Microsporidia are eukaryotic, intracellular protozoa that infect a wide range of vertebrate and invertebrate hosts with almost half of the described genera affecting insects. Nosema locustae, which infects approximately 90 species of grasshoppers, has been used as a biological control agent for these insects and is registered as a microbial insecticide by the Environmental Protection Agency (Becnel and Andreadis, 1999). Antibodies to microsporidia proteins should have utility as reagents for the detection of microsporidia in lysates prepared from insects or in the visualization of microsporidia directly in insect tissues.
Monoclonal antibodies were prepared using N. locustae spores (courtesy of Dr. J. Henry, Bozeman, MT) that were stored in distilled water at 4°C. BALB/c mice were immunized with an intradermal injection of a 108 to 109 glass-bead-disrupted spore suspension using Freund’s incomplete adjuvant. Fusion of mouse spleen cells with myeloma cells was performed 12 weeks postimmunization using standard techniques. Supernatants from hybridomas were screened by immunoblotting using N. locustae spore lysate as the antigen as previously described (Keohane et al, 1996).
Two monoclonal antibodies [mAbs] were identified that reacted with N. locustae spore lysate by immunoblotting: 3B1.23, an IgM mAb that recognized a 40-kDa antigen, and 19 F9.24, an IgG3 mAb that recognized a group of three antigens of 12–18 kDa (Fig. 1). No definitive cross-reactivity of these mAbs to Encephalitozoon cuniculi or E. hellem was observed by immunoblotting under reduced conditions at a 1:500 dilution (Fig. 1). However, a 1:20 dilution of each mAb demonstrated cross-reactivity against high molecular weight antigen(s) of Glugea americanus spore lysate (data not shown). There was no reaction of either antibody with lysates from insect tissues (Culex restuans, Helicovera zea, Lymentra dispar, or Acheta domesticus) under reducing or non-reducing conditions. These mAbs were further characterized using immunogold electron microscopy employing protocols previously described (Keohane et al., 1996). Both mAbs demonstrated a generalized localization to antigens in the spores of N. locustae and cross-reacted with antigens in E. cuniculi and a Pleistophora sp. from the muscle of Callinectes sapidus (Fig. 2). No reaction was seen with either antibody to host cells (e.g., RK13 cells, Callinectes sapidus, or grasshopper tissue fragments) containing the corresponding microsporidia species (data not shown). These mAbs appear to recognize an epitope that in some microsporidia is conformationally determined (i.e., in E. cuniculi this epitope is recognized after immunoEM fixation but not in reduced SDS-PAGE immunoblots).
FIG. 1.
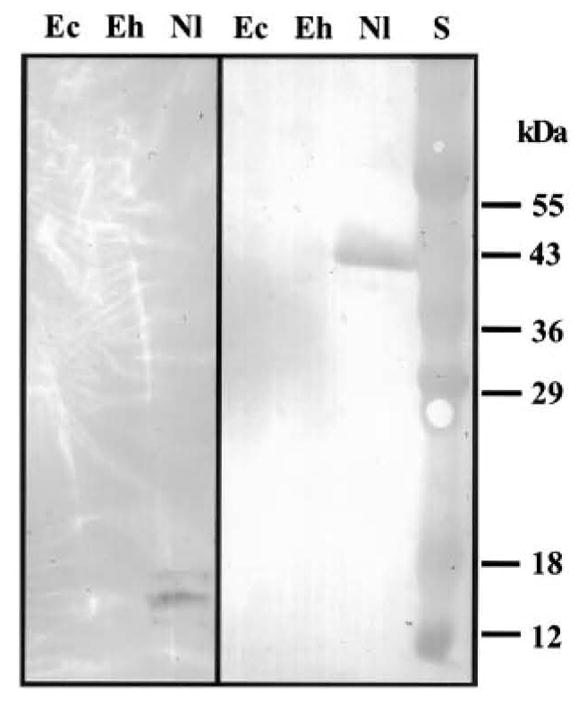
Immunoblot of a 1:500 dilution of mAb 19F9.24 (left) and mAb 3B1.23 (right) against spore lysate of Encephalitozoon cuniculi (Ec), E. hellem (Eh), and Nosema locustae (Nl). S, standard. 19F9.24 recognized a group of three antigens of 12–18 kDa. and mAb 3B1.23 recognized a 40-kDa antigen of Nosema locustae, respectively.Copyright © 2001 by Academic Press All rights of reproduction in any form reserved.
FIG. 2.
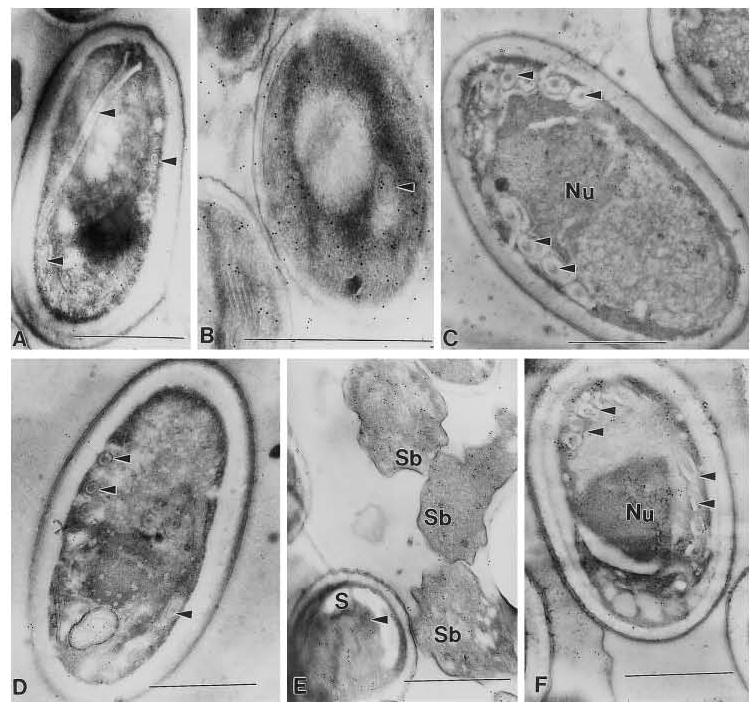
Nosema. locustae monoclonal antibody localization with 10 nm gold (Nanoprobes, Inc., Yaphank NY). Monoclonal antibody 19F.9.24 demonstrated a generalized localization to the spores of A, B, and C and reacted with the polar filament (arrow heads) of A and B. Monoclonal antibody 3B1.23 demonstrated a generalized localization to the spores of D, E. and F and reacted with the polar filament (arrow heads) of D and F. Additionally, a generalized localization in the sporoblasts (Sb) of E is noted. A and D are Nosema locustae, B and E are Encephalitozoon cuniculi, and C and F are Pleistophora sp. from the muscle of Callinectes sapidus. Sections through pelleted spores (A. D) and/or spores in host cells (B, C, E and F) contain spores and sporoblasts that are not necessarily visible in the plane of section, but which can bind antibody resulting in gold labeling with the secondary antibody. In uninfected host cells or tissue no antibody binding, gold labeling, is seen (data not shown).
Monoclonal antibodies to N. locustae were previously described that recognized the spore and extruded polar filament (Knoblett and Youssef, 1996). It appears that the mAbs identified in this study recognize some common microsporidian antigen(s) and may have utility in developing reagents for assays to detect microsporidia in insects.
Acknowledgments
This research was supported by NIH Grants AI31788 and R44 GM60067.
References
- Becnel JJ, Andreadis TG. Microsporidia in insects. In: Wittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. Am. Soc.. Microbiol; Washington, DC: 1999. pp. 447–501. [Google Scholar]
- Keohane EM, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. Identification of a microsporidian polar tube reactive monoclonal antibody. J Euk Microbiol. 1996;43:26–31. doi: 10.1111/j.1550-7408.1996.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Knoblett JN, Youssef NN. Detection of Nosema locustae (Microsporidia:Nosematidae) in frozen grasshoppers (Orthoptera:Acrididae) by using monoclonal antibodies. J Econ Entomol. 1996;89:841–847. doi: 10.1093/jee/89.4.841. [DOI] [PubMed] [Google Scholar]


