. OTU was scored at 12 weeks (webappendix p 14). Briefly, good OTU indicated no clinical or radiological evidence of disease progression, and no major negative treatment effects in terms of toxicity or patient acceptability. Intermediate OTU signified either clinical deterioration but no negative treatment effect, or a significant negative treatment effect but no clinical deterioration. Poor OTU indicated both clinical deterioration and a major negative treatment effect, or death.
We then investigated whether baseline clinicopathological and CHA data can help to predict the probability of a favourable OTU at 12 weeks. Categorical factors and continuous factors with predefined cutoffs for categories were treated as categorical, with all other variables regarded as continuous. Univariate analyses were first done, with ordinal logistic regression, to assess patients' baseline characteristics and CHA data in relation to the OTU score at 12 weeks. All variables, irrespective of their univariate result, were then included in a multivariable analysis with backward stepwise ordinal logistic regression. Results are displayed with odds ratios (ORs) to show the odds of a worse outcome, with effect size (Z value) and statistical significance (p value).
This study is registered, number ISRCTN21221452.
Role of the funding source
The sponsor of the study was the MRC which, as the parent body of the MRC CTU, was involved in the design, conduct, and analysis of the trial. The manufacturers of the drugs used in the study were not involved in the research. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Results
Figure 1 shows the trial profile. 411 of 459 (90%) patients had died at the time of final analysis. Baseline characteristics were well balanced between groups (table 1). Median age was 74 years (range 35–87), with 199 (43%) patients older than 75 years and 60 (13%) older than 80 years. 98 (21%) patients had WHO performance status of 0, 227 (49%) a performance status of 1, and 134 (29%) a performance status of 2. The reason for inclusion in FOCUS2 instead of a full-dose protocol was cited as frailty in 324 (71%) patients and advanced age in 311 (68%). However, dementia was uncommon: full baseline mini-mental health data were obtained in 387 patients, of whom 374 (96%) scored within the normal range and only two (0·5%) fell within the range associated with dementia.
Figure 1.

Trial profile
FU=simplified LV5FU2 regimen of levofolinate, bolus fluorouracil, and 46-h infusion of fluorouracil, repeated every 2 weeks. OxFU=oxaliplatin plus FU. Cap=capecitabine. OxCap=oxaliplatin plus Cap. OxFp=oxaliplatin plus fluoropyrimidine (either fluorouracil or capecitabine).
Table 1.
Baseline patient characteristics
| Group A (n=115) | Group B (n=115) | Group C (n=115) | Group D (n=114) | Total (n=459) | |
|---|---|---|---|---|---|
| Sex | |||||
| Men | 73 (63%) | 69 (60%) | 68 (59%) | 68 (60%) | 278 (61%) |
| Women | 42 (37%) | 46 (40%) | 47 (41%) | 46 (40%) | 181 (39%) |
| Age (years) | |||||
| Median | 75 | 75 | 73 | 75 | 74 |
| IQR | 71–78 | 71–78 | 69–78 | 70–79 | 70–78 |
| Range | 46–86 | 35–87 | 49–84 | 45–85 | 35–87 |
| WHO performance status | |||||
| 0 | 25 (22%) | 23 (20%) | 23 (20%) | 27 (24%) | 98 (21%) |
| 1 | 58 (50%) | 57 (50%) | 58 (50%) | 54 (47%) | 227 (50%) |
| 2 | 32 (28%) | 35 (30%) | 34 (30%) | 33 (29%) | 134 (29%) |
| Primary tumour site | |||||
| Rectum | 25 (22%) | 30 (26%) | 31 (27%) | 34 (30%) | 120 (26%) |
| Colon | 90 (78%) | 85 (74%) | 84 (73%) | 80 (70%) | 339 (74%) |
| Primary tumour not resected | 39 (34%) | 40 (35%) | 40 (35%) | 40 (35%) | 159 (35%) |
| Metastatic sites | |||||
| No distant metastases | 1 (1%) | 2 (2%) | 1 (1%) | 1 (1%) | 5 (1%) |
| Non-liver metastases | 31 (27%) | 25 (22%) | 30 (26%) | 26 (23%) | 112 (24%) |
| Liver-only metastases | 17 (15%) | 14 (12%) | 14 (12%) | 22 (19%) | 67 (15%) |
| Liver+other metastases | 66 (57%) | 74 (64%) | 70 (61%) | 65 (57%) | 275 (60%) |
| Reason for entering FOCUS2 | |||||
| Advanced age alone | 35 (30%) | 28 (24%) | 37 (32%) | 35 (31%) | 135 (29%) |
| Frailty/patient choice alone | 37 (32%) | 36 (31%) | 35 (30%) | 40 (35%) | 148 (32%) |
| Both age and frailty/choice | 43 (37%) | 51 (44%) | 43 (37%) | 39 (34%) | 176 (38%) |
Data are number (%), unless otherwise indicated.
419 (91%) patients were alive and receiving treatment 6 weeks after starting treatment, and so were eligible for discretionary escalation to 100% of standard doses (table 2). Of these patients, 154 (37%) had dose escalation. Dose escalation was more frequent in patients allocated single agent than combination therapy (p=0·01). Only 60 (14% of all patients starting treatment) sustained the higher dose to 12 weeks (table 2). 146 (33%) patients sustained the 80% standard starting dose to 12 weeks, whereas 215 (49%) needed a further dose reduction or stopped (table 2). Capecitabine compliance, assessed by tablet returns, was greater than 97% in the Cap (group C) and OxCap (group D) regimens (data not shown).
Table 2.
Treatment received
| Group A | Group B | Group C | Group D | ||
|---|---|---|---|---|---|
| Allocated first-line treatment | FU | OxFU | Cap | OxCap | |
| Number allocated | 115 | 115 | 115 | 114 | |
| Number started treatment | 111 | 107 | 111 | 111 | |
| Dose escalation at 6 weeks | |||||
| On study at 6 weeks | 100 | 106 | 107 | 106 | |
| Dose escalated | 47 (47%) | 36 (34%) | 39 (36%) | 32 (30%) | |
| Eligible for escalation but not escalated | 31 (31%) | 41 (39%) | 30 (28%) | 33 (31%) | |
| Not escalated because of toxicity | 16 (16%) | 23 (22%) | 23 (21%) | 38 (36%) | |
| Dose delivery during first 12 weeks of treatment | |||||
| Increased at 6 weeks and sustained | 20 (18%) | 14 (13%) | 11 (10%) | 15 (14%) | |
| Sustained starting dose | 32 (29%) | 32 (30%) | 42 (38%) | 40 (36%) | |
| Further dose reduced or stopped | 57 (51%) | 59 (55%) | 54 (48%) | 46 (41%) | |
| Higher dose than intended* | 2 (2%) | 2 (2%) | 4 (4%) | 10 (9%) | |
Data are number (%), unless otherwise indicated. FU=simplified LV5FU2 regimen of levofolinate, bolus fluorouracil, and 46-h infusion of fluorouracil, repeated every 2 weeks. OxFU=oxaliplatin plus FU. Cap=capecitabine. OxCap=oxaliplatin plus Cap.
These patients had a dose increase earlier than the protocol-specified week 6 escalation point.
At the time of analysis 445 (97%) patients had had a PFS event (figure 2A). PFS, measured by intention to treat, was the primary outcome measure for the factorial comparison of treatment with or without oxaliplatin; this comparison suggested some benefit of oxaliplatin but the finding was not significant (figure 2A; table 3). Factorial comparison of fluorouracil versus capecitabine showed no effect on PFS (figure 2B; table 3).
Figure 2.

Kaplan-Meier curves for PFS and OS for each main effect comparison and hazard ratio plots to show tests for heterogeneity for each factorial comparison
(A) PFS by addition of oxaliplatin. (B) PFS by FU versus Cap. (C) OS by addition of oxaliplatin. (D) OS by FU versus Cap. PFS=progression-free survival. OS=overall survival. FU=simplified LV5FU2 regimen of levofolinate, bolus fluorouracil, and 46-h infusion of fluorouracil, repeated every 2 weeks. OxFU=oxaliplatin plus FU. Cap=capecitabine. OxCap=oxaliplatin plus Cap.
Table 3.
Main outcome measures
|
Individual groups |
Factorial comparisons |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Addition of oxaliplatin [A vs B] + [C vs D] |
Fluorouracil vs capecitabine [A vsC] + [B vs D] |
|||||||||||
| A (FU) | B (OxFU) | C (Cap) | D (OxCap) | No oxaliplatin | With oxaliplatin | p | Fluorouracil based | Capecitabine based | p | |||
| Number allocated | 115 | 115 | 115 | 114 | 230 | 229 | .. | 230 | 229 | .. | ||
| Survival and response | ||||||||||||
| Number started treatment | 111 | 107 | 111 | 111 | 222 | 219 | .. | 218 | 222 | .. | ||
| RECIST response at week 12–14 | ||||||||||||
| Response rate: CR + PR (%)* | 12 (11%) | 41 (38%) | 16 (14%) | 36 (32%) | 28 (13%) | 77 (35%) | <0·0001 | 53 (24%) | 52 (23%) | 0·83 | ||
| Disease control: CR + PR + SD (%) | 51 (46%) | 76 (71%) | 56 (50%) | 72 (65%) | 107 (48%) | 148 (68%) | <0·0001 | 127 (58%) | 128 (58%) | 0·90 | ||
| Median PFS (months; IQR)† | 3·5 (2·8–6·2) | 5·8(3·2–7·6) | 5·2(2·8–6·7) | 5·8(3·3–7·4) | .. | .. | .. | .. | .. | .. | ||
| HR (95% CI) | .. | .. | .. | .. | Reference | 0·84 (0·69–1·01) | 0·07 | Reference | 0·99 (0·82–1·20) | 0·93 | ||
| Median OS (months; IQR) † | 10·1(5·1–17·3) | 10·7(5·7–17·2) | 11·0(5·4–18·0) | 12·4(5·8–18·0) | .. | .. | .. | .. | .. | .. | ||
| HR (95% CI) | .. | .. | .. | .. | Reference | 0·99 (0·81–1·18) | 0·91 | Reference | 0·96 (0·79–1·17) | 0·71 | ||
| Improved QoL at week 12–14 | ||||||||||||
| Complete QoL data | 62 | 62 | 65 | 58 | 127 | 120 | .. | 124 | 123 | .. | ||
| Improved global QoL (%) | 37 (60%) | 32 (52%) | 42 (65%) | 27 (47%) | 79 (62%) | 59 (49%) | 0·04 | 69 (56%) | 69 (56%) | 0·94 | ||
| OTU score at 12 weeks | ||||||||||||
| Assessable for OTU | 109 | 107 | 111 | 111 | 220 | 218 | .. | 216 | 222 | |||
| Good (%) | 38 (35%) | 58 (54%) | 41 (37%) | 45 (41%) | 79 (36%) | 103 (47%) | 0·003‡ | 96 (44%) | 86 (39%) | 0·27‡ | ||
| Intermediate (%) | 37 (34%) | 29 (27%) | 33 (30%) | 41 (37%) | 70 (32%) | 70 (32%) | .. | 66 (31%) | 74 (33%) | .. | ||
| Poor (%) | 34 (31%) | 20 (19%) | 37 (33%) | 25 (23%) | 71 (32%) | 45 (21%) | .. | 54 (25%) | 62 (30%) | .. | ||
FU=simplified LV5FU2 regimen of levofolinate, bolus fluorouracil, and 46-h infusion of fluorouracil, repeated every 2 weeks. OxFU=oxaliplatin plus FU. Cap=capecitabine. OxCap=oxaliplatin plus Cap. RECIST=Response Evaluation Criteria In Solid Tumors. CR=complete response. PR=partial response. SD=stable disease. PFS=progression-free survival. OS=overall survival. QoL=quality of life. OTU=overall treatment utility.
Interaction test: Z=−1·18, p=0·238.
Interaction tests done for time-to-event endpoints (figure 2) and OTU score.
χ2 test for trend.
Paired baseline and 12-week QoL data were available in 247 patients, with similar numbers in each group (table 3). QoL improvement was the primary outcome measure for the factorial comparison of fluorouracil versus capecitabine; this comparison showed no difference between groups, with more than half of assessable patients reporting improved QoL in both groups (table 3). Factorial comparison of treatment with and without oxaliplatin was suggestive of a detrimental effect with oxaliplatin regimens (table 3).
RR was assessed with RECIST criteria but only at 12 weeks after randomisation. In the factorial comparisons, we recorded good evidence that oxaliplatin increased the RR (complete response plus partial response) and the rate of disease control (stable disease, complete response, and partial response; table 3). We noted no evidence that the substitution of fluorouracil with capecitabine had an effect on response or disease control (table 3).
Factorial analysis showed no evidence of OS benefit with first-line oxaliplatin (figure 2C). Similarly, factorial analysis showed no difference in OS between fluorouracil and capecitabine (figure 2D).
440 (96%) patients had complete data for toxic effects. The overall risk of having a grade 3 or worse event during the first 12 weeks ranged from 27% of assessed patients (29 of 109) with the FU regimen to 43% (47 of 110) with the OxCap regimen (table 4). In the factorial comparisons, the use of oxaliplatin did not significantly increase the overall risk of toxic effects, but we noted evidence of increased rates of diarrhoea, neurosensory toxicity, nausea, vomiting, and neutropenia, and a lower rate of hand-foot syndrome compared with no use of oxaliplatin (table 4). Compared with fluorouracil, capecitabine increased the overall risk of a grade 3 or worse event (p=0·03), and was specifically associated with increased rates of nausea, vomiting, diarrhoea, anorexia, and hand-foot syndrome.
Table 4.
Toxic effects, weeks 1–12
| Group A (FU; N=109) | Group B (OxFU; N=109) | Group C (Cap; N=112) | Group D (OxCap; N=110) |
Factorial comparisons |
||||
|---|---|---|---|---|---|---|---|---|
|
Addition of oxaliplatin |
Fluorouracil vs capecitabine |
|||||||
| [A vs B] + [C vs D] | p | [A vs C] + [B vs D] | p | |||||
| Any toxicity | ||||||||
| Grade ≥2 | 84 (77%) | 81 (74%) | 86 (77%) | 94 (86%) | 170 (77%) vs 175 (80%) | 0·45 | 165 (76%) vs 180 (81%) | 0·17 |
| Grade ≥3 | 29 (27%) | 36 (33%) | 41 (37%) | 47 (43%) | 70 (32%) vs 83 (38%) | 0·17 | 65 (30%) vs 88 (40%) | 0·03 |
| Nausea | ||||||||
| Grade ≥2 | 8 (7%) | 17 (16%) | 15 (13%) | 27 (25%) | 23 (10%) vs 44 (44%) | <0·0001 | 25 (12%) vs 42 (19%) | 0·03 |
| Grade ≥3 | 1 (1%) | 2 (2%) | 6 (5%) | 5 (5%) | 7 (3%) vs 7 (3%) | 0·99 | 3 (1%) vs 11 (5%) | 0·03 |
| Vomiting | ||||||||
| Grade ≥2 | 5 (5%) | 13 (12%) | 12 (11%) | 21 (19%) | 17 (8%) vs 34 (16%) | 0·01 | 18 (8%) vs 33 (15%) | 0·03 |
| Grade ≥3 | 1 (1%) | 2 (2%) | 3 (3%) | 3 (3%) | 4 (2%) vs 5 (2%) | 0·73 | 3 (1%) vs 6 (3%) | 0·33 |
| Anorexia | ||||||||
| Grade ≥2 | 12 (11%) | 15 (14%) | 19 (17%) | 26 (24%) | 31 (14%) vs 41 (19%) | 0·18 | 27 (12%) vs 45 (20%) | 0·03 |
| Grade ≥3 | 3 (3%) | 3 (3%) | 6 (5%) | 4 (4%) | 9 (4%) vs 7 (3%) | 0·62 | 6 (3%) vs 10 (5%) | 0·33 |
| Stomatitis | ||||||||
| Grade ≥2 | 12 (11%) | 13 (12%) | 6 (5%) | 12 (11%) | 18 (8%) vs 25 (11%) | 0·25 | 25 (12%) vs 18 (8%) | 0·24 |
| Grade ≥3 | 2 (2%) | 3 (3%) | 1 (1%) | 2 (2%) | 3 (1%) vs 5 (2%) | 0·47 | 5 (2%) vs 3 (1%) | 0·46 |
| Diarrhoea | ||||||||
| Grade ≥2 | 20 (18%) | 21 (19%) | 23 (21%) | 38 (35%) | 43 (20%) vs 59 (27%) | 0·06 | 41 (19%) vs 61 (28%) | 0·03 |
| Grade ≥3 | 5 (5%) | 7 (6%) | 10 (9%) | 20 (18%) | 15 (7%) vs 27 (12%) | 0·05 | 12 (6%) vs 30 (14%) | 0·003 |
| Lethargy | ||||||||
| Grade ≥2 | 41 (38%) | 46 (42%) | 40 (36%) | 47 (43%) | 81 (37%) vs 93 (43%) | 0·21 | 89 (40%) vs 87 (39%) | 0·88 |
| Grade ≥3 | 8 (7%) | 10 (9%) | 15 (13%) | 16 (15%) | 23 (10%) vs 26 (12%) | 0·63 | 18 (8%) vs 31 (14%) | 0·06 |
| Pain | ||||||||
| Grade ≥2 | 17 (16%) | 18 (17%) | 24 (21%) | 20 (18%) | 41 (19%) vs 38 (17%) | 0·74 | 35 (16%) vs 44 (20%) | 0·30 |
| Grade ≥3 | 9 (8%) | 5 (5%) | 11 (10%) | 6 (6%) | 20 (9%) vs 11 (5%) | 0·10 | 14 (6%) vs 17 (8%) | 0·61 |
| Neurosensory | ||||||||
| Grade ≥2 | 2 (2%) | 10 (9%) | 4 (4%) | 15 (14%) | 6 (3%) vs 25 (11%) | 0·0005 | 12 (6%) vs 19 (9%) | 0·21 |
| Grade ≥3 | 0 (0%) | 1 (1%) | 0 (0%) | 4 (4%) | 0 (0%) vs 5 (2%) | 0·02 | 1 (1%) vs 4 (2%) | 0·18 |
| HFS | ||||||||
| Grade ≥2 | 1 (1%) | 2 (2%) | 24 (21%) | 13 (12%) | 25 (11%) vs 15 (7%) | 0·10 | 3 (1%) vs 37 (17%) | <0·0001 |
| Grade ≥3 | 0 (0%) | 0 (0%) | 11 (10%) | 2 (2%) | 11 (5%) vs 2 (1%) | 0·01 | 0 (0%) vs 13 (6%) | 0·0001 |
| Platelets | ||||||||
| Grade ≥2 | 0 (0%) | 2 (2%) | 1 (1%) | 2 (2%) | 1 (0·5%) vs 4 (2%) | 0·17 | 2 (1%) vs 3 (1%) | 0·67 |
| Grade ≥3 | 0 (0%) | 1 (1%) | 1 (1%) | 1 (1%) | 1 (0·5%) vs 2 (1%) | 0·56 | 1 (0·5%) vs 2 (1%) | 0·57 |
| Anaemia | ||||||||
| Grade ≥2 | 20 (18%) | 21 (19%) | 14 (13%) | 18 (16%) | 34 (15%) vs 39 (18%) | 0·49 | 41 (19%) vs 32 (14%) | 0·22 |
| Grade ≥3 | 3 (3%) | 3 (3%) | 1 (1%) | 2 (2%) | 4 (2%) vs 5 (2%) | 0·73 | 6 (3%) vs 3 (1%) | 0·30 |
| Neutropenia | ||||||||
| Grade ≥2 | 6 (6%) | 11 (10%) | 3 (3%) | 10 (9%) | 9 (4%) vs 21 (10%) | 0·02 | 17 (8%) vs 13 (6%) | 0·42 |
| Grade ≥3 | 3 (3%) | 6 (6%) | 2 (2%) | 2 (2%) | 5 (2%) vs 8 (4%) | 0·39 | 9 (4%) vs 4 (2%) | 0·15 |
Tests for interaction between the two treatment factors showed no evidence of an interaction (data not shown). FU=simplified LV5FU2 regimen of levofolinate, bolus fluorouracil, and 46-h infusion of fluorouracil, repeated every 2 weeks. OxFU=oxaliplatin plus FU. Cap=capecitabine. OxCap=oxaliplatin plus Cap. HFS=hand-foot syndrome.
438 (95%) patients had complete data to allow scoring of OTU at 12 weeks, of whom 182 (42%) scored good, 140 (32%) intermediate, and 116 (26%) poor. Better OTU was strongly associated with improved PFS and OS (both p<0·0001, log-rank trend test; data not shown). In the factorial comparisons, allocation to receive oxaliplatin was associated with better OTU (p=0·003), but we recorded no significant difference in OTU with fluorouracil or capecitabine (table 3).
Univariate analysis was done with baseline clinicopathological variables, CHA variables, and treatment allocation (figure 3). The strongest predictors of 12-week OTU were: WHO performance status, white blood cell count, EQ5D QoL score, overall symptom score, and allocation to oxaliplatin (all p<0·01; figure 3). We recorded no evidence of interaction between the two treatment factors (p=0·209; data not shown).
Figure 3.
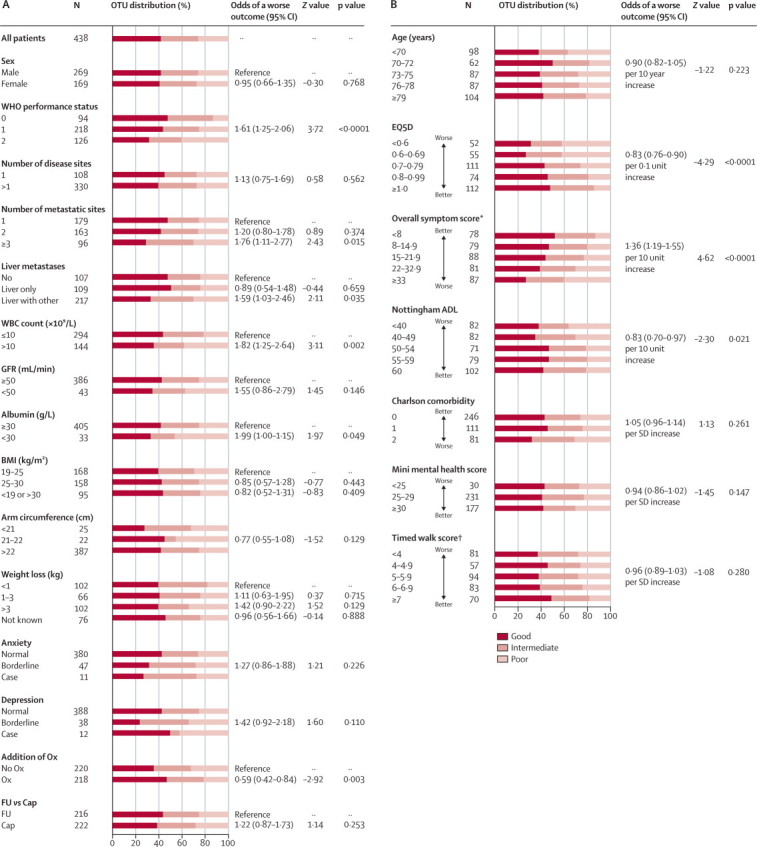
Association of categorical factors (A) and continuous factors (B) associated with OTU outcome
Odds of a worse outcome is expressed with reference to the more normal state, or as an odds ratio proportional across all categories. OTU=overall treatment utility. WBC=white blood cell. GFR=glomerular filtration rate. BMI=body-mass index. FU=simplified LV5FU2 regimen of levofolinate, bolus fluorouracil, and 46-h infusion of fluorouracil, repeated every 2 weeks. Ox=oxaliplatin. Cap=capecitabine. ADL=activities of daily living. *Mean EORTC QLQ-C30 symptom score. †Calculated as 100/time in s to walk 20 m.
Multivariable analyses (table 5) produced a potentially predictive model based on overall symptom score, presence of liver plus extrahepatic metastases, and treatment. WHO performance status and age were included for clinical relevance. On the basis of this model, a 70-year-old patient with performance status of 1, with both liver and extrahepatic metastases, whose overall symptom score is 60, treated with single-agent fluoropyrimidines, has a 61% (95% CI 45–76) probability of a poor OTU and only a 12% (5–20) probability of a good OTU. Conversely, an 80-year-old patient with performance status of 1 and a symptom score 0 and either extrahepatic-only or liver-only disease, treated with combination chemotherapy, has a 66% (56–77) probability of a good OTU and only 10% (5–14) probability of a poor OTU.
Table 5.
Factors associated with OTU outcome (multivariate analysis)
| Odds of a worse outcome (95% CI) | Z value | p value | |
|---|---|---|---|
| Overall baseline symptom score | 1·32* (1·14–1·52) | 3·79 | <0·0001 |
| Additional oxaliplatin | 0·57 (0·39–0·82) | −2·98 | 0·003 |
| Liver with other metastases | 1·51 (1·05–2·19) | 2·22 | 0·026 |
| WHO performance status | 1·28 (0·96–1·70) | 1·70 | 0·090 |
| Age | 1·00 (0·98–1·02) | 0·14 | 0·887 |
Interaction between the two treatment factors was assessed (Z=−1·26, p=0·209). OTU=overall treatment utility.
This odds ratio relates to a 10-point change in the overall symptom score.
Discussion
This is the largest randomised controlled trial so far to have selectively recruited frail and elderly patients with advanced colorectal cancer (panel). With use of reduced starting drug doses, adapted for this population, combination chemotherapy including oxaliplatin seems, on balance, preferable to single-agent fluoropyrimidines, although the primary endpoint of PFS was not met. We did not, however, detect any advantage of capecitabine compared with fluorouracil.
Panel. Research in context.
Systematic review
Previous publications and international meeting abstracts were searched with Ovid Medline and American Society of Clinical Oncology databases to find previous reports of palliative chemotherapy in elderly and frail patients with advanced colorectal cancer. Until now, the major reports of survival data for elderly patients receiving palliative chemotherapy have come from subgroup analyses of older patients participating in standard full-dose trials,4 or from trials of full-dose chemotherapy in selected fit elderly patients.24 These analyses show that elderly patients selected for full-dose treatments achieve survival times similar to younger patients on the same treatments; however, they represent only a small and highly selected proportion of the elderly cancer population.
Interpretation
FOCUS2 adds to the totality of evidence because it is the first large randomised trial in colorectal cancer to have been designed specifically for frail elderly patients and to relate objective baseline measures of geriatric fitness with patient-related outcomes of chemotherapy. Survival, at a median of 11 months, is noticeably shorter than in contemporaneous standard trials. For example, during overlapping recruitment periods two other MRC trials, FOCUS and COIN, were running at many of the same centres as FOCUS2, accruing patients with a median age of 63 years, more than 90% of whom had WHO performance status 0–1, with median survival of 14–17 months.10,25,26 Meanwhile, in France, a selective trial using more intensive therapy achieved median survival of more than 20 months.27 However, a meta-analysis of 6286 patients in nine trials, including FOCUS and the French trial, shows that frailty is a dominant negative prognostic factor, with median survival of only 8·5 months in the subpopulation with WHO performance stats of 2.9 This finding is entirely consistent with the survival recorded in FOCUS2, in which 134 of 459 (29%) patients were of performance status 2, and 324 of 459 (71%) were regarded as too frail to receive standard therapy (table 1).
FOCUS2 successfully recruited an elderly and frail population into a large national trial. Indeed, the trial proved so popular with patients and clinicians that it recruited well ahead of target, showing that age and frailty need not be barriers to research. The decision to start treatments at 80% of standard doses, although arbitrary, mimics common non-trial practice in frail elderly patients. Generally moderate rates of toxic effects and good rates of improvement in QoL in all groups would seem to support this strategy, whereas the relatively low uptake of escalation at 6 weeks, and the fact that only 14% of all patients sustained full-dose therapy to 12 weeks, supports the notion that the trial population was unsuited for full-dose therapy.
We introduced a novel composite endpoint, OTU, to assess the outcome of palliative chemotherapy, and explored the use of objective baseline evaluation to estimate the likelihood of a good or poor outcome with treatment. When confirmed and refined with further studies, this approach could potentially provide valuable guidance for doctors and patients in the difficult decisions between active or symptomatic care or, potentially, between active regimens. The interpretation of clinical trials, especially trials of palliative chemotherapy, often needs subjective synthesis of the objective data. Measures of efficacy are weighed against toxic effects, convenience, and other variables before deciding which treatment is best. For FOCUS2 we developed a simple composite endpoint of treatment outcome, OTU, to reflect both the doctor's question: “In retrospect, am I glad I offered this treatment?”; and the patient's question: “Am I glad I accepted it?”. OTU combines clinical efficacy (“Is my patient alive without disease progression?”), clinical tolerability (“Did we avoid causing major harm?”), and patient opinion (“Was my treatment worthwhile and acceptable?”). We encourage other research groups to adopt and refine this patient-centred approach.
OTU proved useful in comparison of treatment groups, particularly when conventional endpoints were divergent. The addition of oxaliplatin significantly increased RR and suggested some improvement in PFS, although this finding was not significant; however, it also increased some toxic effects and seemed to negatively affect global QoL. So overall, was treatment with oxaliplatin worthwhile? OTU showed unequivocal evidence of overall benefit with oxaliplatin (table 3; figure 3).
For the second factorial question, capecitabine has previously been shown to be non-inferior to fluorouracil,28 and oral therapy is generally thought to be preferred by patients, either because of its convenience or because it is assumed to have low toxicity. However, although analysis of PFS and RR confirmed capecitabine's efficacy, we recorded increased toxicity and no evidence of improved QoL. And despite including a measure of whether treatment interferes with patients' normal activities, the OTU scores for patients receiving capecitabine were not superior; indeed, they tended to favour fluorouracil, although this difference was not significant (table 2B; figure 3A).
Guidelines from the US National Comprehensive Cancer Network recommend use of a comprehensive geriatric assessment (CGA) to guide decision making when considering chemotherapy in elderly patients.29,30 However, there currently exists no evidence-based method to combine the many data items generated by the CGA into one decision about whether to offer chemotherapy, or which regimen to use. The 117-item geriatric assessment used in FOCUS2 was feasible in the oncology clinic, and we have started to identify which elements are of greatest value in prediction of the use of palliative chemotherapy. To develop a working predictive model will need cross-validation with other studies, but this approach offers the potential to better inform oncologists' discussions with patients. For example, a high predicted probability of a good OTU would support encouragement for chemotherapy; conversely, a high predicted probability of poor OTU (eg, in a patient with a high symptom score and widespread metastases) might help the oncologist and patient to consider with confidence the option of non-chemotherapy-based care.
New therapies now present new opportunities to develop treatments with few toxic effects for frail elderly patients with advanced colorectal cancer. For example, investigators of the AGITG MAX trial,31 undertaken in patients of median age 68 years, reported significantly improved PFS without significant extra toxicity from the addition of bevacizumab to single-agent capecitabine. We encourage investigators to continue to design trials using appropriate low-toxicity treatments and patient-centred assessment to expand the evidence base in this important specialty.
Acknowledgments
Acknowledgments
This trial was developed by the UK National Cancer Research Institute Colorectal Clinical Studies Group, funded by Cancer Research UK (C6003/A3830), and ran within the National Health Service supported by the National Institute of Health Research (NIHR) National Cancer Research Network. Discounted levofolinate and infusors were provided, respectively, by Wyeth and Baxter; these companies did not have access to data, and were not involved in any aspect of the research. We thank the 459 participating patients and the large number of clinicians, research nurses, data managers, and other clinical and support staff at the participating centres.
Contributors
MTS was the chief investigator of the trial and participated in its design and analysis, and in preparation of the report. HSW, GM, and TSM contributed to the trial design. AEB and MSO'M advised on geriatric assessment methods. LCT, MP, and REL were responsible for data management, analysis, and interpretation, and contributed to the preparation of the report. SFS, MTS, HSW, GM, AEB, and TSM recruited patients to the trial. All authors commented on the report.
MRC FOCUS2 study group
Trial Management Group M T Seymour (chair), T S Maughan, A Brewster, P Quirke, S Shepherd, H Wasan, S O'Mahony, F Halstead, J Hirst, M Schulpher, R J Stephens, D Smith.
Independent Data Monitoring Committee J Northover (chair), J Brown, M Aapro, R Stout.
MRC Clinical Trials Unit M K B Parmar (Programme Lead), R E Langley, L C Thompson, S Kenny, B Sydes, B May, L van Dyck, A Meade, E Dyer, S Beall, C Murphy, G Griffiths.
Clinical Investigators (institution [number of patients]):
C Topham, G Middleton, S Essapen (Royal Surrey County Hospital, Surrey [47]); R Phillips, C Lowdell, P Riddle, R Ahmed (Charing Cross Hospital, London [30]); H Wasan, C Vernon, C Palmeri, T Powells (Hammersmith Hospital, London [29]); S Karp, J Bridgewater, F Raja (North Middlesex Hospital, Middlesex [28]); D Ferry, S Grumett, D Palmer (Russells Hall Hospital, Dudley [23]); M T Seymour, D Sebag-Montefiore, A Crellin, D A Anthoney, A Melcher, R Cooper (Cookridge Hospital, Leeds [23]); J White, J Carser (Crosshouse Hospital, Kilmarnock [20]); F Daniel, D Sherriff, P Bradbury (Derriford Hospital, Plymouth [15]); A Maraveyas, J Sgouros, S Waters (The Princess Royal Hospital, Hull [14]); A Hartley (Manor Hospital, Walsall [13]); G Wilson (Wigan Royal Infirmary, Wigan [13]); K Hopkins, S Falk (Bristol Haematology and Oncology Centre, Bristol [12]); D Mort, T S Maughan, T Crosby, A Brewster, N Iqbal (Velindre Hospital, Cardiff [12]); J Stewart, C Macmillan, H Eldeeb (Northampton General Hospital, Northampton [11]); I Geh (Birmingham Heartlands Hospital, Birmingham [11]); A O'Callaghan, G Khoury, S Muthuramalingam (St Mary's Hospital, Portsmouth [11]); S Falk (Yeovil Hospital, Yeovil [10]); J Summers, R James, M Hill, S Beesley (Maidstone Hospital, Maidstone [9]); A Makris, R Glynne-Jones, M Harrison (Mount Vernon Hospital, Northwood [8]); R James, C Harper-Wynne, J Hall (William Harvey Hospital, Ashford [8]); S Shepherd, S Elyan, K Benstead, D Farrugia (Cheltenham General Hospital, Cheltenham [6]); L Samuel (Aberdeen Royal Infirmary, Aberdeen [6]); R Mehra, M Churn, D Ferry (New Cross Hospital, Wolverhampton [6]); J Joffe, J Dent (Huddersfield Royal Infirmary, Huddersfield [6]); P Chakraborti, K Shankland (Derbyshire Royal Infirmary, Derby [6]); T Hickish, G Astras (Royal Bournemouth Hospital, Bourenmouth [6]); N Steven, L Medley, V Potter, S Ayres (Queen Elizabeth Hospital, Birmingham [5]); T Iveson (Salisbury District Hospital, Salisbury [5]); M Crawford (Airedale General Hospital, Airedale [5]); K McAdam, K Fife, A Naderi (Peterborough District Hospital, Peterborough [4]); A Robinson, J Prejbisz, P Leonard (Southend Hospital, Southend [4]); N Stuart (Ysbyty Gwynedd, Bangor [4]); D Hochhauser, J Bridgewater, J Ledermann (Middlesex Hospital, London [4); D Smith, S Myint, B Haylock (Clatterbridge Hospital, Wirral [4]); N Wadd (James Cook Hospital, Middlesborough [3]); M Osborne (Royal Devon and Exeter Hospital, Exeter [3]); S Cleator (St Mary's Hospital, London [3]); M Saunders, G Wilson (Christie Hospital, Manchester [3]); T Iveson (Southampton General Hospital, Southampton [3]); D Farrugia (Worcestershire Royal Infirmary, Worcester [3]); C Bradley, (Bradford Royal Infirmary, Bradford [2]); S Shepherd (Hereford County Hospital, Hereford [2]); F McKinna (Eastbourne District General, Eastbourne [2]); J Stewart (Milton Keynes General Hospital, Milton Keynes [2]); M Moody, H Ford (West Suffolk Hospital, Suffolk [2]); H Yosef (Western Infirmary, Hairmyers [2]); F Adab (Staffordshire General Hospital [2]); M Churn, R Mehra (Kidderminster General Hospital [2]); C Blesing (Great Western Hospital, Swindon [2]); M Marples (Weston Park Hospital, Sheffield [1]); S Tahir (Broomfield Hospital, Essex [1]); S Beesley (Conquest Hospital, St Leonard on Sea, East Sussex [1]); M Iqbal (Countess of Chester Healthpark, Chester [1]); J Hasan (Stepping Hill, Stockport [1]); N Hodson (Royal Sussex County Hospital, Sussex [1]).
Conflicts of interest
MTS has received travel and accommodation and departmental research funding (unconnected with FOCUS2) from Roche. HSW has received travel support, honoraria, and educational support from Roche and Sanofi-Aventis. LCT, MP, and REL are employed by the UK MRC, which is also the trial sponsor. MSO'M is the Chair the Academic and Research Committee of the British Geriatrics Society and chairs the Drugs and Prescribing section of the British Geriatrics Society. The British Geriatrics Society has a mission statement that clinical trials be inclusive of older people and frail people. GM, AEB, SFS, and TSM declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Ferlay J, Autier P, Boniol M. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer facts and figures, 2006. American Cancer Society; Atlanta: 2006. http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf (accessed April 26, 2011). [Google Scholar]
- 3.UK Office of National Statistics Mortality statistics. 2007 registrations. http://www.statistics.gov.uk/STATBASE/Expodata/Spreadsheets/D9784.csv (accessed April 26, 2011).
- 4.Folprecht G, Seymour MT, Saltz L. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 5.De Gramont A, Figer A, Seymour MT. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Cunningham D, Roth AD. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 7.Tournigand C, André T, Achille E. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 8.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 9.Sargent DJ, Köhne CH, Sanoff HK. Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:1948–1955. doi: 10.1200/JCO.2008.20.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour MT, Maughan TS, Ledermann JA, for the FOCUS Trial Investigators and the National Cancer Research Institute Colorectal Clinical Studies Group Different strategies of sequential and combination chemotherapy for patients with poor-prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007;370:143–152. doi: 10.1016/S0140-6736(07)61087-3. [DOI] [PubMed] [Google Scholar]
- 11.Simonsick EM, Gardner AW, Poehlman ET. Assessment of physical function and exercise tolerance in older adults: reproducibility and comparability of five measures. Aging Clin Exp Res. 2000;12:274–280. doi: 10.1007/BF03339847. [DOI] [PubMed] [Google Scholar]
- 12.Cort MC, Guranik JM, Saline ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older patients. JAMA. 1994;272:1035–1042. [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. ‘Minimental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Nouri F, Lincoln NB. An extended activities for daily living scale for stroke patients. Clin Rehab. 1998;1:233–238. [Google Scholar]
- 16.Aaronson NK, Ahmedzai S, Bergman B. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Kind P. The EuroQoL instrument: an index of health-related quality of life. In: Spiker B, editor. Quality of life in pharmacoeconomics in clinical trials. 2nd edn. Lippincott-Raven; Philadelphia: 1996. [Google Scholar]
- 19.MRC Good Research Practice. Medical Research Council; London: 2006. http://www.mrc.ac.uk/pdf/good_research_pracice.pdf (accessed Sept 8, 2006). [Google Scholar]
- 20.Cheeseman SL, Joel SP, Chester JD. A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer. 2002;87:393–399. doi: 10.1038/sj.bjc.6600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twelves C, Butts C, Cassidy J. Capecitabine in combination with oxaliplatin as first line therapy for patients with advanced or metastatic cancer: preliminary results of an international multicenter phase II study. Eur J Cancer. 2001;37(suppl 6):272. (1005 abstr). [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA. New guidelines to evaluate the response to treatment in solid tumours. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Seymour MT, Slevin ML, Kerr DJ. A randomized trial assessing the addition of interferon α2a to 5-fluorouracil and leucovorin in advanced colorectal cancer. J Clin Oncol. 1996;14:2280–2288. doi: 10.1200/JCO.1996.14.8.2280. [DOI] [PubMed] [Google Scholar]
- 24.Rosati G, Cordio S, Bordonaro G. Capecitabine in combination with oxaliplatin or irinotecan in elderly patients with advanced colorectal cancer: results of a randomized phase II study. Ann Oncol. 2010;21:781–786. doi: 10.1093/annonc/mdp359. [DOI] [PubMed] [Google Scholar]
- 25.Adams R, Wilson R, Seymour MT. Intermittent versus continuous oxaliplatin-based combination chemotherapy in patients with advanced colorectal cancer: a randomised non-inferiority trial (MRC COIN) Eur J Cancer. 2009;7(suppl 3):10. (15LBA abstr). [Google Scholar]
- 26.Maughan TS, Adams R, Smith CG. Addition of cetuximab to oxaliplatin-based combination chemotherapy in patients with KRAS wild-type advanced colorectal cancer: a randomised superiority trial (MRC COIN) Eur J Cancer. 2009;7(suppl 3):4. (6LBA abstr). [Google Scholar]
- 27.Tournigand C, Andre T, Achille E. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 28.Twelves C, Boyer M, Findlay M. Capecitabine (Xeloda) improves medical resource use compared with 5FU + leucovorin in a phase III trial in patients with advanced colorectal carcinoma. Eur J Cancer. 2001;37:597–604. doi: 10.1016/s0959-8049(00)00444-5. [DOI] [PubMed] [Google Scholar]
- 29.Maas HAAM, Janssen-Heijnen MLG, Rikkert MGMO, Wymenga ANM. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer. 2007;43:2161–2169. doi: 10.1016/j.ejca.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Saif MW, Lichtman SM. Chemotherapy options and outcomes in older adult patients with colorectal cancer. Crit Rev Oncol Hematol. 2009;79:155–169. doi: 10.1016/j.critrevonc.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Tebbutt NC, Gebski V, Wilson K. International randomized phase III study of capecitabine, bevacizumab, and mitomycin C in first-line metastatic colorectal cancer: final results of the AGITG MAX trial. J Clin Oncol. 2009;27(suppl):4023. (abstr). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


