Abstract
The mechanisms of CD4+ T-cell count decline, the hallmark of HIV disease progression, and its relationship to elevated levels of immune activation are not fully understood. Massive depletion of CD4+ T cells occurs during the course of HIV-1 infection, so that maintenance of adequate CD4+ T-cell levels probably depends primarily on the capacity to renew depleted lymphocytes, that is, the lymphopoiesis. We performed here a comprehensive study of quantitative and qualitative attributes of CD34+ hematopoietic progenitor cells directly from the blood of a large set of HIV-infected persons compared with uninfected donors, in particular the elderly. Our analyses underline a marked impairment of primary immune resources with the failure to maintain adequate lymphocyte counts. Systemic immune activation emerges as a major correlate of altered lymphopoiesis, which can be partially reversed with prolonged antiretroviral therapy. Importantly, HIV disease progression despite elite control of HIV replication or virologic success on antiretroviral treatment is associated with persistent damage to the lymphopoietic system or exhaustion of lymphopoiesis. These findings highlight the importance of primary hematopoietic resources in HIV pathogenesis and the response to antiretroviral treatments.
Introduction
HIV disease progression is characterized by a gradual decline in CD4+ T-cell numbers and the eventual onset of immunodeficiency. Colossal progress in our understanding of HIV pathogenesis has been achieved over the past 25 years. It is now well established that chronic immune activation (IA) is linked to and predictive of disease progression in HIV-1 infection.1–5 A number of causative factors for sustained IA and inflammation have been identified, which are both directly or indirectly related to HIV replication. They include the innate and adaptive immune responses against HIV and associated pathogens, the translocation of bacterial products because of the compromised integrity of the mucosal barrier, and the potential bystander stimulation of lymphocytes and macrophages by HIV gene products (reviewed in Appay and Sauce6). However, the potential consequences of IA and its links to CD4+ T-cell decline and thus immunodeficiency in HIV-1 infection remain a matter of debate.
Considering the continuous depletion of CD4+ T cells during HIV-1 infection, the maintenance of adequate levels of CD4+ T cells probably depends on the capacity to renew depleted lymphocytes. Although CD4+ T-cell count decline is the primary hallmark of HIV disease progression, the latter is actually associated with a general lymphopenia. Reduced CD4+ and CD8+ T-cell counts during HIV-1 infection affect particularly the naive T-cell compartment.7 Evidence indicates that both de novo production of new cells and peripheral homeostatic division of existing cells participate to naive T-cell renewal.8–11 However, failure to maintain adequate naive T-cell counts is probably due to deficient production of new cells or reduced thymic output in HIV-infected persons.9,12–14 This has been related to impaired thymopoiesis a conequence of infection of the thymus by HIV or thymic involution, as shown by a number of investigators.15,16 Natural killer (NK)– and B-cell numbers are also reduced during HIV-1 infection. Like for T cells, B cells from HIV-infected patients are characterized by decreased naive B-cell proportions.17 Altogether, this indicates that the production of all lymphocyte populations is defective with HIV disease progression. HIV-associated lymphopenia may therefore have a more profound origin than CD4+ T-cell depletion and weakened thymus, and upstream elements of lymphocyte development may be affected.
This prompted us to investigate further the primary source of all lymphocytes, that is, the CD34+ hematopoietic progenitor cell (HPC) compartment, to reconsider its relevance in HIV pathogenesis. A number of studies have shown that HPCs from HIV-1–infected patient BM present functional alterations, suggesting impaired hematopoiesis in HIV-1 infection.18–21 However, the problematical access to large numbers of human BM samples or the need to rely on animal models have significantly limited our perception of the importance of dysregulated hematopoiesis in HIV pathogenesis. We thus explored the possibility of studying quantitative and qualitative attributes of HPCs directly from the blood (without mobilization), to generate relevant information from a large set of donors, in association with markers of progression. We show here that progression to HIV disease is directly linked to alterations in the HPC compartment, a probable consequence of chronic immune activation in HIV-1–infected patients.
Methods
Study subjects and samples
Blood samples were obtained from patients with chronic HIV-1 infection (aged 25–55 years), treatment naive or receiving antiretroviral therapy (ART) for > 3 years, attending the Infectious Diseases and Internal Medicine Departments of the Hôpital Pitiě Salpêtrière (Paris, France). Patients were divided into distinct groups on the basis of CD4+ T-cell counts (supplemental Figures 1 and 6, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), with a priori–determined cut points. For comparison, blood samples were obtained from age-matched or elderly (75-96 years old) healthy adults. Eight patients with primary HIV-1 infection (based on Western blot results and either positive p24 ELISA or positive proviral DNA) were recruited in the St Vincent's Hospital (Sydney, Australia); 7 of these patients exhibited symptomatic primary infection. Nonprogressing (n = 12) or progressing (n = 10) HIV elite controllers (ie, with plasma HIV RNA levels below the level of detection with the use of conventional assays, in the absence of antiviral therapy) were recruited from established cohorts in San Francisco (SCOPE) and Paris (ANRS EP 36; supplemental Figure 5). Longitudinal cryopreserved samples were obtained from HIV-1–infected patients enrolled in the French Agence Nationale de la Recherche sur le SIDA (ANRS) SEROCO cohort, established in 1988. These patients were selected for presenting spontaneous CD4+ T-cell count decrease (before the ART era) before recovering on ART initiation. All participants gave their written informed consent. The study was approved by the local institutional ethics committee (ie, Comitě de Protection des Personnes of the Pitiě Salpětrière Hospital, Paris). Mononuclear cells were isolated over a Lymphoprep gradient and then either directly stained or cryopreserved until use.
Flow cytometry
Directly conjugated antibodies were obtained from the following vendors: BD Biosciences: CD4 (allophycocyanin [APC]–cyanin7 [Cy7]), CCR7 (PE-Cy7), CD38 (APC), CD19 (PE-Cy7), CD34 (FITC and PE), lineage cocktail (CD3, CD14, CD16, CD19, CD20, CD56/FITC), and CD45RA (V450); Beckman Coulter: CD45RA (PE-Texas RED), CD45 (PE-Texas Red), and CD117 (PE-Cy7); Caltag: CD8 (Alexa Fluor 405); Dako: CD3 (Cascade Yellow); BioLegend: CD27 (Alexa Fluor700), CD10 (APC-Cy7); Sigma-Aldrich: CD56 (FITC). Cell surface marker stainings were performed with standard methods. Stainings were analyzed on an LSR2 flow cytometer (Becton Dickinson), and data were analyzed with FlowJo v8.2 (TreeStar Inc) and DIVA software.
Measure of soluble factors
Measures of the soluble factors IFN-inducible protein 10 (IP-10), and monokine induced by IFN-γ (MIG) in plasma were performed with the use of multiplex bead immunoassays (Biosource) and Luminex instrument. Measures of stromal cell-derived factor 1 α (SDF-1α) and soluble CD14 (sCD14) plasma levels were performed by Quantikine ELISA (R&D Systems).
Precursor CFU assay
Immunomagnetic sorting of CD34+ cells from PBMCs was performed with MACS technology, according to provider's recommendations (Miltenyi Biotech). Purity of enriched populations was > 90%, as determined by flow cytometry. Five hundred pure (ie, adjusted for concentration) CD34+ cells were plated in MethoCult medium (StemCell Technology) and cultured at 37°C and 5% CO2. Colonies were counted at day 14, and their subtypes were evaluated with inverted microscope and gridded scoring dishes.
Statistical analysis
Univariate statistical analysis was performed with GraphPad prism software. Groups were compared with the nonparametric Kruskal-Wallis or Mann-Whitney tests. Spearman rank test was used to determine correlations. Multivariate statistical analysis was performed with JMP software. P values > .05 were considered not significant.
Results
Disruption of the HPC compartment with HIV disease progression
Attributes of circulating HPCs were assessed in treatment-naive HIV-1 chronically infected patients separated into distinct groups according to their CD4+ T-cell count (as a marker of progression state) and age-matched uninfected adults (patient characteristics are described in supplemental Figure 1). Because advanced age is thought to be associated with a declining hematopoietic regenerative capacity, accompanied with quantitative and qualitative changes of the HPCs (reviewed in Effros and Globerson22), we analyzed HPC attributes in blood samples from elderly donors (ie, > 75 years old) for comparison. Although they are rare events, hematopoietic precursors (CD34+ CD45low Lin−) can be readily numerated in the blood (Figure 1A). In line with the decline of mature lymphocyte numbers (ie, CD8+ T, NK, or B cells) (supplemental Figure 1), circulating HPC counts decreased significantly with HIV disease progression (Figure 1B), so that middle-aged HIV-1–infected patients with CD4+ T-cell counts < 200 cells/μL presented circulating HPC levels similar to those of elderly noninfected donors. We found that the number of circulating HPCs were directly correlated with the CD4+ T-cell count (P < .0001, r = 0.43) (Figure 1C), as well as, although less strongly, with CD8+ T-cell (P = .03, r = 0.16), NK-cell (P = .002, r = 0.21), or B-cell (P = .004, r = 0.21) and erythrocyte (P = .002, r = 0.24) or neutrophil (P = .005, r = 0.22) counts (data not shown). To consider the influence of circulating HPC counts together with other variables on HIV disease progression (ie, CD4+ T-cell count), we used multivariate analysis, including CD34+ cell frequency, viral load, sex, and age, as well as CMV, hepatitis C virus (HCV), and hepatitis B virus (HBV) serology, as parameters. This indicated that, along with the HIV-1 load (P < .0001) and the sex (P = .004), the CD34+ cell frequency could be considered as an independent predictive factor of CD4+ T-cell counts in our set of HIV-1–infected donors (P < .0001).
Figure 1.
Attributes of circulating CD34+ HPCs. (A) Representative examples of CD34 and CD45 staining to identify HPCs in PBMC samples. (B) Absolute counts of CD34+ CD45low Lin− cells in middle aged (M; n = 27) or old (O; n = 26) adults, and in treatment-naive HIV-1–infected patients, grouped according to CD4+ T-cell counts: > 500 CD4+ (H; n = 35), between 200 and 500 CD4+ (I; n = 44), or < 200 (L; n = 23) CD4+ T cells/μL. (C) Correlation between CD34+ HPC and CD4+ T-cell counts in treatment-naive HIV-1–infected patients. The Spearman rank test was used to determine the correlation. (D) Numbers of total or white (CFU-GM and CFU-GEMM) progenitor CFUs generated from CD34+-sorted cells of HIV-1–infected patients and healthy donors. (E) Representative stainings for CD117, CD45RA, and CD10 on CD34+-sorted cells from PBMCs of HIV-1–infected patients and healthy controls. Numbers indicate percentages of cells in the different quadrants. (F) Ratio lymphoid (CD38+ CD117− CD45RA+ CD10+) versus myeloid (CD38+ CD117+ CD45RA− CD10−) HPCs within CD34+ cells from PBMCs of HIV-1–infected patients and healthy controls. (G) Frequency of lymphoid HPCs in the blood of HIV-1–infected patients and healthy controls. The Mann-Whitney or Kruskal-Wallis tests were used for comparing 2 groups or ≥ 3 groups, respectively. *P < .05, **P < .01, and ***P < .001. Bars indicate the median.
We next assessed the clonogenic potential of CD34+-sorted cells from the blood of different donors: CFU assays were completed to evaluate the capacity of HPCs to generate “white progenitor colonies” (ie, GM-CFU and granulocyte-erythroid-macrophage-megakaryocyte CFU [CFU-GEMM]), or “red progenitor colonies” (ie, erythroid CFU and erythroid burst-forming unit). Although the total number of progenitor colonies was only marginally affected with the use of circulating HPC cells from HIV-infected donors, the capacity of these cells to produce white CFUs appeared to be preferentially impaired with HIV disease progression, indicating altered clonogenic potential and HPC function (Figure 1D), in line with previous observations made in HIV-1–infected patient BM samples.20 We also assessed the phenotypic distribution of circulating HPCs. Although the phenotypic dissection of HPCs into well-defined subsets is still at an early stage in humans, a number of studies concur with the distinction between CD38+ CD117− CD45RA+ CD10+ HPCs with lymphoid precursor properties (l-HPCs) versus CD38+ CD117+ CD45RA− CD10− with myeloid precursor properties (m-HPCs).23–26 With the use of these markers, identification of these 2 HPC subpopulations was possible directly from blood. Most circulating CD34+ Lin− cells appear to be CD38+-committed HPCs (in contrast to CD38− primitive multipotent stem cells), divided into 2 main subsets: l-HPCs (CD117− CD45RA+ CD10+) or m-HPC (CD117+ CD45RA− CD10−) (supplemental Figure 2; Figure 1E). Decreasing ratios of l-HPCs to m-HPCs were observed in HIV-1–infected donors with lowering CD4+ T-cell counts (Figure 1F), so that circulating l-HPC numbers were clearly reduced with HIV disease progression (Figure 1G). Overall, in addition to low levels of lymphocytes, HIV-1–infected patients with progressing disease present decreased numbers of circulating HPCs, and their remaining CD34+ cells have both functional alterations and a preferential reduction in cells with lymphoid precursor properties. This indicates that HIV disease progression is associated with a disruption of hematopoiesis, which may affect in particular the generation of lymphocytes.
Exhaustion of lymphopoiesis despite elite control of HIV
Several factors could probably participate in the disruption of lymphopoiesis during HIV-1 infection. Although initial evidence indicated that HPCs were poorly susceptible to HIV infection,18,19,27 recent work suggests that HIV-1 can actually infect CD34+ HPCs, resulting in increased apoptosis, as well as the establishment of a latent viral reservoir in these cells.28 Nonetheless, we found no direct correlation between circulating HPC numbers and the plasma HIV load in infected donors (Figure 2A). On the same line, we observed only a modest effect on HPC counts in patients with primary HIV-1 infection compared with HIV-1–infected progressors (ie, with CD4+ T cells < 200 cells/μL) despite comparable viral loads (supplemental Figure 3). Overall, these data indicate that the virus itself is unlikely to be the main cause of HPC frequency reduction in patients with HIV-1. We next assessed whether altered lymphopoiesis could be related to elevated levels of IA in HIV-1–infected donors. For this purpose, we measured the expression of CD38 on memory CD8+ T cells, which is a commonly used marker of systemic IA in HIV infection, and a well-established correlate of disease progression.1,5 Interestingly, circulating HPC counts in HIV-1–infected donors were inversely correlated with CD38 expression levels (Figure 2B). Among variables such as viral load, sex and age, the level of IA (ie, percentage of memory CD8+ T cells expressing CD38) emerged as the most robust predictive factor of the CD34+ cell counts (P = .003) with the use of multivariate analysis, thus suggesting a direct effect of IA on lymphopoiesis.
Figure 2.
Association between immune activation and altered hematopoiesis. (A) Lack of correlation between CD34+ HPC counts and viral load in treatment-naive HIV-1–infected patients. (B) Inverse correlation between CD34+ HPC counts and percentages of CD38-expressing memory CD8+ T cells in treatment-naive HIV-1–infected patients. The Spearman rank test was used to determine correlations. Plasma levels of (C) SDF-1α, IP-10, MIG and (D) sCD14 in middle-aged (M) adults and treatment-naive HIV-1–infected patients with CD4+ T-cell counts > 500 (H), between 200 and 500 (I), or < 200 (L) cells/μL. The Mann-Whitney or Kruskal-Wallis tests were used for comparing 2 groups or ≥ 3 groups, respectively. *P < .05, **P < .01, and ***P < .001. Bars indicate the median.
We next measured plasma levels of soluble proinflammatory factors that are known to influence the mobilization of CD34+ HPCs (Figure 2C). Plasma levels of SDF-1α, a main player of HPC migration,29 were significantly increased in HIV-1–infected donors in particular those progressing. Two other chemokines, IP-10 and MIG, known to affect HPC chemotaxis and to have suppressive effects on HPC proliferation,30,31 were also found in higher concentrations in the plasma of HIV-infected donors. This was particularly obvious for IP-10, whose plasma levels rose with HIV disease progression. Plasma levels of these soluble factors, in particular IP-10, correlated with CD38 expression on T cells in HIV-1–infected patients (supplemental Figure 4A), supporting an association between systemic IA and the release of these soluble factors. We also assessed the plasma concentration of sCD14, which is directly associated with microbial product–mediated activation of monocytes32 and represents a good marker of systemic IA. Plasma sCD14 levels increased with HIV disease progression (Figure 2D) and were correlated positively with IP-10, MIG, and SDF-1α levels (supplemental Figure 4B) and inversely with CD34+ cell counts (supplemental Figure 4C). Altogether, although the present dataset does not show cause and effect, it suggests that the alteration of several parameters related to lymphopoietic activity is closely associated with elevated systemic IA.
To strengthen the potential relationship between lymphopoiesis alteration and systemic immune activation rather than HIV replication, we studied a rare and puzzling group of HIV-elite controllers who are immunologic progressors (correspond to 1 in 25 000 HIV-1–infected patients). These treatment-naive patients are characterized by several years of infection without detectable virus replication, but eventually decreasing CD4+ T-cell counts (< 350 cells/μL). In contrast to typical HIV-elite controllers (see supplemental Figure 5 for donor characteristics), “elite controller progressors” presented overall markers of exhausted lymphopoiesis, similarly to elderly uninfected donors (Figure 3): significantly altered qualitative and quantitative HPC attributes (ie, decreased CD34+ total and m-HPC counts and reduced clonogenic potential), as well as reduced counts of lymphocyte subsets (naive CD4+ or CD8+ T, B, NK cells). HIV-elite controllers represent a particularly interesting group to study because viral replication is unlikely to be the cause of exhausted lymphopoiesis and progressive disease in these donors. Instead, they are known to present elevated levels of immune activation,33 which could thus contribute to damaging the hematopoietic system and lymphocyte renewal capacity in the long run. The present findings provide, for the first time, important insights into HIV disease progression despite elite control of the virus. They suggest that exhausted lymphopoiesis, as a potential consequence of chronic IA, may be the cause of disease progression in HIV-elite controller progressors.
Figure 3.
Exhausted lymphopoiesis in HIV-elite controller progressors. (A) Absolute counts of CD34+ CD45low Lin− cells and frequency of lymphoid (CD45RA+ CD10+ CD117− CD38+) HPCs in HIV-elite controller nonprogressors (Cnp; CD4+ T-cell count > 500 cells/μL; n = 12) and progressors (Cp; CD4+ T-cell count < 350 cells/μL; n = 10). For comparison, counts in middle-aged (M; n = 27) and elderly (O; n = 26) adults are also shown. (B) Numbers of white (CFU-GM and CFU-GEMM) progenitor CFUs generated from CD34+-sorted cells of HIV-elite controller nonprogressors or progressors, and (C) absolute naive CD4+ or CD8+ T-, B-, and NK-cell counts in HIV-elite controller or comparative donor groups. Bars indicate the median. The Kruskal-Wallis test was used for group comparison. *P < .05, **P < .01, and ***P < .001.
CD4 reconstitution under ART is linked to reestablished lymphopoiesis
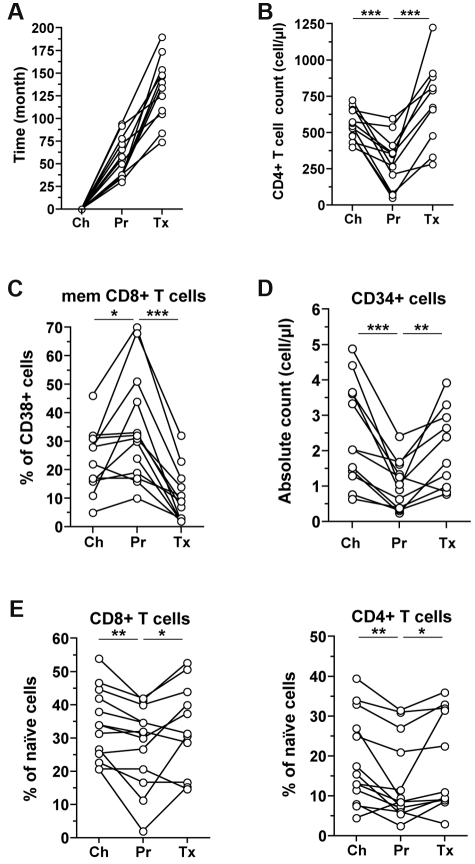
We next assessed circulating HPC attributes in the context of prolonged ART, which, through its potent inhibition of viral replication, results in a significant reduction of IA in treated patients and thus represents a strong immune “deactivator” in HIV-infected people. We first performed a longitudinal analysis on selected HIV-1–infected donors who had evidence of progression (patients recruited before the ART era) and who recovered with prolonged ART (ie, increasing CD4+ T-cell count) (Figure 4A-B). This confirmed the significant decrease of circulating HPCs with progression and showed an increase in circulating CD34+ cell numbers with reduced IA as patients were treated (Figure 4C-D). The latter was accompanied with increasing proportions of naive T cells (Figure 4E). Overall, these data suggest that CD4+ T-cell reconstitution may be linked to the recovery of upstream hematopoietic resources.
Figure 4.
Longitudinal follow-up during chronic infection and ART. Time interval (A), CD4+ T-cell counts (B), percentages of CD38-expressing memory CD8+ T cells (C), blood CD34+ HPC counts (D), and percentages of naive (CD45RA+ CCR7+ CD27+) CD8+ or CD4+ T cells (E) are shown for 11 HIV-1–infected patients followed longitudinally: between chronic infection (Ch), progression before treatment (Pr), and prolonged ART (Tx). The Wilcoxon test was used for comparing time points. *P < .05, **P < .01, and ***P < .001.
This may help in understanding the failure to reconstitute the CD4+ T-cell compartment despite effective ART, which remains unclear. For this purpose, we performed a comprehensive study of HIV-1–infected patients undergoing successful therapy (ie, potent inhibition of viral replication) separated into 3 groups according to CD4+ T-cell counts or levels of CD4 reconstitution. Importantly, all patients had matching age and equivalent CD4+ T-cell nadir, < 200 cells/μL, before therapy (supplemental Figure 6). Successful CD4+ T-cell reconstitution on ART was associated with increased CD34+; naive CD4+ or CD8+ T, B, NK-cell counts; and reequilibration of CD4/CD8 ratios, supporting a link between these different compartments and indicating a restoration of the global lymphocyte production machinery (Figure 5A-C). The clonogenic potential and m-HPC counts were significantly improved in treated patients with good versus poor CD4+ T-cell reconstitution (Figure 6A-B). We did not find any association between the lack of CD4+ T-cell recovery on therapy and the “duration of illness” (ie, considering CD4+ T-cell nadir, time since presumed infection, and time on ART). On the whole, successful CD4+ T-cell reconstitution with therapy appears thus to be directly dependent on the reestablishment of general lymphopoiesis and linked to the regeneration of all lymphocyte compartments.
Figure 5.
Recovery of lymphopoiesis with ART. (A) Absolute counts of CD34+ CD45low Lin− cells in HIV-1–infected patients with low CD4+ T-cell nadir before treatment (< 200 cells/μL) and treated for > 3 years with ART, grouped according to CD4+ T-cell counts: > 500 (Hx; n = 13), between 200 and 500 (Ix; n = 28), or < 200 (Lx; n = 13) cells/μL. For comparison, counts in middle-aged control adults (M; n = 27), treatment-naive HIV-1–infected patients with CD4+ T-cell count < 200 cells/μL (L;, n = 23), and treated HIV-1–infected patients with high CD4+ T-cell nadir and counts (> 500 cells/μL) (Hxh; n = 21) are also shown. (B) CD4-to-CD8 ratios and (C) absolute naive CD4+ or CD8+ T-, B-, and NK-cell counts in ART-treated HIV-1–infected patients or comparative donor groups. Bars indicate the median. The Kruskal-Wallis test was used for group comparison. *P < .05, **P < .01, and ***P < .001.
Figure 6.
CD4+ T-cell reconstitution and lymphopoiesis. (A) Numbers of total or white (CFU-GM and CFU-GEMM) progenitor CFUs generated from CD34+-sorted cells of treated HIV-1–infected patients (with CD4+ T-cell nadir before treatment < 200 cells/μL and for > 3 years on ART) grouped according to CD4+ T-cell counts: > 500 (Hx) or < 200 (Lx) cells/μL. Middle-aged control adults (M), treatment-naive HIV-1–infected patients with CD4+ T-cell count < 200 cells/μL (L), and treated HIV-1–infected patients with CD4+ T-cell nadir > 500 cells/μL (Hxh) are shown for comparison. (B) Frequency of lymphoid (CD45RA+ CD10+ CD117− CD38+) HPCs from PBMCs of treated HIV-1–infected patients or comparative donor groups. (C) Percentages of CD38-expressing memory CD8+ T cells and (D) plasma levels of sCD14, IP-10, MIG, and SDF-1α in treated HIV-1–infected patients and comparative donor groups. The Mann-Whitney or Kruskal-Wallis tests were used for comparing 2 groups or ≥ 3 groups, respectively. *P < .05, **P < .01, and ***P < .001. Bars indicate the median.
Of interest, despite ART, donors with low CD4+ T-cell numbers retained attributes equivalent to those of uninfected elderly or HIV-elite controller progressors. Failure to reestablish lymphopoiesis was not due to the use of antiviral medications with documented marrow suppressive effects (ie, zidovudine [AZT], lamivudine [3TC], or valganciclovir) as patients treated with these molecules did not present particular bias toward lower CD34+ cell levels or CD4+ T-cell recovery (data not shown). It was not due to higher immune activation levels in these patients either. As expected, markers of immune activation (ie, percentages of CD38-expressing memory CD8+ T cells and plasma levels of IP-10, MIG, and SDF-1α and less apparent for sCD14) decreased significantly with prolonged ART, but there was no differences between the different groups of treated patients (Figure 6C). Overall, this suggests that failure to reconstitute the CD4+ T-cell compartment despite low viral replication and inflammatory conditions on ART due to of irreversible damage to the lymphopoietic system in HIV-infected patients. In keeping with this possibility, we studied one particular untreated HIV-1–infected person who started progressing despite a relatively controlled low viral load (< 1000 copies/mL; Figure 7). Declining CD4+ T-cell count in this patient was associated with a profound exhaustion of primary immune resources before therapy (despite relatively high CD4+ T-cell count, ie, > 500 cells/μL, at that time). Importantly, these attributes, including the CD4+ T-cell count, were not improved with ART. These data emphasize further the need to maintain adequate lymphopoiesis to prevent progression and to enable CD4+ T-cell recovery on ART.
Figure 7.
Exhausted lymphopoiesis and persistent CD4+ T-cell count decline. Circulating CD34+, lymphoid HPCs, naive CD4+ T-cell, or CD8+ T-, B-, and NK-cell counts and percentages of CD38+ memory CD8+ T cells are shown for one donor at 2 different time points to highlight the association between declining CD4+ T-cell counts and exhaustion of lymphopoietic capacity despite low viral load before and after initiation of ART.
Discussion
Direct infection by HIV, apoptosis of activated cells, and killing of infected cells by cytotoxic CD8+ T cells can result in a massive depletion of CD4+ T cells during HIV-1 infection.2 Moreover, other lymphocyte populations (eg, B cells) may be depleted during HIV-1 infection because of the establishment of a proapoptotic environment.34 Maintenance of adequate levels of lymphocytes, in particular CD4+ T cells, is therefore highly dependent on the capacity to renew these depleted lymphocytes. During HIV-1 infection, T-cell renewal may be impaired due to deficient thymopoiesis, as previously described.15,16 Our data indicate that it can also have a more upstream cause. The present work is the largest analysis of hematopoietic parameters in relation to the onset of immunodeficiency in HIV-1 infection. This was made possible owing to the assessment of HPC attributes directly from blood, which allows the analysis of a large number of donors. It represents an original and relevant approach to study hematopoietic parameters in humans, as shown here in the context of HIV-1 infection. With the use of this method, we were able to show that the onset of HIV disease progression is closely related to the state of the primary lymphoid resources. Compared with healthy donors or HIV-1–infected nonprogressors, patients progressing toward AIDS have decreased numbers of circulating HPCs, and their remaining CD34+ cells present both functional alterations and a preferential reduction in cells with lymphoid precursor capacity. Our findings are in line with the impairment of T-cell progenitor function as shown by a reduced capacity of CD34+ HPCs from HIV-1–infected progressors to generate both CD4+ and CD8+ T cells, in culture experiments of HPCs on fetal thymus from T-cell–deficient (RAG-1 knockout) mice (ie, fetal thymic organ cultures).35,36 Of note, a former study showed also that the progenitor cell function of HIV-negative infants of HIV-positive mothers was impaired compared with control infants of HIV-negative mothers.37 The capacity to generate new T cells ultimately depends on the availability of functional lymphoid precursors. Studies in animal models suggest indeed that declining thymopoiesis in HIV-1 infection may be related to impaired lymphopoiesis.19,21 Disrupted lymphopoiesis may significantly limit the production of the CD4+ T-cell population and thus the maintenance of adequate CD4+ T-cell numbers during HIV-1 infection. Although altered lymphopoiesis shows effects beyond the CD4+ T-cell compartment, CD4+ T-cell counts were more closely related to CD34+ cell levels, compared with CD8+ T-, NK-, and B-cell numbers (as well as erythrocytes and neutrophils). This probably reflects the strong dependence of the CD4+ T-cell compartment on lymphopoietic-replenishing capacity because of their continuous depletion in HIV-1 infection. Considering that the maintenance of the naive T-lymphocyte compartment is related to both de novo production and peripheral self-renewal of lymphocytes, it will be interesting to study the potential influence of lymphopoiesis exhaustion on peripheral lymphoid homeostatic division.
Deterioration of the hematopoietic system with advanced age is thought to result from life-long mobilization of resources and intrinsic cellular impairments (of both HPCs and stromal cells). The fine mechanisms underlying the exhaustion of lymphopoiesis during untreated HIV-1 infection will need to be addressed in future studies. Altered lymphopoiesis in HIV-1–infected patients probably results from the combination of multiple factors. Convincing evidence for the infection by HIV-1 and apoptosis of hematopoietic progenitors has been recently reported,28 which might result in HPC depletion. Moreover, Nef was shown to have inhibitory effects on HPC multipotent potential.38,39 Finally, HIV may infect and deplete BM stromal auxiliary cells, which has been suggested as a potential cause of disrupted hematopoiesis.40 In addition, our data indicate that elevated systemic IA may have a major effect on lymphopoietic activity. In fact, the lack of correlation between viral replication and CD34+ cell levels suggests that a direct effect of the virus on lymphopoiesis disruption may only be secondary, in comparison with the effect of IA. The association between plasma sCD14 levels and CD34+ cell counts in untreated patients suggests that high levels of microbial products such as lipopolysaccharide (LPS; associated with bacterial translocation) or IFN-α (associated with activation of plasmacytoid dendritic cells), which result in monocyte activation and are both associated with disease progression in HIV or SIV infection,32,41,42 might participate in disrupting lymphopoiesis. For instance, LPS may act directly on HPCs, which express TLRs, including TLR4.43 Alternatively, LPS or IFN-α or both might have indirect hematosuppressive effects by inducing the release of proinflammatory factors (such as IP-10, MIG, and SDF-1α) that cause exacerbated mobilization and eventually exhaustion of primary lymphoid resources. LPS- or IFN-α–activated monocytes, whose importance in HIV-1 pathogenesis is raising interest, are known producers of IP-10 and MIG.44 It was recently shown that high levels of bacterial products (ie, LPS and 16S rDNA) during therapy correlate with reduced recovery of the CD4+ T-lymphocyte count, irrespective of plasma HIV RNA levels.45 Finally, in support for a potential influence of type I IFN on hematopoiesis, IFN-α treatment is known to have lympho-hematopoiesis suppressive effects.46,47 Although a strong association between IA and the loss of progenitor function emerges from our data, it remains to be established if the occurrence early of IA can predict the subsequent development of lymphopoiesis exhaustion in HIV infection in prospective longitudinal studies. The disruption of lymphopoiesis and ensuing suboptimal capacity to replenish lymphocyte compartments as consequences of elevated IA and inflammation could be central in the relationship between systemic IA and the decline of CD4+ T-cell counts with HIV disease progression.
Our observations of a partial normalization of hematopoietic activity with the introduction of art are in line with previous reports that show improved T-cell progenitor function in fetal thymic organ culture,36 and clonogenic potential of HPCs from the BM48,49 or G-CSF–mobilized blood50 of HIV-1–infected patients on therapy. Altogether this suggests that, through its potent inhibitory effects on HIV replication and therefore IA, ART results in a partial reestablishment of lymphopoiesis and thus the capacity to reconstitute the CD4+ T-cell compartment. However, some treated patients fail to reestablish adequate lymphopoiesis, displaying attributes similar to those of the uninfected elderly person. HIV-1 infection can thus result in profound and persistent impairments of the lymphopoietic system, which cannot be reversed with ART. This is the probable reason for the limited recovery in CD4+ T-cell numbers in these patients. It may be due to severe damage at the BM level, as suggested in former studies.51,52 Our findings suggest therefore that modifications of the antiretroviral drug regimen in these patients are unlikely to result in significant improvements of their CD4+ T-cell counts or HIV disease status. Resolving determining factors and predictive markers for the lack of lymphopoiesis recovery under ART will be central to improve the clinical management of HIV infected donors. Although our study did not underline the “duration of illness” as a potential correlate of exhausted lymphopoiesis, the development of profound and lasting damage to lymphopoiesis is probably related to the duration and intensity of HIV-mediated IA. Persistent IA may eventually result in permanent exhaustion of lymphopoiesis and failure to maintain the CD4+ T-cell compartment. This is supported by our findings in HIV-elite controllers, which confer enlightenment into the puzzling progression of these rare patients toward disease, and emphasize further the central role of IA in HIV disease progression.1–5 For the general HIV-1–infected population, the precautionary principle would suggest that potent antiretroviral treatment should be initiated as early as possible to limit HIV replication–related IA and thus prevent the development of hematopoietic damage.
The identification of lymphopoiesis disruption as an important facet of HIV disease progression opens interesting avenues for the treatment of HIV-1–infected patients with low CD4+ T-cell counts, with the focus on molecules that target the hematopoietic system. For instance, the use of growth hormone, a known stimulatory factor of hematopoiesis,53,54 has been shown to enhance thymopoiesis and the production of lymphocytes in HIV-1–infected donors.55 It will be interesting to determine whether the effect of this molecule enhances the attributes of HPCs in HIV–infected donors. In addition, the influence of disrupted lymphopoiesis on anti-HIV immunity (eg, maintenance of diverse pool of B cells or renewal of effective virus-specific CD8+ T cells) represents a relevant area of investigation. Finally, future studies will also need to determine the potential effect of IA and inflammation on other stem cell compartments, because it may be directly relevant in the establishment of the various disorders associated with HIV-1 infection.
Supplementary Material
Acknowledgments
We thank the patients and staff of the infectious diseases and internal medicine departments of the Hôpital Pitiě Salpêtrière in Paris, the St. Vincent's Hospital in Sydney, and the ANRS SEROCO cohort. We thank Rachid Aigher, Laurent Arnaud, Loic Desquilbet, and Pascale Ghillani Dalbin for assistance with the patient database, statistical analysis, and luminex measurements, respectively, and we thank François Lemoine (CNRS UMR 7087, hospital Pitiě Salpêtrière, Paris) for constructive discussion. We apologize to the authors of many excellent and relevant studies that we have been unable to cite because of formatting constraints.
This work was supported by an Inserm AVENIR grant, the French ANRS, the ANR (project ANR-09-JCJC-0114-01), Sidaction, and ORVACS. M.L. is supported by the European Union (ATTACK project LHS-CT-2005-018914). P.W.H. is supported by K23 AI065244 and a Doris Duke Clinical Scientist Development Award. Financial support for the HIV elite controller SCOPE cohort is provided by the UCSF Centers for AIDS Research at UCSF (PO AI27763), the UCSF Clinical and Translational Science Institute (UL1 RR024131), the NIAID (RO1 AI087145, K24AI069994), the American Foundation for AIDS Research (106710-40-RGRL), and the Ragon Institute.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.S. designed and performed research, analyzed data, and wrote the paper; M.L. designed and performed the research and analyzed data; S.F. and M.B. performed research; M.P., F.B., and J.Z. analyzed data; H.A.-M., L.S., A.G., C.D., A.D.K., A.S., L.M., S.G.D., O.L., and P.W.H. analyzed data and recruited patients; M.I. recruited patients; G.G. and G.C. contributed vital analytical tools; D.C. and B.A. designed the research; C.K. designed the research and recruited patients; and V.A. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Victor Appay, Infections and Immunity, Inserm U945, Hôpital Pitiě-Salpêtrière, 75013 Paris, France; e-mail: victor.appay@upmc.fr.
References
- 1.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 2.Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1(4):285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 3.Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8(4):319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 4.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17(13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 6.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214(2):231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 7.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95(5):2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazenberg MD, Borghans JA, de Boer RJ, Miedema F. Thymic output: a bad TREC record. Nat Immunol. 2003;4(2):97–99. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- 9.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21(6):757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Vrisekoop N, den Braber I, de Boer AB, et al. Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc Natl Acad Sci U S A. 2008;105(16):6115–6120. doi: 10.1073/pnas.0709713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vrisekoop N, van Gent R, de Boer AB, et al. Restoration of the CD4 T cell compartment after long-term highly active antiretroviral therapy without phenotypical signs of accelerated immunological aging. J Immunol. 2008;181(2):1573–1581. doi: 10.4049/jimmunol.181.2.1573. [DOI] [PubMed] [Google Scholar]
- 12.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 13.Douek DC, Betts MR, Hill BJ, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001;167(11):6663–6668. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 14.Dion ML, Bordi R, Zeidan J, et al. Slow disease progression and robust therapy-mediated CD4+ T-cell recovery are associated with efficient thymopoiesis during HIV-1 infection. Blood. 2007;109(7):2912–2920. doi: 10.1182/blood-2006-09-047308. [DOI] [PubMed] [Google Scholar]
- 15.Kalayjian RC, Landay A, Pollard RB, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003;187(12):1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 16.Stanley SK, McCune JM, Kaneshima H, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178(4):1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marandin A, Katz A, Oksenhendler E, et al. Loss of primitive hematopoietic progenitors in patients with human immunodeficiency virus infection. Blood. 1996;88(12):4568–4578. [PubMed] [Google Scholar]
- 19.Jenkins M, Hanley MB, Moreno MB, Wieder E, McCune JM. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multilineage hematopoiesis in vivo. Blood. 1998;91(8):2672–2678. [PubMed] [Google Scholar]
- 20.Moses A, Nelson J, Bagby GC., Jr The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91(5):1479–1495. [PubMed] [Google Scholar]
- 21.Thiebot H, Vaslin B, Derdouch S, et al. Impact of bone marrow hematopoiesis failure on T-cell generation during pathogenic simian immunodeficiency virus infection in macaques. Blood. 2005;105(6):2403–2409. doi: 10.1182/blood-2004-01-0025. [DOI] [PubMed] [Google Scholar]
- 22.Effros RB, Globerson A. Hematopoietic cells and replicative senescence. Exp Gerontol. 2002;37(2–3):191–196. doi: 10.1016/s0531-5565(01)00183-8. [DOI] [PubMed] [Google Scholar]
- 23.Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38- progenitor cells. Blood. 1991;77(6):1218–1227. [PubMed] [Google Scholar]
- 24.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3(4):459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13(3):205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 26.Six EM, Bonhomme D, Monteiro M, et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med. 2007;204(13):3085–3093. doi: 10.1084/jem.20071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Luca A, Teofili L, Antinori A, et al. Haemopoietic CD34+ progenitor cells are not infected by HIV-1 in vivo but show impaired clonogenesis. Br J Haematol. 1993;85(1):20–24. doi: 10.1111/j.1365-2141.1993.tb08640.x. [DOI] [PubMed] [Google Scholar]
- 28.Carter CC, Onafuwa-Nuga A, McNamara LA, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16(4):446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185(1):111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarris AH, Broxmeyer HE, Wirthmueller U, et al. Human interferon-inducible protein 10: expression and purification of recombinant protein demonstrate inhibition of early human hematopoietic progenitors. J Exp Med. 1993;178(3):1127–1132. doi: 10.1084/jem.178.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GN, Liao F, Gress RE, Farber JM. Suppressive effects of recombinant human monokine induced by IFN-gamma (rHuMig) chemokine on the number of committed and primitive hemopoietic progenitors in liquid cultures of CD34+ human bone marrow cells. J Immunol. 1997;159(2):895–904. [PubMed] [Google Scholar]
- 32.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 33.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moir S, Malaspina A, Pickeral OK, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200(5):587–599. doi: 10.1084/jem.20032236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark DR, Ampel NM, Hallett CA, Yedavalli VR, Ahmad N, DeLuca D. Peripheral blood from human immunodeficiency virus type 1-infected patients displays diminished T cell generation capacity. J Infect Dis. 1997;176(3):649–654. doi: 10.1086/514086. [DOI] [PubMed] [Google Scholar]
- 36.Clark DR, Repping S, Pakker NG, et al. T-cell progenitor function during progressive human immunodeficiency virus-1 infection and after antiretroviral therapy. Blood. 2000;96(1):242–249. [PubMed] [Google Scholar]
- 37.Nielsen SD, Jeppesen DL, Kolte L, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood. 2001;98(2):398–404. doi: 10.1182/blood.v98.2.398. [DOI] [PubMed] [Google Scholar]
- 38.Dorival C, Brizzi F, Lelievre JD, et al. HIV-1 Nef protein expression in human CD34+ progenitors impairs the differentiation of an early T/NK cell precursor. Virology. 2008;377(1):207–215. doi: 10.1016/j.virol.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Prost S, Le Dantec M, Auge S, et al. Human and simian immunodeficiency viruses deregulate early hematopoiesis through a Nef/PPARgamma/STAT5 signaling pathway in macaques. J Clin Invest. 2008;118(5):1765–1775. doi: 10.1172/JCI33037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moses AV, Williams S, Heneveld ML, et al. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood. 1996;87(3):919–925. [PubMed] [Google Scholar]
- 41.Mandl JN, Barry AP, Vanderford TH, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14(10):1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 42.Jacquelin B, Mayau V, Targat B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119(12):3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackwell SE, Krieg AM. CpG-A-induced monocyte IFN-gamma-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-alpha. J Immunol. 2003;170(8):4061–4068. doi: 10.4049/jimmunol.170.8.4061. [DOI] [PubMed] [Google Scholar]
- 45.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199(8):1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185(3):517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin Q, Dong C, Cooper MD. Impairment of T and B cell development by treatment with a type I interferon. J Exp Med. 1998;187(1):79–87. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dam Nielsen S, Kjaer Ersboll A, Mathiesen L, Nielsen JO, Hansen JE. Highly active antiretroviral therapy normalizes the function of progenitor cells in human immunodeficiency virus-infected patients. J Infect Dis. 1998;178(5):1299–1305. doi: 10.1086/314464. [DOI] [PubMed] [Google Scholar]
- 49.Adams GB, Pym AS, Poznansky MC, McClure MO, Weber JN. The in vivo effects of combination antiretroviral drug therapy on peripheral blood CD34+ cell colony-forming units from HIV type 1-infected patients. AIDS Res Hum Retroviruses. 1999;15(6):551–559. doi: 10.1089/088922299311079. [DOI] [PubMed] [Google Scholar]
- 50.Baillou C, Simon A, Leclercq V, et al. Highly active antiretroviral therapy corrects hematopoiesis in HIV-1 infected patients: interest for peripheral blood stem cell-based gene therapy. AIDS. 2003;17(4):563–574. doi: 10.1097/00002030-200303070-00012. [DOI] [PubMed] [Google Scholar]
- 51.Thiebot H, Louache F, Vaslin B, et al. Early and persistent bone marrow hematopoiesis defect in simian/human immunodeficiency virus-infected macaques despite efficient reduction of viremia by highly active antiretroviral therapy during primary infection. J Virol. 2001;75(23):11594–11602. doi: 10.1128/JVI.75.23.11594-11602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isgro A, Leti W, De Santis W, et al. Altered clonogenic capability and stromal cell function characterize bone marrow of HIV-infected subjects with low CD4+ T cell counts despite viral suppression during HAART. Clin Infect Dis. 2008;46(12):1902–1910. doi: 10.1086/588480. [DOI] [PubMed] [Google Scholar]
- 53.French RA, Broussard SR, Meier WA, et al. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology. 2002;143(2):690–699. doi: 10.1210/endo.143.2.8612. [DOI] [PubMed] [Google Scholar]
- 54.Hanley MB, Napolitano LA, McCune JM. Growth hormone-induced stimulation of multilineage human hematopoiesis. Stem Cells. 2005;23(8):1170–1179. doi: 10.1634/stemcells.2004-0322. [DOI] [PubMed] [Google Scholar]
- 55.Napolitano LA, Schmidt D, Gotway MB, et al. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118(3):1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.