Abstract
There is no licensed vaccine available against Moraxella catarrhalis, an exclusive human pathogen responsible for otitis media in children and respiratory infections in adults. We previously developed conjugate vaccine candidates based on lipooligosaccharides (LOSs) of M. catarrhalis serotypes A, B, and C, each of which was shown to cover a portion of the clinical strains. To generate conserved LOS antigens and eliminate a potential autoimmune response to a similar epitope between M. catarrhalis LOS moiety Galα1-4Galβ1-4Glc and human Pk antigen, two LOS mutants from strain O35E were constructed. Mutant O35Elgt5 or O35EgalE revealed a deletion of one or two terminal galactose residues of wild type O35E LOS. Each LOS molecule was purified, characterized, detoxified, and coupled to tetanus toxoid (TT) to form conjugates, namely dLOS-TT. Three subcutaneous immunizations using dLOS-TT from O35Elgt5 or O35EgalE elicited significant increases (a 729- or 1263-fold above the preimmune serum levels) of serum immunoglobulin (Ig)G against O35E LOS in rabbits with an adjuvant or without an adjuvant (an 140- or 140-fold above the preimmune serum levels). Rabbit antisera demonstrated elevated complement-mediated bactericidal activities against the wild type strain O35E. The rabbit sera elicited by O35Elgt5 dLOS-TT were further examined and showed cross bactericidal activity against all additional 19 M. catarrhalis strains and clinical isolates studied. Moreover, the rabbit sera displayed cross-reactivity not only among three serotype strains but also clinical isolates in a whole-cell enzyme-linked immunosorbent assay (ELISA), which was further confirmed under transmission electron microscopy. In conclusion, O35Elgt5 dLOS-TT may act as a vaccine against most M. catarrhalis strains and therefore can be used for further in vivo efficacy studies.
Keywords: Moraxella catarrhalis, lipooligosaccharide mutant, conjugate vaccine, cross-reactivity, conserved antigen
1. Introduction
Moraxella (Branhamella) catarrhalis is a Gram-negative aerobic diplococcus, a frequent pathogen of otitis media in children or respiratory tract infections in adults [1]. It causes 15%–20% of acute otitis media episodes with at least 50% of recurrences in children below 2 years [1–3]. M. catarrhalis also induces an estimated 2–4 million exacerbations of chronic obstructive pulmonary disease in adults annually, which is the fourth highest cause of mortality in the United States [4]. Otitis media accounts for more than 13 million antibiotic prescriptions and approximately $6 billion in health care costs in the United States annually [5]. Despite the availability of antibiotics for treatment, frequent use of antibiotics might result in antibiotic resistance since greater than 90% of the clinical isolates express a drug-resistance enzyme, beta-lactamase [6, 7]. Thus, vaccine development is a key approach to preventing primary and recurrent M. catarrhalis infections, which would have an enormous human and economic impact [3, 6, 8].
Vaccine development against M. catarrhalis has focused primarily on surface antigens including outer membrane proteins (OMPs) and lipooligosaccharides (LOSs) [8, 9]. OMP antigens such as ubiquitous surface protein A (UspA) [10], catarrhalis outer membrane protein B (CopB) [11], and CD [12] elicit bactericidal antibodies. Furthermore, immunization with UspA [10], CopB [13], or recombinant CD [14] has been reported to enhance pulmonary clearance of M. catarrhalis in a mouse model. These functional activities may predict a potential efficacy of a candidate vaccine in humans since there is no appropriate animal model available. M. catarrhalis LOS is another major surface antigen, which is not only an important virulence factor in the bacterial pathogenesis but also a potential vaccine candidate due to its relatively conserved structure[15, 16]. There are three major LOS serotypes, A, B, and C. The ‘A’ serotype accounts for 61.3%, the ‘B’ serotype for 28.8% and the ‘C’ serotype for 5.3% of the 302 tested strains [17]. Serotypes A and C share a common N-acetyl-D-glucosamine (GlcNAc) residue in their LOS branches and induce cross-reactive antibodies [18–20]. We previously developed conjugate vaccine candidates derived from LOSs of M. catarrhalis serotypes A, B and C [21–23]. Our data indicate that these conjugates are immunogenic and the antibodies towards them have bactericidal activity. Mice immunized with a conjugate vaccine derived from the serotype A LOS clear both a homologous and a heterologous bacterial challenges in a mouse model of pulmonary clearance [24]. However, each of these conjugates is implicated to cover only a portion of pathogenic strains of M. catarrhalis [21–24]. In this study, we aim to develop conjugate vaccines with broad coverage, which may act as substitutes for any of the conjugates from M. catarrhalis serotype A, B, and C.
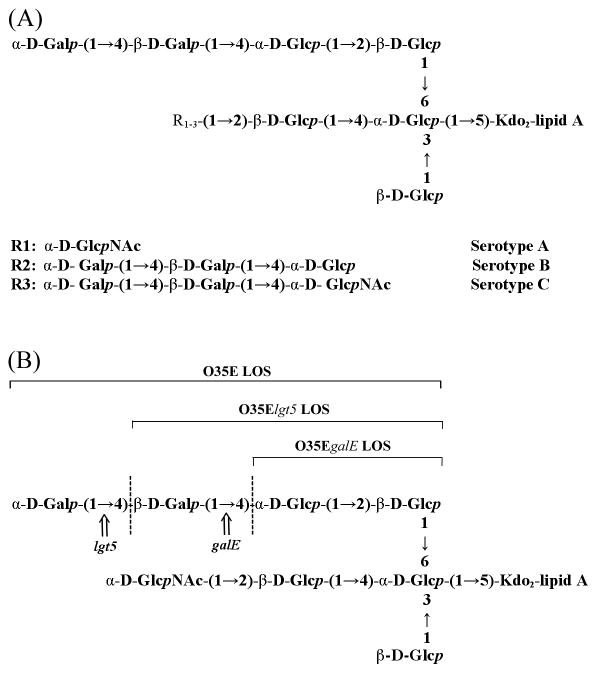
Like other non-enteric Gram-negative bacteria, M. catarrhalis produces LOSs containing an oligosaccharide (OS) linked to lipid A without an O-specific polysaccharide. Structural studies indicate that the LOSs from all three serotypes are branched with a common inner core, and the lipid A portion is similar to that of other Gram-negative bacteria (Fig. 1A) [18, 19, 25, 26]. The LOS glycosyltransferase (lgt)5 gene and the UDP-glucose-4-epimerase (galE) gene are responsible for the catalysis of glycosidic linkage in the LOS biosynthesis of M. catarrhalis strains (Fig. 1B) [27, 28]. Because O35E is a major clinical isolate of serotype A that makes up 61.3% of M. catarrhalis strains, we chose O35E as a parental strain to generate mutants, from which conjugate vaccines were derived. To create conserved LOS antigens, two O35E LOS mutants, O35Elgt5 with the lgt5 gene knockout and O35EgalE with the galE gene knockout, were constructed. The O35Elgt5 or O35EgalE had the deletion of one or two terminal galactose (Gal) residues, respectively, in the LOS oligosaccharide (OS) branch, Galα1-4Galβ1-4Glc (Fig. 1B) [27–29]. In addition, there is a similarity between the LOS epitope Galα1-4Galβ1-4Glc and human Pk antigen [30–32]. Our conjugate vaccines derived from the mutant LOSs will eliminate a possible cross-reaction with the human antigen. In this study, we extracted LOSs from both mutants O35Elgt5 and O35EgalE, and coupled the LOSs to a common carrier, tetanus toxoid (TT) to form carbohydrate-based conjugates. The composition, antigenicity, immunogenicity, efficacy, and coverage of the conjugates were then examined.
Fig. 1.
Schematic structures of the LOS moieties on the surface of M. catarrhalis and the proposed LOS structures of M. catarrhalis serotype A strain O35E and its mutants, O35Elgt5 and O35EgalE. Three main serotypes, A, B and C, are presented with different R groups (A) [18, 19, 25, 26]. One- or two-Gal-residue deletion on the O35Elgt5 or the O35EgalE LOS branch is displayed, respectively (B) [27–29]. Abbreviations: Gal, galactose; Kdo, 3-deoxy-D-manno-octulosonic acid; Glc, glucose; GlcNAc, N-acetyl-D-glucosamine; p, pyranose.
2. Materials and methods
2.1. Bacterial strains
M. catarrhalis strains O35E (serotype A) and TTA24 (serotype B′) were kindly provided by Eric J. Hansen at University of Texas, Dallas, TX. O35ElpxA, an O35E LOS-deficient mutant, was previously developed in Dr. Gu’s laboratory [33]. M. catarrhalis serotype A strains 26395 and 26394, serotype B strains 26397, 3292, and 26400, and serotype C strains 26404 and 26391 were obtained from the Culture Collection of the University of Goteborg, Department of Clinical Bacteriology, Goteborg, Sweden. M. catarrhalis clinical strains that only bind to or show strong binding activity to a monoclonal antibody 8E7 against strain O35E LOS (serotype A) were assigned as serotype A/C’ as previously described [23]. Clinical strains that only bind to or display strong binding activity to the rabbit serum against serotype B LOS were assigned as serotype B′ as previously described [23]. M. catarrhalis strains 25238 (serotype A), 25239 (serotype B′), 43627 (serotype B′), and 49143 (serotype B′) were purchased from the American Type Culture Collection (Manassas, VA). Seven other M. catarrhalis clinical isolates M1 (serotype A/C’), M3 (serotype A/C’), M4 (serotype B′), M6 (serotype A/C’), M7 (serotype B′), M9 (serotype A/C’), and M10 (serotype B′) were kindly provided by Goro Mogi, Oita Medical University, Oita, Japan.
2.2. Construction of O35Elgt5 and O35EgalE mutants and the glycosyl composition and linkage analyses of their LOSs
Unless otherwise specified, all reagents were obtained from Sigma Chemical Co. (St. Louis, MO). M. catarrhalis O35E LOS mutants were constructed as previously described [29, 34]. The mutant with an inactivated lgt5 or galE replaced by a Kanamycin resistant cassette was verified by nucleotide sequence analysis and named O35Elgt5 or O35EgalE. The LOSs from strains O35E, O35Elgt5, and O35EgalE were extracted with phenol-water extraction as described [21, 26]. The protein or nucleic acid content of the LOSs was less than 2% of the total weight. The OS from the LOS of O35E, O35Elgt5, or O35EgalE was prepared for glycosyl composition and linkage analyses. Briefly, the purified LOSs were washed with a 9:1 ethanol / water (v / v) mixture to remove phospholipids. The OSs were prepared by mild acid hydrolysis of LOSs in 1% aqueous acetic acid (v / v) for 2.5 h at 100°C and gel-filtration chromatography using Bio-Gel P-2 with deionized water as the eluent. The glycosyl compositions of OSs in O35E, O35Elgt5, and O35EgalE were determined by the preparation and gas chromatography-mass spectrometry (GC-MS) analysis of trimethylsilyl methylglycosides [35]. The glycosyl linkages were determined by the preparation and GC-MS analysis of partially methylated aldiol acetates [36]. Analysis of GC-MS was performed on an HP-5890 GC interfaced to a mass selective detector 5970 MSD using a Supelco DB1 fused silica capillary column (30 mm × 0.25 mm internal diameter, J & W Scientific, Folsom, CA).
2.3. Detoxification of LOS, derivatization of dLOS, and conjugation of the derivatized dLOS to carrier protein
Each LOS (300 mg) from O35E, O35Elgt5, or O35EgalE was detoxified with anhydrous hydrazine and purified as previously described [37]. The final carbohydrate-containing fractions were freeze-dried and designated as detoxified LOS (dLOS). Each dLOS (80 mg) was derivatized with a linker, adipic acid dihydrazide (ADH; Aldrich Chemical Co., Milwaukee, WI) [38]. The purified derivative containing both carbohydrate and adipic hydrazide (AH) was lyophilized and named as AH-dLOS. Each AH-dLOS (20 mg) was coupled to TT (Connaught Laboratories Inc., Swiftwater, PA) to form conjugates as described [38]. The purified compound containing both carbohydrate and protein was pooled and designated as dLOS-TT. All conjugates were analyzed for their composition of carbohydrate and protein, using AH-dLOS and bovine serum albumin (BSA) as standards [39, 40].
2.4. Preparation of hyperimmune sera
Female New Zealand White rabbits (2 to 3 kg) were obtained from Taconic Farms Inc. (Germantown, NY). The rabbits were handled according to National Institutes of Health (NIH) guidelines under Animal Study Proposal 1214. Two rabbits were injected subcutaneously at four injection sites (1 dose every 4 weeks, 3 doses total) with 109 colony forming unit (CFU)/ml heat-killed (60°C for 1 h) bacterial suspension of strain O35E, O35Elgt5, or O35EgalE in Dulbecco’s phosphate-buffered saline (D-PBS) plus Sigma Adjuvant System (250 mg each of monophosphoryl lipid A and synthetic trehalose dicorynomycolate). Blood samples were collected before the immunization and 2 weeks after each injection.
2.5. Antigenicity of the conjugates
Antigenicity of the conjugates and O35E LOS was tested with enzyme-linked immunosorbent assay (ELISA) using rabbit hyperimmune serum against O35E whole cells [41]. Briefly, the 96-well plate was coated with each conjugate or O35E LOS (10 μg of carbohydrate per ml) overnight followed by blocking with 1% BSA in PBS for 1 h. The rabbit serum against strain O35E (1/8,000) was added to the plate for 3 h of incubation before alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin (Ig)G (Sigma) was added for 2 h of incubation. PBS containing 0.025% Tween 20 was used for washings between steps. After the enzyme substrate was added and the mixture was incubated for 30 min, the reactions were read by a microplate autoreader (BioTek, Winooski, VT) at A405.
2.6. Immunogenicity of the conjugates
Immunogenicity of the conjugates was examined in rabbits. Two rabbits per group were injected subcutaneously (1 dose every 4 weeks, 3 doses total) with dLOS-TT (50 μg of carbohydrate each rabbit) with or without Sigma Adjuvant System. Two rabbits as controls were injected simultaneously with a mixture of dLOSs from O35E, O35Elgt5, and O35EgalE, and TT (50 μg of each for one rabbit) with the adjuvant. The rabbits were bled before the immunization and 2 weeks after each injection. Serum anti-LOS IgG levels were detected with ELISA as described above and expressed as ELISA units, using LOS from strain O35E, O35Elgt5, or O35EgalE as a coating antigen. The rabbit hyperimmune sera against strain O35E, O35Elgt5, and O35EgalE whole cells were used as references. Rabbit antisera against conjugate 26397 (B) or 26404 (C) dLOS-TT plus the adjuvant with 3 injections as positive controls were prepared as previously described [22, 23].
2.7. Bactericidal assay
Rabbit pre- and post-immune sera were inactivated at 56°C for 30 min and tested for bactericidal activity against M. catarrhalis by a microbactericidal assay [21] with modifications. M. catarrhalis strains were grown on chocolate plates at 37°C under 5% CO2 overnight and 20 single colonies were spread to another plate and incubated at 37°C under 5% CO2 for 4 h. The sera were diluted at 1:5 followed by two-fold serial dilutions with Dulbecco’s PBS containing calcium, magnesium, and 0.1% gelatin (DPBSG) in duplicate. Each well of a 96-well plate contained 50 μl of serially diluted sera. By prescreening, the 4 h-log-phase bacteria were adjusted to 6 × 103 CFU/ml in DPBSG with 70% transmission at 540 nm against viable counts, and 30 μl of bacterial suspension was added to each well (approximately 100–200 CFU per well for convenient counting). After incubation at 37°C for 60 min, 50 μl of the mixture was plated onto chocolate agar plates. The plates were incubated at 37°C under 5% CO2 overnight, and the colonies were counted. Controls included complement, inactive complement, and a positive serum sample. The highest serum dilution causing 50% killing was expressed as the reciprocal bactericidal titer.
2.8. Limulus amebocyte lysate (LAL) assay
The LOSs and dLOSs were tested by the LAL QCL-1000 (Cambrex Bio Science Walkersville, Inc., Walkersville, MD). The sensitivity of the LAL assay is 0.1 endotoxin unit (EU)/μl.
2.9. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining
SDS-PAGE and silver staining were performed as previously described [42]. Lipopolysaccharides (LPSs) from Salmonella minnesota Ra and Rc mutants (Sigma) were loaded as molecular markers.
2.10. Whole-cell ELISA
To examine the cross-reactivity of IgG in rabbit sera against the conjugates, each strain was suspended in PBS at an optical density of 65% transmission at 540 nm. Microtiter plates were coated with 100 μl of the suspensions and incubated at 50°C overnight for evaporation. The following steps were the same as described in the LOS ELISA except that 3% BSA-PBS was used for blocking. Rabbit sera against whole cells of strain O35E (A), 26397 (B), or 26404 (C) were used as references [22, 23].
2.11. Immuno-electron microscopy of bacteria
Strains O35E, 25238, 26397, and 26404 were suspended in 100 μl of saline on slides. Formvar-coated 200-mesh grids (Electron Microscopy Sciences, Hatfield, PA) were floated on the bacterial suspensions for 1 min and blotted dry. The grids were subsequently blocked with 0.5% BSA in 0.01 M PBS for 30 min and incubated with a 1:200 dilution of the rabbit serum against O35Elgt5 dLOS-TT or a corresponding rabbit preimmune serum for 15 min at room temperature. The grids were then incubated with 1:20 gold (5-nm diameter)-conjugated goat anti-rabbit IgG (Ted Pella, Inc., Redding, CA) for 60 min. The grids were blotted and washed three times with PBS between steps. After the final washing, the bacteria were negatively stained with a mixture of equal volumes of 2% ammonium acetate and 2% ammonium molybdate (Sigma). The grids were viewed with a JEOL JEM-1010 transmission electron microscope (JEOL Ltd., Tokyo, Japan).
2.12. Statistical analysis
Rabbit serum IgG level and cross-reactivity against M. catarrhalis strains were expressed as geometric mean of ELISA units of two independent observations. Bactericidal activity was expressed as titers of two independent observations and positive percentage. The nonparametric correlation between the IgG ELISA units and bactericidal titers were analyzed by Spearman test. P values less than 0.05 were considered significant.
3. Results
3.1. Glycosyl composition and linkage analyses of LOS from O35Elgt5 or O35EgalE mutant
Glycosyl composition and linkage analyses showed that the OS from the parental O35E contained Gal, Glc, GlcNAc, and Kdo. The O35E LOS had the presence of 4-substituted Glc (4-Glc) with terminal substituted Glc (t-Glc) and Gal (t-Gal) in 1:1 molar ratio. However, the O35Elgt5 LOS lacked the 4-subtituted Gal (4-Gal), indicating one Gal-residue loss when compared with the O35E LOS. On the other hand, the O35EgalE LOS lacked detectable levels of Gal, suggesting two Gal-residues were missing (Table 1; Fig. 1B).
Table 1.
Glycosyl linkage analysis of OSs isolated from O35E, O35Elgt5, and O35EgalE
| OS | Composition (relative mole, %)a |
||||||
|---|---|---|---|---|---|---|---|
| t-Glc | 2-Glc | 4-Glc | 3,4,6-Glc | t-Gal | 4-Gal | t-GlcNAc | |
| O35E | 14 | 26 | 12 | 12 | 14 | 12 | 10 |
| O35Elgt5 | 15 | 28 | 15 | 15 | 15 | -b | 12 |
| O35EgalE | 31 | 33 | - | 15 | - | - | 20 |
Abbreviations: t-Glc, 1,5-di-O-acetyl-1-deuterio-2,3,4,6-tetra-O-methyl-D-glucitol; 2-Glc, 1,2,5-tri-O-acetyl-1-deuterio-3,4,6-tri-O-methyl-D-glucitol; 4-Glc, 1,4,5-tri-O-acetyl-deuterio-2,3,6-tri-O-methyl-D-glucitol; 3,4,6-Glc, 1,3,4,5,6-penta-O-acetyl-1-deuterio-2-O-methyl-d-glucitol; t-Gal, 1,5-di-O-acetyl-1-deuterio-2,3,4,6-tetra-O-methyl-D-galactitol; 4-Gal, 1,4,5-tri-O-acetyl-deuterio-2,3,6-tri-O-methyl-D-galactitol; t-GlcNAc, 1,5-di-O-acetyl-2-(acetylmethylamino)-2-deoxy-1-deuterio-3,4,6-tri-O-methyl-D-glucitol. Kdo is also present, but not quantified.
Absent.
3.2. Characterization of the dLOSs
Because LOS is too toxic to be used as an antigen for humans, an alkaline anhydrous hydrazine was employed to detoxify the LOSs. After the treatment, the dLOSs and their LOSs were characterized with a silver-stained SDS-PAGE assay, which displayed a single band for 200 ng of each LOS from strains O35E, O35Elgt5, and O35EgalE (Fig. 2A). In contrast, as much as 10 μg of each dLOS did not show a band at the migration of each LOS, indicating that the residual LOS was less than 2%. By the LAL assay, each LOS from O35E, O35Elgt5 or O35EgalE displayed the endotoxin content of 24,400, 36,650 or 32,475 EU/μg, respectively. But each corresponding dLOS showed only 1.32, 0.07 or 0.91 EU/μg, accounting for an 18,538, 479,690 or 40,497-fold reduction of toxicity, respectively (Fig. 2B).
Fig. 2.
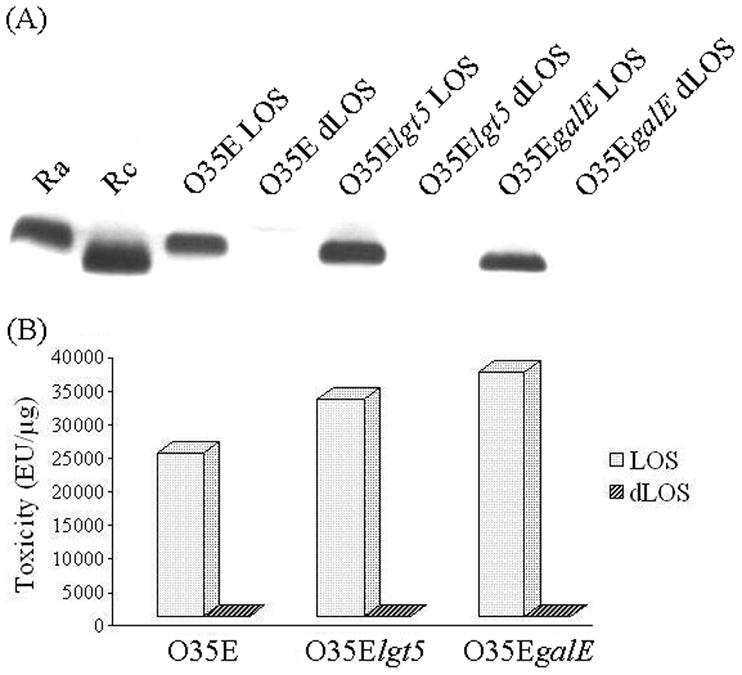
Silver-stained SDS-PAGE patterns (A) and the toxicity (B) of the LOSs and dLOSs from M. catarrhalis O35E, O35Elgt5, and O35EgalE. Each of the LOSs (200 ng/lane) and the dLOSs (10 μg/lane) was loaded with LPSs from Salmonella minnesota Ra (200 ng/lane) and Rc (200 ng/lane) mutants as molecular controls (A). LAL assay was employed to detect the endotoxin unit of the LOS and the dLOS from each strain (B).
3.3. Composition and antigenicity of the conjugates
After the synthesis reaction of each dLOS derivative with a carrier to form a dLOS-TT conjugate, the contents of carbohydrate and protein in the conjugate were analyzed (Table 2). For the conjugates derived from strains O35E, O35Elgt5, and O35EgalE, the molar ratios of dLOS to TT were 45:1, 62:1, and 56:1, respectively. The yields of the dLOS-TT derived from strains O35E, O35Elgt5, and O35EgalE were 30%, 41%, and 36%, respectively, on the basis of carbohydrate content. The antigenicity of all conjugates was similar to that of the O35E LOS (Table 2).
Table 2.
Composition, yield, and antigenicity of conjugate vaccines
| Conjugate | Conc. (μg/ml)
|
Yielda (%) | dLOS/TT ratio
|
A405c (Hyperimmune serum) | ||
|---|---|---|---|---|---|---|
| dLOS | TT | Wt | Molarb | |||
| O35E dLOS-TT | 276 | 306 | 30 | 0.9 | 45 | 1.6 |
| O35Elgt5 dLOS-TT | 382 | 342 | 41 | 1.1 | 62 | 1.6 |
| O35EgalE dLOS-TT | 319 | 328 | 36 | 1.0 | 56 | 1.7 |
Based on the starting amount of AH-dLOS and the AH-dLOS contained in the conjugates as measured by a phenol-sulfuric acid method [39].
Expressed as moles of dLOS per mole of protein, with molecular weights of 3,000 Da for dLOS and 150,000 Da for TT.
The antigenicity of conjugates was expressed as ELISA reactivity at A405 when the conjugates were used as coating antigens (10 μg of carbohydrate per ml) and a rabbit immune serum against O35E whole cells was used as a binding antibody (1/8,000). O35E LOS (10 μg of carbohydrate per ml) showed an A405 value of 0.8 under the same conditions.
3.4. Conjugate-induced rabbit serum IgG level evaluated with the homologous LOS and O35E LOS
The immunogenicity of the conjugates was studied by immunizing rabbits with different conjugates and detecting rabbit antiserum IgG with an ELISA assay (Table 3). A control mixture of dLOSs and TT with the adjuvant elicited a very low level of anti-LOS IgG after injections. In contrast, O35E dLOS-TT, a positive control, induced significant increases of anti-O35E LOS IgG after the second and the third injections (2187- and 6561-fold above the preimmune serum levels, respectively). Mutant O35Elgt5 dLOS-TT induced high levels of anti-LOS IgG after two and three injections (729- and 2187-fold against the homologous O35Elgt5 LOS, and 243- and 140-fold against O35E LOS, above the preimmune serum levels). Mutant O35EgalE dLOS-TT also enhanced the anti-LOS IgG level after two and three injections (16- and 27-fold against homologous O35EgalE LOS, and 16- and 140-fold against O35E LOS, above the preimmune serum levels). The adjuvant enhanced the levels of anti-LOS IgG in all conjugate groups. Notably, O35Elgt5 or O35EgalE dLOS-TT with the adjuvant all elicited high levels of serum IgG against O35E LOS after two and three injections (729- and 729-fold or 243- and 1263-fold above the preimmune serum levels, respectively).
Table 3.
ELISA reactivity of conjugate-induced rabbit serum IgG evaluated with corresponding homologous LOS and the wild type O35E LOS
| Immunogena | Injection no. | Geometric mean (rabbit 1, rabbit 2) of ELISA unitsb |
|
|---|---|---|---|
| Binding to homologous LOS | Binding to O35E LOS | ||
| O35E dLOS-TT | 0 | 1 (1, 1) | 1 (1, 1) |
| 2 | 2187 (2187, 2187) | 2187 (2187, 2187) | |
| 3 | 6561 (6561, 6561) | 6561 (6561, 6561) | |
| O35Elgt5 dLOS-TT | 0 | 1 (1, 1) | 1 (1, 1) |
| 2 | 729 (729, 729) | 243 (243, 243) | |
| 3 | 2187 (2187, 2187) | 140 (243, 81) | |
| O35EgalE dLOS-TT | 0 | 1 (1, 1) | 1 (1, 1) |
| 2 | 16 (27, 9) | 16 (9, 27) | |
| 3 | 27 (27, 27) | 140 (243, 81) | |
| O35E dLOS-TT + Adjuvant | 0 | 1 (1, 1) | 1 (1, 1) |
| 2 | 6561 (6561, 6561) | 6561 (6561, 6561) | |
| 3 | 19683 (19683, 19683) | 19683 (19683, 19683) | |
| O35Elgt5 dLOS-TT + Adjuvant | 0 | 1 (1, 1) | 1 (1, 1) |
| 2 | 3788 (2187, 6561) | 729 (729, 729) | |
| 3 | 11364 (6561, 19683) | 729 (729, 729) | |
| O35EgalE dLOS-TT + Adjuvant | 0 | 1 (1, 1) | 1 (1, 1) |
| 2 | 243 (81, 729) | 243 (81, 729) | |
| 3 | 11364 (6561, 19683) | 1263 (729, 2187) | |
| dLOSs + TT + Adjuvant | 0 | 1 (1, 1) | 1 (1, 1) |
| 2 | 1 (1, 1) | 1 (1, 1) | |
| 3 | 3 (3, 3) | 3 (3, 3) | |
Two rabbits per group were injected subcutaneously (1 dose every 4 weeks, 3 doses total) with dLOS-TT (50 μg of carbohydrate each rabbit) with or without Sigma Adjuvant System. Two rabbits as controls were injected simultaneously with a mixture of dLOSs from O35E, O35Elgt5, and O35EgalE, and TT (50 μg of each for one rabbit) plus the adjuvant. The rabbits were bled before the immunization and 2 weeks after each injection.
ELISA units were based on a reference rabbit serum against the whole cells of strain O35E, O35Elgt5, or O35EgalE, respectively. Each homologous LOS was employed as a coating antigen for its corresponding dLOS-TT-elicited sera and the O35E LOS was also used as a coating antigen for O35Elgt5 or O35EgalE dLOS-TT-elicited sera.
3.5. Bactericidal activity of rabbit sera against the conjugates
To explore the biological functions of the rabbit antisera, a complement–mediated bactericidal assay was used for measurement. 50% to 100% of rabbit sera against each conjugate with or without the adjuvant displayed bactericidal activity toward the wild type strain O35E after two and three injections (Table 4). There was a correlation between anti-O35E LOS IgG ELISA units and the bactericidal titers among 14 rabbits (r =0.420, P =0.0261), suggesting that the serum IgG might be a potential factor for the complement-mediated bactericidal activity of the rabbit sera.
Table 4.
Bactericidal activity against M. catarrhalis O35E for rabbit sera elicited by conjugate vaccines
| Immunogena | Injection no. | Bactericidal titer (rabbit 1, rabbit 2)b | No. of rabbit positive/no. in group (positive rate (%))c |
|---|---|---|---|
| O35E dLOS-TT | 0 | <5, <5 | 0/2 (0) |
| 2 | 5, 5 | 2/2 (100) | |
| 3 | 5, 5 | 2/2 (100) | |
| O35Elgt5 dLOS-TT | 0 | <5, <5 | 0/2 (0) |
| 2 | <5, 5 | 1/2 (50) | |
| 3 | <5, 10 | 1/2 (50) | |
| O35EgalE dLOS-TT | 0 | <5, <5 | 0/2 (0) |
| 2 | <5, 5 | 1/2 (50) | |
| 3 | <5, 5 | 1/2 (50) | |
| O35E dLOS-TT + Adjuvant | 0 | <5, <5 | 0/2 (0) |
| 2 | 5, <5 | 1/2 (50) | |
| 3 | 20, 10 | 2/2 (100) | |
| O35Elgt5 dLOS-TT + Adjuvant | 0 | <5, <5 | 0/2 (0) |
| 2 | 5, 5 | 2/2 (100) | |
| 3 | 10, <5 | 1/2 (50) | |
| O35EgalE dLOS-TT + Adjuvant | 0 | <5, <5 | 0/2 (0) |
| 2 | 10, 5 | 2/2 (100) | |
| 3 | 5, <5 | 1/2 (50) | |
| dLOSs + TT + Adjuvant | 0 | <5, <5 | 0/2 (0) |
| 2 | <5, <5 | 0/2 (0) | |
| 3 | <5, <5 | 0/2 (0) |
See table 3, footnote a.
The reciprocal of the highest serum dilution causing >50% killing was expressed as the bactericidal titer.
Based on a bactericidal titer of an individual serum of ≥5.
Two rabbit sera with higher bactericidal titers against each conjugate, O35E dLOS-TT (both with three injections), O35Elgt5 dLOS-TT (rabbit 1 with three injections and rabbit 2 with two injections), and O35EgalE dLOS-TT (both with two injections), plus the adjuvant were then chosen. They were further tested for bactericidal activities toward heterologous-prototype strains. The sera against O35E dLOS-TT displayed titers of 20 and 40, <5 and <5, and <5 and 20 against strains 25238 (A), 26397 (B), and 26404 (C), respectively. The sera against O35Elgt5 showed titers of 5 and 5, 5 and 5, 5 and <5 against strains 25238 (A), 26397 (B), and 26404 (C), respectively. The sera against O35EgalE dLOS-TT revealed titers of 5 and <5, <5 and <5, and 10 and <5 against strains 25238 (A), 26397 (B), and 26404 (C), respectively. As positive controls, previous rabbit sera against 26397 (B) or 26404 (C) dLOS-TT plus the adjuvant with three injections both showed bactericidal titers of 20 toward their homologous strains.
Since the rabbit sera induced by O35Elgt5 dLOS-TT showed broad bactericidal activity against 3 prototype strains of M. catarrhalis, a serum (rabbit 1) against O35Elgt5 dLOS-TT plus the adjuvant with three injections was selected to test its bactericidal activity toward additional heterologous M. catarrhalis strains. It displayed bactericidal titers of 10, 5, 5, 10, 5, 10, 5, 10, 10, 5, 5, 10, 5, 5, 10, and 5 against strains 26395, 26394, M1, M3, M6, M9, 26391, 3292, 26400, 25239, 49143, 43627, TTA24, M4, M7, and M10, respectively. Thus, O35Elgt5 dLOS-TT-elicited rabbit sera exhibited a broad bactericidal spectrum against all tested M. catarrhalis three-serotype strains and clinical isolates.
3.6. Cross-reactivity of rabbit sera against conjugates from O35Elgt5 or O35EgalE
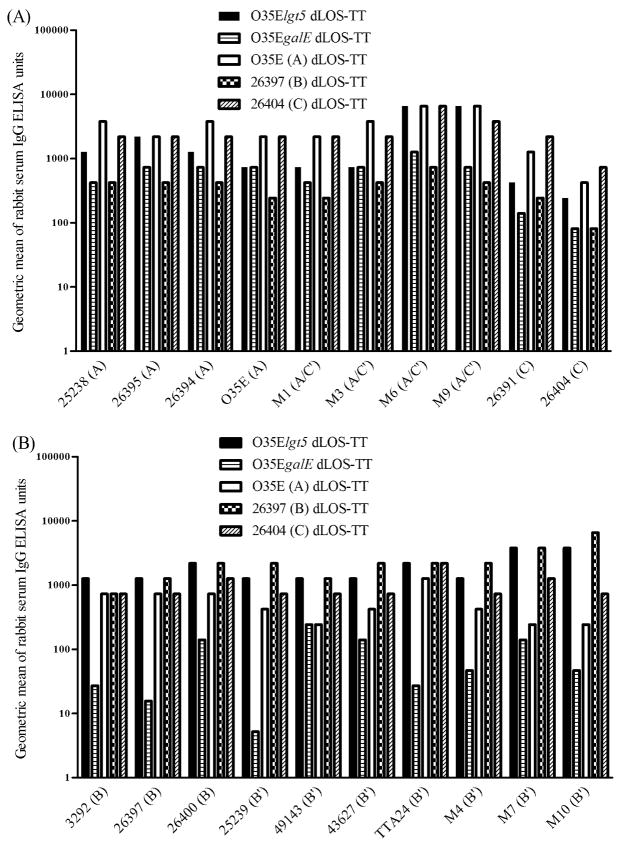
A whole-cell ELISA was further used to explore the cross-reactivity of rabbit sera against conjugates from O35Elgt5 or O35EgalE by comparing them with positive rabbit sera toward each conjugate from strains O35E (A), 26397 (B), and 26404 (C) (Fig. 3). Two rabbit sera elicited by each conjugate plus the adjuvant with three injections were tested. An O35E LOS-deficient mutant, O35ElpxA, had very low cross-reactions (geometric mean units ≤3) with all the rabbit sera. This observation demonstrated that the whole-cell ELISA reactions between the rabbit sera and all the strains were mainly on M. catarrhalis LOS antigen. As for binding with serotype A and C strains, rabbit sera against O35Elgt5 dLOS-TT showed higher reactivity than that against 26397 (B) dLOS-TT. Rabbit sera toward O35EgalE dLOS-TT also displayed better binding activity than that against 26397 (B) dLOS-TT for most serotype A and C strains (Fig. 3A). As for binding with serotype B strains, rabbit sera against O35Elgt5 dLOS-TT had a higher reactivity than that toward O35E (A) dLOS-TT or 26404 (C) dLOS-TT while rabbit sera induced by O35EgalE dLOS-TT showed low binding activity (Fig. 3B). Therefore, O35Elgt5 dLOS-TT-elicited rabbit sera demonstrated the broadest spectrum of cross-reactivity against the M. catarrhalis strains among all the tested sera.
Fig. 3.
Whole-cell ELISA binding activity of rabbit sera elicited by dLOS-TT conjugates from O35Elgt5, O35EgalE, O35E (type A), 26397 (type B), and 26404 (type C) among M. catarrhalis clinical strains. Two rabbits were injected subcutaneously (1 dose every 4 weeks, 3 doses total) with each dLOS-TT (50 μg of carbohydrate each rabbit) from O35Elgt5, O35EgalE, O35E (type A), 26397 (type B), and 26404 (type C), plus Sigma Adjuvant System. The rabbits were bled before the immunization and 2 weeks after each injection. The binding activity of the rabbit sera toward serotype A and C strains (A) or B strains (B) is shown as a geometric mean of ELISA units for two rabbits after three injections with each conjugate plus the adjuvant. O35ElpxA, an O35E LOS-deficient mutant displayed geometric means of ELISA units ≤3 when binding with all rabbit sera against the conjugates.
3.7. Immuno-electron microscopy of M. catarrhalis binding with O35Elgt5 dLOS-TT-vaccinated rabbit serum IgG
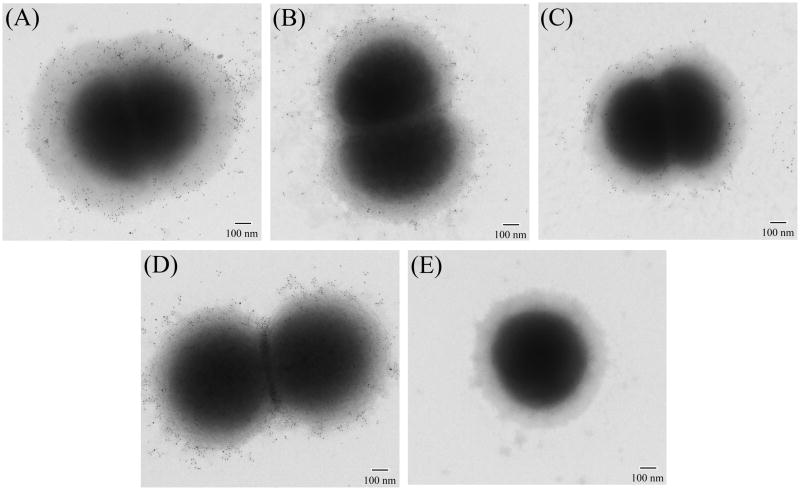
To determine if rabbit serum IgG induced by the O35Elgt5 dLOS-TT recognizes surface-exposed epitopes on the intact M. catarrhalis, immuno-electron microscopy was conducted on fixed whole cells. Rabbit serum IgG bound to the surface of all representative strains O35E (type A, parental), 25238 (type A, heterologous), 26397 (type B, heterologous), and 26404 (type C, heterologous), as indicated by the electron dense gold particles scattered across the surface of the cells (Fig. 4). This confirmed the broad cross-reactivity of the O35Elgt5 dLOS-TT-induced rabbit serum binding with all three serotypes of M. catarrhalis strains.
Fig. 4.
Binding of the rabbit serum against O35Elgt5 dLOS-TT to the surface of M. catarrhalis as visualized by immuno-electron microscopy. Rabbits were injected subcutaneously (1 dose every 4 weeks, 3 doses total) with O35Elgt5 dLOS-TT (50 μg of carbohydrate each rabbit) and Sigma Adjuvant System. The rabbits were bled before the immunization and 2 weeks after each injection. Cells of strains O35E (type A) (A), 25238 (type A) (B), 26397 (type B) (C), and 26404 (type C) (D) were incubated with a rabbit serum against O35Elgt5 dLOS-TT plus the adjuvant after three injections (1:200 dilution). Cells of strain 25238 (E) were also incubated with a corresponding rabbit preimmune serum (1:200 dilution) as a control. Data are representative of three independent experiments. Gold particles are 5 nm in diameter. Scale bars, 100 nm.
4. Discussion
Conjugate vaccine candidates from M. catarrhalis serotype A, B, and C were previously synthesized and elicited antibodies with bactericidal activity in mice and rabbits. However, each of these conjugates was observed to protect against only partial M. catarrhalis clinical strains. In this study, we synthesized conjugate vaccines based on M. catarrhalis mutant LOSs with the Pk epitope disruptions, which may have broader coverage with the probability of autoimmune responses in humans eliminated. These conjugates were designed to act as potential vaccine candidates to substitute any of the conjugates from M. catarrhalis wild type strains. Our prior observations indicate that conjugate vaccines generally induce a higher level of serum antibodies with a positive or higher level of complement-mediated bactericidal titers in rabbits than that in mice [21–23]. We therefore chose rabbit model instead of mouse model in current study, though it may limit the number of animals that could be used per group. All dLOS-TT conjugates from the mutants O35Elgt5 and O35EgalE induced high levels of serum anti-LOS IgG in rabbits. The rabbit sera against the O35Elgt5 dLOS-TT showed a higher specific binding reactivity and bactericidal activity to all tested M. catarrhalis strains. Our findings demonstrate that dLOS-TT conjugate from O35Elgt5 mutant may act as a vaccine candidate with broad coverage against most M. catarrhalis strains and clinical isolates.
Conserved LOS epitope(s) can exist among different strains, species and genera of non-enteric Gram-negative human pathogens [30]. But it is not clear in our case why the dLOS-TT derived from the O35Elgt5 mutant would serve as a conserved antigen among different M. catarrhalis serotype strains. One explanation is that the Galβ1-4Glcα1-2Glc at the 6-linked position of the trisubstituted Glc residue of the LOS forms a conserved epitope to elicit IgGs toward all serotypes of M. catarrhalis. The deletion of the terminal Gal residue resulted in the exposure of the Galβ1-4Glcα1-2Glc epitope on the O35Elgt5 LOS, which may be concealed in the natural O35E OS branch. Moreover, the removal of the terminal Gal residue might eliminate the latent steric effect interfering with the epitope exposure on the LOS, which may contribute to forming such a highly conserved epitope among all serotype strains. Our observation is in agreement with a report of Schwingel et al., which indicates that lgt5 mutant LOSs from M. catarrhalis serotype A, B and C strains all have high cross-reactivity with human serum IgGs against any of serotype A, B and C clinical strains [43].
The core OS of some Gram-negative bacterial LOS, Galα1-4Galβ1-4Glc, has been found to be immunochemically identical to the Pk antigen in humans. This molecular imitation may be involved in the pathogenesis of mucosal infections by the colonization and spread of bacteria [30–32]. The mimicry has been of particular concern to investigators using bacterial products from these human pathogens as vaccines, because it may possibly cause autoimmune responses in the host [32]. We constructed the O35Elgt5 and O35EgalE mutants with the Pk epitope disrupted to remove the potentiality of the cross-reaction with human antigen for our LOS-derived conjugate vaccines. The disruption of the Pk epitope on the LOS branch ensured the safety of our mutant-dLOS-based conjugates as vaccines used on humans. In theory, the O35EgalE dLOS-TT is a shorter and more conserved antigen than the O35Elgt5 dLOS-TT but it displayed less immunogenicity, which was consistent with a previous study in meningococcal LOS-mutant conjugates [44].
In summary, dLOS-TT conjugates derived from lgt5- and galE-knockout LOS mutants of M. catarrhalis O35E were synthesized and all elicited high serum anti-LOS IgG with bactericidal activity against strain O35E in rabbits. Rabbit sera against the O35Elgt5 dLOS-TT demonstrated a broad spectrum of bactericidal activity and cross-reactivity against most tested M. catarrhalis clinical strains. Therefore, O35Elgt5 dLOS-TT conjugate can be further investigated as a candidate vaccine against M. catarrhalis for clinical applications.
Acknowledgments
We are grateful to Eric J. Hansen at University of Texas, Dallas, TX and Goro Mogi (deceased) at Oita Medical University, Oita, Japan for providing strains. We thank Anup Datta at Glycobiology Research and Training Center, University of California, San Diego, La Jolla, California for the composition and structure analyses of LOSs. We thank the NIH Fellows Editorial Board for carefully reviewing and providing critical comments during the preparation of the manuscript. This research was supported by the Intramural Research Program of the National Institute on Deafness and Other Communication Disorders (NIDCD), NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez Vidakovics ML, Riesbeck K. Virulence mechanisms of Moraxella in the pathogenesis of infection. Curr Opin Infect Dis. 2009;22:279–85. doi: 10.1097/qco.0b013e3283298e4e. [DOI] [PubMed] [Google Scholar]
- 2.Corbeel L. What is new in otitis media? Eur J Pediatr. 2007;166:511–9. doi: 10.1007/s00431-007-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy TF, Parameswaran GI. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis. 2009;49:124–31. doi: 10.1086/599375. [DOI] [PubMed] [Google Scholar]
- 4.Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med. 2005;172:195–9. doi: 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–65. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 6.Verduin CM, Hol C, Fleer A, van Dijk H, van Belkum A. Moraxella catarrhalis: from emerging to established pathogen. Clin Microbiol Rev. 2002;15:125–44. doi: 10.1128/CMR.15.1.125-144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardle JK. Branhamella catarrhalis as an indirect pathogen. Drugs. 1986;31 (Suppl 3):93–6. doi: 10.2165/00003495-198600313-00020. [DOI] [PubMed] [Google Scholar]
- 8.Mawas F, Ho MM, Corbel MJ. Current progress with Moraxella catarrhalis antigens as vaccine candidates. Expert Rev Vaccines. 2009;8:77–90. doi: 10.1586/14760584.8.1.77. [DOI] [PubMed] [Google Scholar]
- 9.Murphy TF. Vaccine development for Moraxella catarrhalis: rationale, approaches and challenges. Expert Rev Vaccines. 2009;8:655–8. doi: 10.1586/erv.09.28. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, McMichael JC, VanDerMeid KR, Hahn D, Mininni T, Cowell J, et al. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–5. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aebi C, Cope LD, Latimer JL, Thomas SE, Slaughter CA, McCracken GH, Jr, et al. Mapping of a protective epitope of the CopB outer membrane protein of Moraxella catarrhalis. Infect Immun. 1998;66:540–8. doi: 10.1128/iai.66.2.540-548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang YP, Myers LE, McGuinness U, Chong P, Kwok Y, Klein MH, et al. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–99. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 13.Helminen ME, Maciver I, Latimer JL, Cope LD, McCracken GH, Jr, Hansen EJ. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–10. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy TF, Kyd JM, John A, Kirkham C, Cripps AW. Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J Infect Dis. 1998;178:1667–75. doi: 10.1086/314501. [DOI] [PubMed] [Google Scholar]
- 15.Rahman M, Holme T, Jonsson I, Krook A. Lack of serotype-specific antibody response to lipopolysaccharide antigens of Moraxella catarrhalis during lower respiratory tract infection. Eur J Clin Microbiol Infect Dis. 1995;14:297–304. doi: 10.1007/BF02116522. [DOI] [PubMed] [Google Scholar]
- 16.Fomsgaard JS, Fomsgaard A, Hoiby N, Bruun B, Galanos C. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect Immun. 1991;59:3346–9. doi: 10.1128/iai.59.9.3346-3349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaneechoutte M, Verschraegen G, Claeys G, Van Den Abeele AM. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J Clin Microbiol. 1990;28:182–7. doi: 10.1128/jcm.28.2.182-187.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edebrink P, Jansson PE, Rahman MM, Widmalm G, Holme T, Rahman M, et al. Structural studies of the O-polysaccharide from the lipopolysaccharide of Moraxella (Branhamella) catarrhalis serotype A (strain ATCC 25238) Carbohydr Res. 1994;257:269–84. doi: 10.1016/0008-6215(94)80040-5. [DOI] [PubMed] [Google Scholar]
- 19.Edebrink P, Jansson PE, Rahman MM, Widmalm G, Holme T, Rahman M. Structural studies of the O-antigen oligosaccharides from two strains of Moraxella catarrhalis serotype C. Carbohydr Res. 1995;266:237–61. doi: 10.1016/0008-6215(94)00276-l. [DOI] [PubMed] [Google Scholar]
- 20.Hu WG, Chen J, McMichael JC, Gu XX. Functional characteristics of a protective monoclonal antibody against serotype A and C lipooligosaccharides from Moraxella catarrhalis. Infect Immun. 2001;69:1358–63. doi: 10.1128/IAI.69.3.1358-1363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu XX, Chen J, Barenkamp SJ, Robbins JB, Tsai CM, Lim DJ, et al. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect Immun. 1998;66:1891–7. doi: 10.1128/iai.66.5.1891-1897.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu S, Gu XX. Synthesis and characterization of lipooligosaccharide-based conjugate vaccines for serotype B Moraxella catarrhalis. Infect Immun. 2005;73:2790–6. doi: 10.1128/IAI.73.5.2790-2796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu S, Gu XX. Biological and immunological characteristics of lipooligosaccharide-based conjugate vaccines for serotype C Moraxella catarrhalis. Infect Immun. 2007;75:2974–80. doi: 10.1128/IAI.01915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiao X, Hirano T, Hou Y, Gu XX. Specific immune responses and enhancement of murine pulmonary clearance of Moraxella catarrhalis by intranasal immunization with a detoxified lipooligosaccharide conjugate vaccine. Infect Immun. 2002;70:5982–9. doi: 10.1128/IAI.70.11.5982-5989.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edebrink P, Jansson PE, Widmalm G, Holme T, Rahman M. The structures of oligosaccharides isolated from the lipopolysaccharide of Moraxella catarrhalis serotype B, strain CCUG 3292. Carbohydr Res. 1996;295:127–46. doi: 10.1016/s0008-6215(96)90132-9. [DOI] [PubMed] [Google Scholar]
- 26.Masoud H, Perry MB, Richards JC. Characterization of the lipopolysaccharide of Moraxella catarrhalis. Structural analysis of the lipid A from M. catarrhalis serotype A lipopolysaccharide. Eur J Biochem. 1994;220:209–16. doi: 10.1111/j.1432-1033.1994.tb18616.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JC, Collins PM, Klipic Z, Grice ID, Peak IR. Identification of a novel glycosyltransferase involved in LOS biosynthesis of Moraxella catarrhalis. Carbohydr Res. 2006;341:2600–6. doi: 10.1016/j.carres.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Zaleski A, Scheffler NK, Densen P, Lee FK, Campagnari AA, Gibson BW, et al. Lipooligosaccharide P(k) (Galalpha1–4Galbeta1–4Glc) epitope of moraxella catarrhalis is a factor in resistance to bactericidal activity mediated by normal human serum. Infect Immun. 2000;68:5261–8. doi: 10.1128/iai.68.9.5261-5268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng D, Hu WG, Choudhury BP, Muszynski A, Carlson RW, Gu XX. Role of different moieties from the lipooligosaccharide molecule in biological activities of the Moraxella catarrhalis outer membrane. FEBS J. 2007;274:5350–9. doi: 10.1111/j.1742-4658.2007.06060.x. [DOI] [PubMed] [Google Scholar]
- 30.Campagnari AA, Spinola SM, Lesse AJ, Kwaik YA, Mandrell RE, Apicella MA. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990;8:353–62. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 31.Mandrell RE, Apicella MA. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402. doi: 10.1016/S0171-2985(11)80352-9. [DOI] [PubMed] [Google Scholar]
- 32.Moran AP, Prendergast MM, Appelmelk BJ. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol. 1996;16:105–15. doi: 10.1111/j.1574-695X.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 33.Peng D, Hong W, Choudhury BP, Carlson RW, Gu XX. Moraxella catarrhalis bacterium without endotoxin, a potential vaccine candidate. Infect Immun. 2005;73:7569–77. doi: 10.1128/IAI.73.11.7569-7577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu S, Xie H, Datta A, Naidu N, Gu XX. Galactose residues on the lipooligosaccharide of Moraxella catarrhalis 26404 form the epitope recognized by the bactericidal antiserum from conjugate vaccination. Infect Immun. 2008;76:4251–8. doi: 10.1128/IAI.01570-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.York WS, AG, Darvill MM, Stevenson TT, Albersheim P. Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 1985;118:3–40. [Google Scholar]
- 36.Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1984;131:209–17. [Google Scholar]
- 37.Gupta RK, Szu SC, Finkelstein RA, Robbins JB. Synthesis, characterization, and some immunological properties of conjugates composed of the detoxified lipopolysaccharide of Vibrio cholerae O1 serotype Inaba bound to cholera toxin. Infect Immun. 1992;60:3201–8. doi: 10.1128/iai.60.8.3201-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu XX, Tsai CM. Preparation, characterization, and immunogenicity of meningococcal lipooligosaccharide-derived oligosaccharide-protein conjugates. Infect Immun. 1993;61:1873–80. doi: 10.1128/iai.61.5.1873-1880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 40.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 41.Gu XX, Tsai CM, Ueyama T, Barenkamp SJ, Robbins JB, Lim DJ. Synthesis, characterization, and immunologic properties of detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins. Infect Immun. 1996;64:4047–53. doi: 10.1128/iai.64.10.4047-4053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–9. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 43.Schwingel JM, Edwards KJ, Cox AD, Masoud H, Richards JC, St Michael F, et al. The Use of Moraxella catarrhalis Lipooligosaccharide Mutants to Identify Specific Oligosaccharide Epitopes Recognized by Human Serum Antibodies. Infect Immun. 2009;77:4548–58. doi: 10.1128/IAI.00294-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox AD, Zou W, Gidney MA, Lacelle S, Plested JS, Makepeace K, et al. Candidacy of LPS-based glycoconjugates to prevent invasive meningococcal disease: developmental chemistry and investigation of immunological responses following immunization of mice and rabbits. Vaccine. 2005;23:5045–54. doi: 10.1016/j.vaccine.2005.06.011. [DOI] [PubMed] [Google Scholar]