Summary
OBJECTIVE
To identify risk factors for uncomplicated malaria in highland areas of East Africa at higher risk of malaria epidemics, in order to design appropriate interventions.
METHODS
Prospective, population-based, case–control study in the Nandi Hills, a highland area of western Kenya, to identify environmental, sociodemographic and behavioural factors associated with clinical malaria. Data were collected using field observation, a structured questionnaire, and a global positioning system device.
RESULTS
We interviewed 488 cases of slide-confirmed malaria and 980 age-matched controls. Multivariate analyses associated higher malaria risk with living <250 m of a forest [OR = 3.3 (95% CI 1.5, 7.1)], <250 m of a swamp [2.8 (1.3, 5.9)], <200 m of maize fields [2.0 (1.2, 3.4)], in the absence of trees <200 m [1.6 (1.2, 2.2)], on flat land [1.6 (1.2, 2.2)], in houses without ceilings [1.5 (1.1, 2.2)], in houses with a separate kitchen building [1.8 (1.4, 2.3)] and in households where the female household head had no education [1.9 (1.1, 3.1)]. Travelling out of the study site [2.2 (1.2, 4.1)] was also associated with increased risk.
CONCLUSIONS
In this East African highland area, risk of developing uncomplicated malaria was multifactorial with a risk factor profile similar to that in endemic regions. Households within close proximity to forest and swamp borders are at higher risk of malaria and should be included in indoor residual spraying campaigns.
Keywords: highland malaria, Plasmodium falciparum, case–control, risk factors, environmental, household-level
Introduction
Historically, malaria transmission in the East African highlands (>1500 m) has been low and unstable but notable increases have occurred in the past few decades. Transmission is spatially focal in the highlands. Cases and anopheline mosquitoes cluster in low-lying valley bottoms, often in close proximity to swamps or rivers (Brooker et al. 2004; Minakawa et al. 2004; Zhou et al. 2004, 2007; Ernst et al. 2006; Githeko et al. 2006). Although overall risk may be higher in these areas, behavioural, micro-environmental and socioeconomic factors likely modify the relationship between malaria risk and environmental suitability for transmission. Little research has been conducted to examine household-level environmental and behavioural factors that mediate risk in the East African highlands. Studies have been restricted to children despite significant incidence of clinical malaria in adults in these areas (Ghebreyesus et al. 2000; Brooker et al. 2004), and were conducted during an epidemic when risk factors may have differed from those during normal seasonal transmission (Brooker et al. 2004). The clinical malaria age distribution, climate, agricultural activities, cultural practices and biophysical environments in highland regions differ from the endemic lowlands, potentially resulting in a different risk profiles.
To identify risk factors for malaria in the highlands that differ from the lowlands, a prospective, population-based, case–control study was conducted in a highland area of western Kenya. Living close to swamps and forests and at lower elevations was associated with greater malaria risk (Ernst et al. 2006) in this area but did not fully explain malaria risk. Accordingly, additional environmental factors that may influence vector abundance, density or activity, as well as sociodemographic and behavioural factors potentially related to developing clinical malaria were investigated.
Methods
Study sites
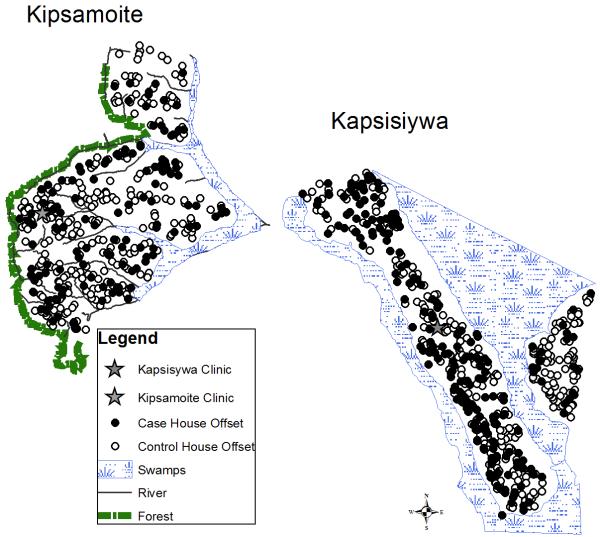
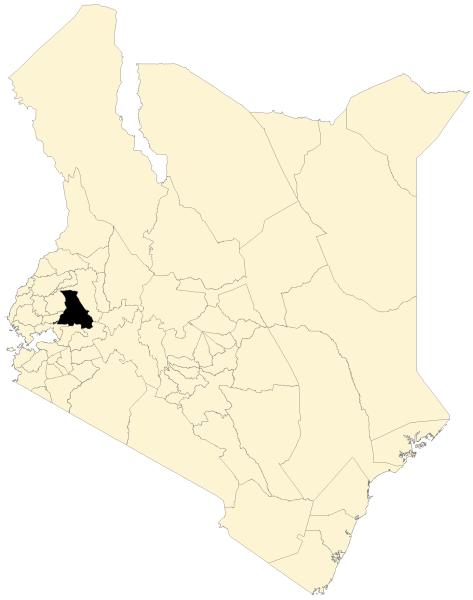
The study was conducted in Kipsamoite and Kapsisiywa (sub)locations (pop. ~7000) in Nandi District, western Kenya (Figure 1), at elevations of 1887–2100 m. North Nandi forest borders Kipsamoite’s western side. Kimondi swamp borders much of the remaining study area (Ernst et al. 2006 for details). Primary occupations are subsistence farming and animal husbandry.
Figure 1.
Nandi District in western Kenya with map of study site (Kipsamoite Sublocation and Kapsisiywa Location).
Study design
A prospective, population-based, case–control study design was used. Cases were identified as individuals with Plasmodium species identified in thick or thin blood smears who had presented to either of the two local health centres with symptoms consistent with malaria (fever, chills, headache or severe malaise).
Quarterly demographic surveillance was conducted in a concurrent study during which residents were assigned permanent unique study identification numbers. Using the enumerated population, malaria cases were frequency-matched to two controls by site (Kispsamoite or Kapsisiywa) and age category (<5, 5–14, 15–44, or >45 years old). Three visits on three separate days, at different times of day, were made to homes of potential controls to determine eligibility and request participation. Exclusion criteria for selected controls were (1) prior malaria diagnosis during the study period, (2) symptoms of malaria in the preceding month, or (3) moved or primary residence out of study area. Controls deemed ineligible or refusing to participate were replaced. Controls that later became cases were reinterviewed and replaced.
Exposure measurements
Environmental data were collected for cases and controls from direct household observations, Global Positioning System (GPS) measurements (Trimble Navigation, Sunnyvale, CA, USA), and a questionnaire. The dwelling in which the participant normally slept was assessed and distances (GPS corrected 1 m resolution) to mapped forest edge, swamps, rivers, roads, and health centres were calculated using ordinary Euclidean distance in ArcGIS V 9.0 (Redlands, CA, USA). Distances from the perimeter of the sleeping place to agricultural fields and penned livestock within a radius of 200 m were measured using steel tape. Type of vegetation within 200 m of the house was recorded (Earth Institute 2005).
A questionnaire was orally administered to cases and controls in the local language, Kalenjin, to ascertain sociodemographic and behavioural characteristics such as number of occupants, travel history, malaria prevention practices, and presence of livestock and other assets.
Variables
Bednet use was defined as sleeping under a net each day of the previous week. Individuals who reported using mosquito coils (at least three times a week), taking malaria medication as prophylaxis (at least once a month) and clearing brush (at least once a month) were classified as exposed to those factors. Distances from household to swamp, forest, river and roadsides were divided into four categories <250, 250–500, 500–1000, >1000 m [average flight range of Anopheles gambiae (Costantini et al. 1996)]. Elevation and distance from the household to the nearest clinic were divided into equal quartiles. Tea, maize, trees and bushes were classified as present if located within 200 m of the structure where the participant slept. Exposure to channelled swamp water was defined as participants who lived within 250 m of a swamp and reported channelling swamp water. Geographic slope was categorized by the field assistants into four classes (flat land, gentle slope, medium slope, and steep slope). The final variable for slope was dichotomous; sloped, including any grade of slope, or flat. Similarly, field assistants used a model illustration to determine if there were no trees, few trees, some trees or many trees within 200 m of the house. The final variable was also dichotomous; no trees vs. trees. Overnight travel was restricted to people sleeping outside the home 7–21 days prior to interview, adjusting for the average incubation period of malaria. Travel to any area outside the study sites was included.
Household Wealth Index
Principal component analysis (PCA) was conducted using sas V 9.1 (Cary, NC, USA) to estimate a relative household wealth index from a combination of household and asset variables (Filmer & Pritchett 2001). Household characteristics included building materials used for wall, floor, separate kitchen and roof. Assets included television, radio, bicycle, pressure lamp, car, sofa set, and charcoal cooking stove. The first component accounted for 30% of the total variance. A linear combination of the original asset variables (standardized to mean = 0 and variance = 1) multiplied by their respective standardized scoring coefficients (Table 1) was used to create the household wealth variable which was then divided into quartiles.
Table 1.
Standardized scoring coefficients
| Variable | Pressure lamp |
Stove | Sofa | Lantern | Bike | Radio | TV | Cement Floor |
Mud Walls |
Metal Roof |
No Separate Kitchen |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Standardized Scoring Coefficient |
0.17 | 0.23 | 0.09 | 0.26 | 0.36 | 0.37 | 0.17 | 0.17 | −0.17 | 0.02 | −0.10 |
Statistical analyses
Bivariate analyses, logistic regression, and then backwards stepwise regression with generalized estimating equations (GEE) were used to create multivariable models using sas V 9.1. All explanatory variables were initially included in the model and variables making the smallest contribution were sequentially dropped until all variables included in the model were P < 0.10. Matching variables, site and age were kept in the model with week of interview which was used to control for the average 2-week time difference in interviews for controls vs. cases. An exchangeable correlation matrix was used to adjust for correlation among members of the same household. People from different households were considered independent of each other. Model residuals were assessed for spatial autocorrelation using Moran’s I statistics (ArcGIS ver. 9.1, ESRI, Redlands, CA, USA).
Ethical considerations
All cases and controls, or the guardians of subjects <18 years old, provided written, informed consent before participation. Approval to undertake this study was obtained from the Institutional Review Board (IRB) committees at the University of Michigan (Ann Arbor, MI, USA), Kenya Medical Research Institute (KEMRI, Nairobi, Kenya), and U.S. Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA). Approval for the demographic and malaria surveillance in this study was obtained from the IRB committees at Case Western Reserve University (Cleveland, OH, USA), CDC, University of Michigan and KEMRI.
Results
Study population characteristics
From March through September 2004, 492 malaria cases were identified, of whom 99.1% (n = 488) agreed to be in the study. Participation was similarly high (98.9%, n = 980) for the 990 eligible controls. Malaria cases were equally distributed between the sexes (M:F = 251:237) and were identified in all age groups [<5 years of age, n = 100 (20.5%); 5–14 years, n = 163 (33.4%); 15–44 years, n = 182 (37.5%); >45 years, n = 43 (8.8%)].
Associations with sociodemographic and environmental variables
In bivariate analyses, increased malaria risk was associated with various sociodemographic and behavioural factors (Table 2) including low education levels of female household heads, overnight travel, living near channelled swamp water and keeping livestock near the house at night. Living in a house with a metal roof, no ceiling or separate kitchen also were related to higher risk, while open eaves and uncovered windows did not appear to have an effect. Environmental risks included living on flat land, living close to forests, and having bushes but not trees <200 m from the house (Table 2).
Table 2.
Bivariate analysis of association between malaria risk (cases/controls) and various demographic, social and environmental variables in Kipsamoite and Kapsisiywa, Kenya, March–September 2004
| Potential risk factors | No. cases (n = 488) |
No. controls (n = 980) |
Crude odds ratio (95% CI) |
P |
|---|---|---|---|---|
| Socio-demographics | ||||
| Female Household Head Education | ||||
| None | 78 (16) | 155 (16) | 1.85 (1.09, 3.13) | 0.02 |
| Primary/some secondary | 386 (79) | 737 (75) | 1.92 (1.20, 3.07) | 0.006 |
| Completed Secondary + | 22 (5) | 84 (9) | 1 | |
| Household Wealth Index | ||||
| First quartile (poorest) | 123 (25) | 244(25) | 0.85 (0.62, 1.16) | 0.31 |
| Second quartile | 146 (26) | 253 (30) | 0.95 (0.70, 1.30) | 0.75 |
| Third quartile | 115 (24) | 240 (24) | 1.14 (0.85, 1.54) | 0.37 |
| Fourth quartile | 104 (21) | 243 (25) | 1 | |
| People sleeping in room | ||||
| <3 | 168(35) | 309 (31) | 1 | |
| 3–5 | 271 (45) | 571 (48) | 0.87 (0.69, 1.11) | 0.26 |
| >5 | 49 (20) | 100 (20) | 0.90 (0.61, 1.31) | 0.60 |
| Prevention activities | ||||
| Malaria prophylaxis | 4 (1) | 10 (1) | 0.82 (0.25, 2.56) | 0.71 |
| Mosquito coils | 10 (2) | 28 (3) | 0.71 (0.34, 1.48) | 0.36 |
| Bednet use | 15 (3) | 37 (4) | 0.81 (0.44, 1.49) | 0.49 |
| Clearing of bushes | 167 (34) | 398 (38) | 0.84 (0.64, 1.09) | 0.17 |
| Burning herbs at night | 55 (11) | 95 (9) | 1.12 (0.63, 1.99) | 0.69 |
| Travel/residential history | ||||
| Overnight travel 7–21 days prior | 21 (4) | 20 (2) | 2.16 (1.16, 4.02) | 0.02 |
| Immigrated from outside study | 22 (5) | 36 (4) | 1.24 (0.72, 2.13) | 0.44 |
| Agricultural and animal husbandry activities | ||||
| Channelled swamp water | 50 (10) | 44 (4) | 2.43 (1.59, 3.70) | <0.001 |
| Animals sleeping in compound | ||||
| Insecticide treated cattle | 254 (52) | 509 (52) | 1.00 (0.81, 1.25) | 0.97 |
| Insecticide free cattle | 84 (17) | 131 (13) | 1.35 (1.00, 1.82) | 0.05 |
| Goats | 43 (9) | 48 (5) | 1.88 (1.23, 2.88) | 0.003 |
| Dogs | 252 (52) | 435 (44) | 1.34 (1.08, 1.66) | 0.01 |
| House construction | ||||
| Metal roof | 284 (58) | 483 (49) | 1.43 (1.15, 1.78) | 0.001 |
| Mud walls | 430 (88) | 867 (88) | 0.97 (0.69, 1.35) | 0.84 |
| Absence of ceiling | 409 (84) | 771 (79) | 1.40 (1.05, 1.87) | 0.02 |
| Open eaves | 387 (79) | 795 (81) | 0.89 (0.68, 1.17) | 0.41 |
| Uncovered windows | 52 (11) | 119 (12) | 1.06 (0.74, 1.51) | 0.76 |
| Separate kitchen | 129 (74) | 356 (64) | 1.59 (1.25, 2.02) | 0.001 |
| Surrounding environment | ||||
| Livestock holding area <100 m | 206 (42) | 374 (38) | 1.18 (0.95,1.48) | 0.14 |
| House on flat land | 121 (25) | 179 (18) | 1.47 (1.13, 1.91) | 0.004 |
| Location at bottom of hill | 52 (11) | 85 (9) | 1.26 (0.87, 1.81) | 0.22 |
| Elevation (quartiles) | ||||
| < 1932 m | 110(23) | 260 (27) | 0.95 (0.69, 1.30) | 0.74 |
| 1932–1947 m | 114 (23) | 247 (25) | 1.03 (0.76, 1.41) | 0.84 |
| 1947–1983 m | 151 (31) | 220 (22) | 1.53 (1.13, 2.08) | 0.06 |
| > 1983 m | 113 (23) | 253 (26) | 1 | |
| Distance from forest | ||||
| < 250 m | 33 (7) | 40 (4) | 1.77 (1.10, 2.85) | 0.02 |
| 250–500 m | 37 (8) | 50 (5) | 1.58 (1.02, 2.47) | 0.04 |
| 500–1000 m | 55 (11) | 113 (12) | 1.02 (0.71, 1.45) | 0.82 |
| > 1000 m | 363 (74) | 777 (79) | 1 | |
| Distance from swamp | ||||
| < 250 m | 166 (34) | 263 (27) | 1.36 (0.94, 1.98) | 0.10 |
| 250–500 m | 198 (41) | 421 (43) | 1.02 (0.71, 1.45) | 0.93 |
| 500–1000 m | 68 (14) | 175 (18) | 0.84 (0.55, 1.28) | 0.42 |
| > 1000 m | 56 (11) | 121 (12) | 1 | |
| Distance from clinics (quartiles) | ||||
| 1190 m | 115 (23) | 253 (26) | 0.78 (0.57, 1.06) | 0.11 |
| 1190–2081 m | 125 (26) | 241 (24) | 0.89 (0.66, 1.20) | 0.44 |
| 2081–2711 m | 112 (23) | 253 (26) | 0.76 (0.56, 1.03) | 0.08 |
| >2711 m | 136 (28) | 233 (24) | 1 | |
| Distance from Stream | ||||
| < 250 m | 97 (19) | 186 (20) | 1.19 (0.92, 1.53) | 0.19 |
| 250–500 m | 83 (17) | 113 (12) | 1.79 (1.23, 2.59) | 0.002 |
| 500–1000 m | 6 (1) | 12 (1) | 1.11 (0.41, 2.97) | 0.84 |
| > 1000 m | 302 (62) | 669 (68) | 1 | |
| Absence of trees within 200 m | 394 (81) | 703 (72) | 1.65 (1.27, 2.15) | 0.0002 |
| Presence of bushes within 200 m | 124 (25) | 194 (20) | 1.38 (1.07, 1.79) | 0.01 |
| Presence of tea within 200 m | 243 (50) | 522 (53) | 0.87 (0.70, 1.08) | 0.21 |
| Presence of maize within 200 m | 445 (91) | 887 (90) | 1.09 (0.74, 1.59) | 0.67 |
The final multivariable model included many of the same variables, indicating that greater malaria risk was associated with lower levels of education of female household heads, recent overnight travel, living near channelled swamp water and near forests, lack of ceiling in the house, a separate kitchen and having goats in the compound. External environmental influences such as living on flat land, in close proximity to maize fields, and on land lacking nearby trees also increased malaria risk (Table 3).
Table 3.
Multivariate model of associations between measured variables and malaria risk in Kipsamoite and Kapsisiywa, western Kenyan highlands, March–September 2004
| Potential risk factors | Adjusted* odds ratio (95% CI) |
P |
|---|---|---|
| Female Household Head Education | ||
| None | 2.02 (1.08, 3.75) | 0.03 |
| Primary/some secondary | 2.01 (1.16, 3.05) | 0.01 |
| Cmpleted Secondary + | 1 | |
| Mosquito coils | 0.48 (0.21, 1.10) | 0.08 |
|
Overnight travel 7–21
days prior |
2.17 (1.15, 4.11) | 0.02 |
| Channelled swamp water | 1.75 (0.96, 3.20) | 0.07 |
| Goats sleeping in compound | 1.61 (0.95, 2.74) | 0.07 |
| Absence of ceiling | 1.53 (1.08, 2.18) | 0.02 |
| Separate kitchen | 1.77 (1.35, 2.32) | <0.0001 |
| House on flat land | 1.61 (1.19, 2.18) | 0.002 |
| Elevation (quartiles) | ||
| < 1932 m | 1.35 (0.60, 3.04) | 0.47 |
| 1932–1947 m | 1.68 (0.76,3.71) | 0.20 |
| 1947–1983 m | 2.02 (1.08, 3.79) | 0.03 |
| > 1983 m | 1 | |
| Distance from forest | ||
| < 250 m | 3.26 (1.49, 7.12) | 0.003 |
| 250–500 m | 2.23 (1.02, 4.85) | 0.04 |
| 500–1000 m | 1.26 (0.72, 2.20) | 0.41 |
| > 1000 m | 1 | |
| Distance from swamp | ||
| < 250 m | 2.81 (1.34, 5.89) | 0.006 |
| 250–500 m | 2.31 (1.15, 4.65) | 0.02 |
| 500–1000 m | 1.52 (0.80,2.89) | 0.20 |
| > 1000 m | 1 | |
|
Absence of trees
within 200 m |
1.59 (1.15, 2.19) | 0.005 |
|
Presence of maize
within 200 m |
2.02 (1.21, 3.37) | 0.007 |
Model adjusted for age, site and week of interview.
Discussion
Previous analyses of passive malaria surveillance data from Kipsamoite suggested ‘hotspots’ of transmission associated with distance from swamps and forests (Ernst et al. 2006). Even in these apparent hotspots, there were malaria-free households. Although shared bio-physical environments can produce clusters of higher transmission, other factors at the individual and household levels can mediate this risk. Accordingly, associations between malaria risk and environmental, socio-demographic, and behavioural variables were explored.
Environment around the home was important. Closer proximity to swamps and forest border was associated with increased malaria risk and is consistent with findings in other highland areas (Lindblade et al. 2000; Staedke et al. 2003; Brooker et al. 2004; Minakawa et al. 2004; Zhou et al. 2004, 2007). These habitats may represent enhanced Anopheles breeding sites or microclimates that prolong adult vector survival. Our analyses also demonstrated a 500-m threshold for these relationships. Absence of trees and presence of maize near dwellings also were associated with increased risk. Anopheles gambiae s.s. prefer breeding in open areas (Minakawa et al. 2004) and exposure to sunlight increases water temperatures potentially speeding up the development rate of the aquatic stages of An. gambiae s.s. (Bayoh & Lindsay 2003). Maize pollen is a good nutritional source for An. gambiae larvae (Ye-Ebiyo et al. 2003) and has been related to higher malaria incidence (Kebede et al. 2005). The presence of many mixed vegetation classes within 200 m of the house may have obscured further relationships.
Living on flat ground, where water is most likely to accumulate, was associated with increased risk corroborating results found by Cohen et al. (2008) that demonstrated topography, when used as a predictor of wetness, is highly correlated with malaria risk. Absolute elevation, however, was not associated with risk. This is counter to results from a study by Brooker et al. (2004), who found a significant relationship between malaria risk and elevation. The narrower range of elevation across this study area (213 m) as compared to theirs (400 m) may have prevented a similar association.
Similarly to the study by Brooker et al. (2004), we found no association between household wealth and malaria risk. Our index may not have accurately differentiated levels of household wealth or variation may have been too limited to impact risk. Having a kitchen separate from the sleeping area was strongly associated with increased malaria odds, perhaps because smoke repels vector mosquitoes, although sleeping in a smoky room was not associated with risk in another study in the Kenyan highlands (Brooker et al. 2004). Many relatives and older children reported sleeping in the kitchen area and there was a tendency to keep fires burning at night for warmth in this study area which may differ from other highland communities. House construction also influences mosquito access to people sleeping inside and we found that having a ceiling in the home was associated with decreased risk of malaria. Open eaves have been associated with increased risk in some past studies (e.g. Ghebreysus et al. 2000) but not by Brooker et al. (2004) or in our study.
The education level of female heads of household was inversely associated with malaria risk. Other studies have shown that use of malaria prevention measures has been consistently related to higher maternal education (e.g. Keating et al. 2005; Noor et al. 2006), yet we saw no notable associations between use of preventive measures, including bednets, and malaria risk except for a modest decreased risk among those using mosquito coils. In our study, as in that by Brooker et al. (2004), practice of preventive measures was quite low. Environmental control (removal of tins and brush) was the most commonly reported activity, indicating effective environmental management techniques could be well-received. Prevalence of bednet use was low and we did not test the nets for insecticide or examine them for holes, potentially obscuring their true association with malaria risk. Although people expressed interest in bednets, anecdotal evidence suggests that even those who owned bednets did not use them, primarily because vector density, hence perceived risk, was low. Some believed that only those living near the swamps were at risk of malaria. Education regarding the importance of net use, even when vector density is low, could improve effectiveness of bednets in this area.
In our study, like others (e.g. Shanks et al. 2005), overnight travel increased malaria risk, even though such movement was primarily to neighbouring highland communities. Increased malaria risk may be related to increased vector-human contact in such settings. Indeed, some interviewees informally reported that when attending funerals it was common to sleep in the open air. The relationship between malaria risk and travel should be interpreted with caution, however, as frequency of travel was low and positive bias could have arisen if residents temporarily living outside the study area were included as cases (perhaps at home on holiday and seen at the clinic) versus controls (replaced if residing out of area for work or school).
This study had both strengths and potential entries of bias. High participation rates and a fully enumerated population permitting strata-specific random selection of controls created a highly representative sample. Recall bias, a concern in case–control studies, was minimized by interviewing shortly after case and control identification. The need to replace ineligible and unavailable controls put their interview times an average of 2 weeks later than cases; however, week of interview was adjusted for in the multivariable model. Although all cases were slide-confirmed, logistical constraints precluded the testing of controls and asymptomatic cases may have been misclassified as controls, thus biasing associations. Past studies, however, demonstrated that the level of asymptomatic malaria in this region is low (John et al. 2005). The large sample size required multiple field assistants, potentially resulting in differential information bias particularly for subjectively measured variables such as slope and tree cover. To minimize such bias, an intensive training program was combined with spot checks of 10% of all questionnaires by the study coordinator and by dichotomizing highly subjective variables (tree cover and slope).
Results of our study underscore the complexity of highland malaria transmission. Prior studies have assessed some of the factors that we evaluated, but our investigation adds to these earlier reports by assessing multiple environmental, social and behavioural factors in a single multivariate model. The report by Brooker et al. (2004) is the only other study of this nature from the east African highlands, but their study focused on children during an epidemic and a different set of variables. Although our results indicate that malaria risk may cluster near specific land covers, considerable time and resources are needed to identify such high risk areas, and only some of the malaria cases are included. Moreover, predictors of high risk may vary among regions. The resources needed to map high risk areas may be better allocated, for example, toward achieving universal coverage of indoor residual spraying. Workers administering the spraying should keep in mind that not all members of the household sleep in the main house and spraying of outbuildings, particularly kitchen buildings, may be necessary to ensure complete coverage.
Acknowledgements
We thank the communities of Kipsamoite and Kapsisiywa for their incredible support of this research. The work would not have been possible without the contribution of the field assistants (Rosebella Chepchumba, Paul Lelei, Peter Cheboiywo, Usillah Biwott, Haron Rugut, Moses Sawe, Gideon Kurgat, Josphat Koech, Raymond Bungei, Steven Koros, Japheth Koech, Jeruto Ogla, Jepn’getich Melly, Celestine Rotich, Japhet Kipleting, Simeon Kipleting, Dorothy Kirwa), the microscopists (John Oluoch, Joseph Otieno), the study coordinators (Lillian Kipkagat, Peter Siwat), and the clinical officer (Willy Rotich). Financial support was provided by USPHS grants (AI-056184 and AI-01572) and the Global Health Program at the University of Michigan. The findings and conclusions in this [presentation/report] are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- Bayoh M, Lindsay S. Effect of water temperature on the development of the aquatic stages of Anopheles gambiae ss. Bulletin of Entomological Research. 2003;93:375–381. doi: 10.1079/ber2003259. [DOI] [PubMed] [Google Scholar]
- Brooker S, Clarke S, Njagi JK, et al. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Tropical Medicine and International Health. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Cohen J, Ernst K, Lindblade K, Vulule J, John C, Wilson M. Topography-derived wetness indices are associated with household-level malaria risk in two communities in the western Kenyan highlands. Malaria Journal. 2008;7:40–52. doi: 10.1186/1475-2875-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini C, Li SG, Della Torre A, Sagnon N, Coluzzi M, Taylor CE. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a west African Sudan savanna village. Medical and Veterinary Entomology. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Earth Institute – Tropical Agriculture Program Annual report: Millennium research villages the first year July 2004 to June 2005. Columbia University; Dec 12, 2005. < http://www.earthinstitute.columbia.edu/tropag/documents/MVP_Annual_Report_05.pdf>. [Google Scholar]
- Ernst K, Adoka S, Kowuor D, Wilson M, John C. Malaria hotspot areas in highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malaria Journal. 2006;5:78–87. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- Ghebreyesus TA, Haile M, Witten KH, et al. Household risk factors for malaria among children in the Ethiopian highlands. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94:17–21. doi: 10.1016/s0035-9203(00)90424-3. [DOI] [PubMed] [Google Scholar]
- Githeko A, Ayisi J, Odada P, et al. Topography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malaria Journal. 2006;10:107. doi: 10.1186/1475-2875-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CC, McHugh MM, Moormann AM, Sumba PO, Ofulla AV. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99:780–786. doi: 10.1016/j.trstmh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Keating J, Macintyre K, Mbogo CM, Githure JI, Beier JC. Self-reported malaria and mosquito avoidance in relation to household risk factors in a Kenyan coastal city. Journal of Biosocial Science. 2005;37:761–771. doi: 10.1017/S0021932005007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebede A, McCann JC, Kiszewski AE, Ye-Ebiyo Y. New evidence of the effects of agro-ecologic change on malaria transmission. American Journal of Tropical Medicine and Hygiene. 2005;73:676–680. [PubMed] [Google Scholar]
- Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land-use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Tropical Medicine & International Health. 2000;5:263–274. doi: 10.1046/j.1365-3156.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Sonye G, Mogi M, Yan G. Habitat characteristics of Anopheles gambiae s.s. larvae in a Kenyan highland. Medical and Veterinary Entomology. 2004;18:301–305. doi: 10.1111/j.0269-283X.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Noor AM, Omumbo JA, Amin AA, Zurovac D, Snow RW. Wealth, mother’s education and physical access as determinants of retail sector net use in rural Kenya. Malaria Journal. 2006;5:5. doi: 10.1186/1475-2875-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks GD, Biomndo K, Guyatt HL, Snow RW. Travel as a risk factor for uncomplicated Plasmodium falciparum malaria in the highlands of western Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99(1):71–74. doi: 10.1016/j.trstmh.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedke SG, Nottingham EW, Cox J, Kamya MR, Rosenthal PJ, Dorsey G. Proximity to mosquito breeding sites as a risk factor for clinical malaria episodes in an urban cohort of Ugandan children. American Journal of Tropical Medicine and Hygiene. 2003;69:244–246. [PubMed] [Google Scholar]
- Ye-Ebiyo Y, Pollack RJ, Kiszewski A, Spielman A. Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. American Journal of Tropical Medicine and Hygiene. 2003;68:748–752. [PubMed] [Google Scholar]
- Zhou G, Minakawa N, Githeko A, Yan G. Spatial distribution patterns of malaria vectors and sample size determination in spatially heterogeneous environments: A case study in the west Kenyan highlands. Journal of Medical Entomology. 2004;41:1001–1009. doi: 10.1603/0022-2585-41.6.1001. [DOI] [PubMed] [Google Scholar]
- Zhou G, Munga S, Minakawa N, Githeko A, Yan G. Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. American Journal of Tropical Medicine and Hygiene. 2007;77:29–35. [PubMed] [Google Scholar]