Abstract
Twin studies have found that global brain volumes, including total intracranial volume (ICV), total gray matter, and total white matter volumes are highly heritable in adults and older children. Very little is known about genetic and environmental contributions to brain structure in very young children and whether these contributions change over the course of development. We performed structural imaging on a 3T MR scanner of 217 neonatal twins, 41 same‐sex monozygotic, 50 same‐sex dizygotic pairs, and 35 “single” twins—neonates with brain scans unavailable for their co‐twins. Tissue segmentation and parcellation was performed, and structural equation modeling was used to estimate additive genetic, common environmental, and unique environmental effects on brain structure. Heritability of ICV (0.73) and total white matter volume (0.85) was high and similar to that described in older children and adults; the heritability of total gray matter (0.56) was somewhat lower. Heritability of lateral ventricle volume was high (0.71), whereas the heritability of cerebellar volume was low (0.17). Comparison with previous twin studies in older children and adults reveal that three general patterns of how heritability can change during postnatal brain development: (1) for global white matter volumes, heritability is comparable to reported heritability in adults, (2) for global gray matter volume and cerebellar volume, heritability increases with age, and (3) for lateral ventricle volume, heritability decreases with age. More detailed studies of the changes in the relative genetic and environmental effects on brain structure throughout early childhood development are needed. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: magnetic resonance imaging, cortex, white matter, gray matter, lateral ventricle, cerebellum, structural equation modeling
INTRODUCTION
There has been a great deal of recent interest in using twins to understand the contributions of genetic and environmental factors to brain structure and morphology in adults. Overall, studies have found that global brain volumes (total intracranial, total gray, total white) are highly heritable (reviewed in Schmitt et al. [2007a] and Peper et al. [2007]). For example, Baaré et al. [2001b] found that variance in whole brain, gray matter, and white matter volumes was all strongly influenced by genetic factors (0.90, 0.82, and 0.88, respectively). There is some evidence of differential heritability in cortical regions. Geschwind et al. [2002] found reduced heritability of the occipital lobe (0.28) compared to other cortical lobes (0.40–0.56). Voxel‐based approaches find high levels of genetic influence in frontal and Broca's and Wernicke's areas [Thompson et al.,2001] and in frontal cortex, Heschl's gyrus, and left occipital and left posterior cingulate [Hulshoff Pol et al.,2006]. Hulshoff Pol et al. [2006] also found high heritability in the white matter density of the corpus callosum, corticospinal tract, superior occipital‐frontal fascicle, and optic radiation. A recent diffusion tensor imaging study found overall high heritability of lobar fractional anisotropy as well [Chiang et al.,2009]. In contrast to tissue volumes, lateral ventricle volume has low heritability [Baaré et al.,2001; Wright et al.,2002] and may be a marker of both shared and nonshared environmental events that influence brain development.
In studies of elderly twins, heritability estimates for total brain volume are similar to those described in studies of younger adult twins [Geschwind et al.,2002; Pfefferbaum et al.,2000], evidence that either genetic factors determine initial brain structure and that aging has similar effects on this original similar structure, or that genetic factors similarly influence the aging process, or a combination of both [Geschwind et al.,2002].
To date, there have been two twin studies in typically developing children [Peper et al.,2009; Wallace et al.,2006]. These reports indicate that overall heritability of global structures in children are similar to those observed in adults, with heritability estimates for total cerebral volume of 0.89–0.91, gray matter volume 0.77–0.82, and for white matter volumes of 0.84–0.85 [Peper et al.,2009; Wallace et al.,2006]. Heritability estimates for the lateral ventricle was also very low at 0.31–0.35. A multivariate analysis revealed that the majority of variation in the volume of the cerebrum, cerebellum, thalamus, and basal ganglia was due to a single genetic factor; common environmental effects contributed significantly to the volume of the lateral ventricles, basal ganglia, and thalamus [Schmitt et al.,2007b].
Heritability tends to decrease in total gray matter with increasing age in late childhood and adolescence, while it increased in white matter [Wallace et al.,2006]. A more recent cortical thickness analysis revealed that primary and sensory cortex show greater heritability early in development, while in dorsal prefrontal cortex and temporal lobes, heritability increases with maturation [Lenroot et al.,2009]. This suggests that the genetic effects on developmental programs for brain structures underlying complex cognitive processes become stronger through later childhood development. Multivariate analysis of this data indicates that a single genetic factor accounts for 60% of the genetic variability in cortical thickness [Schmitt et al.,2007a].
Very little is known about genetic and environmental contributions to human brain development in the first years of life, a period which is likely to be critical in neurodevelopmental disorders including schizophrenia [Lewis and Levitt,2002] and autism [Hazlett et al.,2005]. This is a time of dramatic growth and development of brain structure [Gilmore et al.,2007; Knickmeyer et al.,2008]; the concurrent rapid development of a wide range of cognitive and motor functions [Kagan and Herschkowitz,2005] suggests that this may be a period in which gene‐environment interactions are of great importance. There have been no imaging studies of twin brain development in early childhood. We are conducting such a longitudinal imaging study and report the results from the neonatal period.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the University of North Carolina (UNC) School of Medicine and Duke University Medical Center (DUMC). Mothers with same‐sex twin pregnancies were recruited from the outpatient OB‐GYN clinics at UNC Hospitals between 2004 and 2008 and from the OB‐GYN clinics at DUMC from 2004 to 2006. Exclusion criteria included maternal HIV infection, major congenital abnormality on fetal ultrasound, and chromosomal abnormalities of fetuses. Informed consent was obtained from the parents of all subjects. Magnetic resonance imaging (MRI) scans were performed at term, ∼40 weeks gestational age. For zygosity testing, PCR‐STR analysis of 14 loci was performed on DNA isolated from buccal swab cell collection (BRT Laboratories, Baltimore, MD).
The study sample consisted of 91 twin pairs and 35 unrelated “singleton” twins—twins from a twin pair in which a usable scan was not obtained from the co‐twin. Two twin pairs from a single mother are included, though treated as independent pairs in the statistical analysis. Demographic and clinical variables are presented in Table I.
Table I.
Sample demographics
| MZ twins | DZ twins | Single twin | Total | |
|---|---|---|---|---|
| Gender | ||||
| Male [N (%)] | 36 (43.90) | 54 (54.00) | 16 (45.71) | 106 |
| Female [N (%)] | 46 (56.10) | 46 (46.00) | 19 (54.29) | 111 |
| Ethnicity | ||||
| Caucasian [N (%)] | 56 (68.29) | 74 (74.00) | 26 (74.29) | 156 |
| African‐American [N (%)] | 22 (26.83) | 24 (24.00) | 8 (22.86) | 54 |
| Other [N (%)] | 4 (4.88) | 2 (2.00) | 1 (2.86) | 7 |
| Gestational age at birth (days*); mean (SD) | 246.05 (18.95) | 249.56 (15.42) | 244.69 (1942) | 247.45 (17.52) |
| Gestational age at MRI (days*); mean (SD) | 288.00 (15.41) | 288.98 (16.90) | 291.29 (24.20) | 288.98 (17.70) |
| Birth weight (g); mean (SD) | 2,292.52 (539.98) | 2,404.27(486.65) | 2,292.57 (593.13) | 2,344.03 (525.73) |
*[Correction made here after initial online publication.]
Image Acquisition
MRI scans were done on a Siemens 3T head‐only scanner (Allegra, Siemens Medical System, Erlangen, Germany). Neonates were scanned unsedated; subjects were fed before scanning, swaddled, given ear protection, and held in place with a vacuum‐fixation device for the head. A nurse was present during all scans, and heart rate and oxygen saturation were monitored with a pulse oximeter. T1‐weighted structural pulse sequences were either a 3D magnetization prepared rapid gradient echo (MP‐RAGE TR/TI/TE/Flip Angle 1820/400/4.38 ms/7°) or a 3D spoiled gradient (FLASH TR/TE/Flip Angle 15/7 ms/25°). Proton density and T2‐weighted images were obtained with a turbo spin echo sequence (TSE TR/TE1/TE2/Flip Angle 6200/20/119 ms/150°). Spatial resolution was 1 × 1 × 1‐mm voxel for T1‐weighted images, 1.25 × 1.25 × 1.5‐mm voxel with 0.5‐mm interslice gap for proton density/T2‐weighted images. MRIs were clinically reviewed by a neuroradiologist (JKS).
Image Analysis
Brain tissue was automatically classified as gray matter, nonmyelinated white matter, myelinated white matter, and cerebrospinal fluid using an atlas‐moderated iterative expectation maximization segmentation algorithm as previously described [Gilmore et al.,2007; Prastawa et al.,2005]. Parcellation of the brain was achieved by nonlinear warping of a parcellation atlas template as previously described [Gilmore et al.,2007]. Left and right hemispheres were subdivided into four regions along the anterior–posterior axis (prefrontal, frontal, parietal, and occipital) and into infra‐ and supratentorial regions. The cerebellum, brainstem, and combined sets of subcortical structures are represented separately. This parcellation template is registered to each individual subject's MRI by volumetric nonlinear deformation, combined with the individual tissue segmentation masks, and results in an estimate of gray, white and CSF volume for each region along the anterior–posterior axis. The volume of the lateral ventricles was determined using a user‐supervised, highly automated level‐set evolution segmentation on the individual CSF probabilistic segmentation map via ITK‐SNAP (http://www.itksnap.org).
Statistical Analysis
The volumetric measures were imported into the statistical package R [R Development Core Team,2005] for visualization and for the calculation of basic statistics. QQ plots were constructed to determine whether the raw data were normally distributed. Bivariate scatterplots and linear regression also were generated to examine the relationships between brain volumes and key demographic variables. Brain measures were subsequently residualized via linear regression models to control for the effects of age at MRI scan, age at birth, and gender on mean volumes.
The data were then imported into Mx, a statistical package designed for the analysis of genetically informative multi‐group data [Neale et al.,1999]. Saturated models were then used to test for group differences in means and variances (via likelihood ratio χ2 tests) and to generate maximum likelihood cross‐twin correlations by zygosity. From the derived correlations, preliminary estimates of heritability were calculated using Falconer estimation [Falconer and Mackay,1996]. Classical univariate ACE structural equation models (SEM) were then generated for all ROIs (Supporting Information Fig. 1). The classical twin design allows for the decomposition of phenotypic variance into variance due to additive genetic (A), shared environmental (C), and unique environmental (E) sources [Neale and Cardon,1992]. Such estimates are possible due to the differences in the degree of genetic similarity between monozygotic (MZ) and dizygotic (DZ) twin pairs; whereas MZ twins are genetically identical, DZ twins share, on average, only half of their genes identical by descent. SEMs are preferable to Falconer estimation for several reasons, including (1) they incorporate the level of precision in MZ and DZ correlations into the estimates of heritability, which is particularly important when there are group differences in sample size, (2) they allow data from singleton twins to be included in the analysis, which improves the precision of phenotypic variance estimates, and (3) they allow for straightforward hypothesis testing and generation of confidence intervals on parameters of interest. As noted in Panizzon et al.1, differences in total variance between MZ and DZ twin pairs can cause divergence between Falconer and Maximum Likelihood estimates (MLE). In addition, when the DZ twin correlation is less than half that of the MZ twins, Falconer estimates are typically inflated relative to maximum likelihood (indeed, it is possible to observe a falconer's estimate greater than one, which cannot happen with maximum likelihood estimation). These two effects likely explain the differences between the two sets of estimates; the MLEs are generally to be preferred.
To decompose the phenotypic variance, we constructed SEMs of expected variance–covariance matrices for each ROI. Three variance components parameters were estimated that quantified the relationship between A, C, E, and the observed volumetric measures. The proportion of variance due to each component was then derived (a 2, c 2, and e 2, respectively) by dividing each variance component by the total phenotypic variance. Optimum model fit was determined using maximum likelihood [Edwards,1972], which produces unbiased parameter estimates and allows the identification of statistically significant parameters in the model [Neale and Miller,1997]. Statistical significance of variance components was determined by comparing the likelihood from models with or without the parameter; the difference in −2 times the log likelihood (−2 LL) asymptotically follows a 50:50 mixture of zero and χ2 distribution with one degree of freedom. Likelihood ratio 95% confidence intervals also were calculated for all variance components [Neale and Miller,1997].
RESULTS
Both qualitative and quantitative tests suggested that the population distribution of most ROIs approximated a normal distribution, with the notable exceptions of the corpus callosum and lateral ventricles. Similarly, there were no statistically differences in means and variances between MZ and DZ twins for most structures, except the lateral ventricle and corpus callosum. Group means and variances are presented in Table II. Cross‐twin correlations and Falconer's estimates are presented in Table III. In general, MZ twin pairs had substantially increased correlations when compared with DZ twin pairs. Parameter estimates and tests of the statistical significance from SEMs are presented in Table IV, which includes estimates of genetic and shared environmental effects on the variation of intracranial volume (ICV), lateral ventricle volume, and cerebellum volume, as well as total, cortical, and regional gray and white matter volumes. Overall, there was high heritability of ICV and global tissue volumes. The heritability of total (0.85) and cortical (0.85) unmyelinated white matter volume was higher than that of total (0.56) and cortical (0.58) gray matter. There was a relatively small contribution of common environmental factors to global tissue volumes, though the contribution of the common environment to total (c 2 = 0.29) and cortical (c 2 = 0.27) gray matter volume was greater than that for total (c 2 = 0.05) and cortical (c 2 = 0.05) unmyelinated white matter.
Table II.
Group means and variances
| Mean | Variance | P value | ||||
|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | Mean | Variance | |
| ICV | 460.11 | 465.99 | 3889.01 | 3674.23 | 0.6390 | 0.8415 |
| Lateral ventricles | 4.35 | 4.64 | 2.21 | 4.25 | 0.3984 | 0.0113 |
| Total GM | 233.07 | 238.81 | 987.89 | 917.21 | 0.3623 | 0.7913 |
| Total WM | 156.59 | 160.86 | 433.45 | 525.33 | 0.3375 | 0.4884 |
| Total EM | 10.64 | 10.30 | 13.98 | 12.94 | 0.5740 | 0.7312 |
| Total CSF | 59.80 | 56.09 | 180.02 | 151.33 | 0.1435 | 0.5023 |
| Cortical GM | 193.93 | 198.04 | 692.46 | 610.95 | 0.4310 | 0.6547 |
| Cortical WM | 148.68 | 153.16 | 368.11 | 466.73 | 0.2774 | 0.3897 |
| SubCortGM | 15.90 | 16.08 | 8.69 | 7.87 | 0.7345 | 0.6929 |
| Cerebellum | 23.21 | 24.68 | 17.62 | 21.75 | 0.1076 | 0.4511 |
| Prefrontal GM | 26.48 | 26.73 | 27.09 | 22.61 | 0.7988 | 0.4925 |
| Frontal GM | 42.03 | 43.35 | 36.35 | 34.01 | 0.2655 | 0.8034 |
| Parietal GM | 57.98 | 59.93 | 65.55 | 52.81 | 0.2031 | 0.4166 |
| Occipital GM | 67.60 | 68.12 | 111.53 | 96.33 | 0.8065 | 0.5839 |
| Prefrontal WM | 24.18 | 24.37 | 13.41 | 16.11 | 0.8097 | 0.5014 |
| Frontal WM | 37.03 | 37.61 | 23.00 | 27.27 | 0.5552 | 0.5230 |
| Parietal WM | 45.78 | 48.24 | 41.88 | 53.47 | 0.0769 | 0.3652 |
| Occipital WM | 40.09 | 41.50 | 39.71 | 49.31 | 0.2876 | 0.4215 |
| Total prefrontal | 51.48 | 51.76 | 76.76 | 68.63 | 0.8625 | 0.6801 |
| Total frontal | 80.74 | 82.31 | 108.72 | 111.29 | 0.4543 | 0.9203 |
| Total parietal | 105.99 | 110.53 | 202.47 | 208.70 | 0.1160 | 0.8875 |
| Total Occipital | 110.16 | 112.07 | 276.17 | 265.72 | 0.5657 | 0.8875 |
| RHem GM | 99.16 | 101.37 | 189.04 | 166.31 | 0.4131 | 0.6468 |
| LHem GM | 94.79 | 96.68 | 161.41 | 143.51 | 0.4503 | 0.6714 |
| RHem WM | 71.44 | 73.88 | 87.67 | 109.89 | 0.2253 | 0.4131 |
| LHem WM | 75.61 | 77.77 | 95.09 | 124.18 | 0.3055 | 0.3323 |
| RHem Tot | 174.06 | 178.56 | 522.00 | 520.18 | 0.3323 | 1.0000 |
| LHem Tot | 174.17 | 177.99 | 501.24 | 510.88 | 0.4028 | 0.9203 |
| Corpus callosum | 1.75 | 1.56 | 0.25 | 0.13 | 0.0288 | 0.0131 |
Abbreviations: CSF, cerebrospinal fluid; EM, early myelinated white matter; GM, gray matter; ICV, intracranial volume; LHem, left hemisphere; RHem, right hemisphere; SubCortGM, subcortical gray matter; WM, unmyelinated white matter.
Table III.
Maximum likelihood correlations and Falconer's estimatesa
| Maximum likelihood correlation | Falconer estimates | ||||
|---|---|---|---|---|---|
| MZ | DZ | a 2 | c 2 | e 2 | |
| ICV | 0.84 | 0.55 | 0.57 | 0.27 | 0.16 |
| Lateral Ventricles | 0.62 | 0.26 | 0.72 | −0.10 | 0.38 |
| Total GM | 0.82 | 0.62 | 0.40 | 0.42 | 0.18 |
| Total WM | 0.87 | 0.53 | 0.69 | 0.18 | 0.13 |
| Total EM | 0.25 | −0.02 | 0.53 | −0.28 | 0.75 |
| Total CSF | 0.63 | 0.25 | 0.76 | −0.13 | 0.37 |
| Cortical GM | 0.82 | 0.60 | 0.44 | 0.38 | 0.18 |
| Cortical WM | 0.86 | 0.52 | 0.68 | 0.19 | 0.14 |
| SubCortGM | 0.63 | 0.22 | 0.83 | −0.19 | 0.37 |
| Cerebellum | 0.72 | 0.75 | −0.05 | 0.77 | 0.28 |
| Prefrontal GM | 0.66 | 0.51 | 0.29 | 0.36 | 0.34 |
| Frontal GM | 0.65 | 0.53 | 0.24 | 0.41 | 0.35 |
| Parietal GM | 0.74 | 0.46 | 0.56 | 0.18 | 0.26 |
| Occipital GM | 0.74 | 0.52 | 0.43 | 0.31 | 0.26 |
| Prefrontal WM | 0.79 | 0.58 | 0.41 | 0.38 | 0.21 |
| Frontal WM | 0.81 | 0.32 | 0.99 | −0.18 | 0.19 |
| Parietal WM | 0.73 | 0.46 | 0.53 | 0.20 | 0.27 |
| Occipital WM | 0.81 | 0.46 | 0.69 | 0.12 | 0.19 |
| Total Prefrontal | 0.79 | 0.59 | 0.40 | 0.39 | 0.21 |
| Total Frontal | 0.71 | 0.46 | 0.50 | 0.21 | 0.29 |
| Total Parietal | 0.77 | 0.48 | 0.58 | 0.19 | 0.23 |
| Total Occipital | 0.82 | 0.52 | 0.59 | 0.23 | 0.18 |
| RHem GM | 0.76 | 0.62 | 0.28 | 0.48 | 0.24 |
| LHem GM | 0.86 | 0.54 | 0.65 | 0.21 | 0.14 |
| RHem WM | 0.83 | 0.55 | 0.57 | 0.26 | 0.17 |
| LHem WM | 0.80 | 0.51 | 0.58 | 0.22 | 0.20 |
| RHem Tot | 0.85 | 0.61 | 0.48 | 0.37 | 0.15 |
| LHem Tot | 0.86 | 0.57 | 0.59 | 0.27 | 0.14 |
| Corpus Callosum | 0.61 | 0.45 | 0.31 | 0.29 | 0.39 |
Abbreviations: ICV, intracranial volume; GM, gray matter; WM, unmyelinated white matter; EM, early myelinated white matter; CSF, cerebrospinal fluid; SubCortGM, subcortical gray matter; RHem, right hemisphere; LHem, left hemisphere.
Adjusted for gender, gestational age at birth, and gestational age at MRI.
Table IV.
Univariate ACE model maximum likelihood parameter estimates and P values
| Variance components (95% CI) | Hypothesis test (P values) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| a 2 | c 2 | e 2 | A | C | A and C | ||||
| ICV | 0.73 | (0.68, 0.92) | 0.15 | (0.00, 0.48) | 0.13 | (0.13, 0.23) | 0.0001 | 0.4976 | <0.0001 |
| Lateral ventricles | 0.71 | (0.68, 0.84) | 0.00 | (0.00, 0.00) | 0.29 | (0.16, 0.53) | 0.0045 | 1.0000 | 0.0001 |
| Total GM | 0.56 | (0.56, 0.90) | 0.29 | (0.00, 0.59) | 0.14 | (0.08, 0.26) | 0.0017 | 0.1659 | <0.0001 |
| Total WM | 0.85 | (0.85, 0.94) | 0.05 | (0.01, 0.05) | 0.09 | (0.05, 0.17) | <0.0001 | 0.8065 | <0.0001 |
| Total EM | 0.19 | (0.05, 0.44) | 0.00 | (0.00, 0.00) | 0.81 | (0.56, 1.00) | 0.3833 | 1.0000 | 0.3867 |
| Total CSF | 0.63 | (0.58, 0.77) | 0.00 | (0.00, 0.00) | 0.37 | (0.23, 0.59) | 0.0200 | 1.0000 | <0.0001 |
| Cortical GM | 0.58 | (0.41, 0.90) | 0.27 | (0.00, 0.58) | 0.15 | (0.09, 0.26) | 0.0017 | 0.2131 | <0.0001 |
| Cortical WM | 0.85 | (0.50, 0.94) | 0.05 | (0.00, 0.40) | 0.10 | (0.06, 0.18) | <0.0001 | 0.8065 | <0.0001 |
| SubCortGM | 0.62 | (0.48, 0.76) | 0.00 | (0.00, 0.00) | 0.38 | (0.24, 0.61) | 0.0191 | 1.0000 | <0.0001 |
| Cerebellum | 0.17 | (0.00, 0.53) | 0.62 | (0.28, 0.82) | 0.21 | (0.12, 0.36) | 0.3125 | 0.0015 | <0.0001 |
| Prefrontal GM | 0.30 | (0.00, 0.77) | 0.36 | (0.00, 0.69) | 0.34 | (0.21, 0.57) | 0.2815 | 0.1859 | <0.0001 |
| Frontal GM | 0.31 | (0.25, 0.78) | 0.36 | (0.00, 0.69) | 0.32 | (0.20, 0.54) | 0.2207 | 0.1435 | <0.0001 |
| Parietal GM | 0.65 | (0.65, 0.86) | 0.12 | (0.00, 0.51) | 0.23 | (0.14, 0.41) | 0.0089 | 0.6242 | <0.0001 |
| Occipital GM | 0.57 | (0.51, 0.86) | 0.21 | (0.00, 0.58) | 0.23 | (0.13, 0.40) | 0.0179 | 0.3897 | <0.0001 |
| Prefrontal WM | 0.53 | (0.53, 0.88) | 0.29 | (0.00, 0.63) | 0.18 | (0.11, 0.33) | 0.0117 | 0.2435 | <0.0001 |
| Frontal WM | 0.84 | (0.72, 0.91) | 0.00 | (0.00, 0.00) | 0.16 | (0.09, 0.28) | <0.0001 | 1.0000 | <0.0001 |
| Parietal WM | 0.72 | (0.72, 0.88) | 0.07 | (0.00, 0.45) | 0.21 | (0.12, 0.37) | 0.0033 | 0.7518 | <0.0001 |
| Occipital WM | 0.86 | (0.58, 0.92) | 0.00 | (0.00, 0.00) | 0.14 | (0.08, 0.25) | 0.0001 | 1.0000 | <0.0001 |
| Total prefrontal | 0.42 | (0.42, 0.86) | 0.38 | (0.00, 0.70) | 0.20 | (0.12, 0.36) | 0.0421 | 0.1336 | <0.0001 |
| Total frontal | 0.64 | (0.64, 0.85) | 0.11 | (0.00, 0.51) | 0.25 | (0.15, 0.43) | 0.0121 | 0.6468 | <0.0001 |
| Total parietal | 0.74 | (0.61, 0.89) | 0.09 | (0.00, 0.45) | 0.18 | (0.11, 0.32) | 0.0011 | 0.7083 | <0.0001 |
| Total occipital | 0.74 | (0.36, 0.91) | 0.11 | (0.00, 0.47) | 0.15 | (0.09, 0.27) | 0.0004 | 0.6242 | <0.0001 |
| RHem GM | 0.44 | (0.44, 0.86) | 0.36 | (0.00, 0.67) | 0.20 | (0.12, 0.35) | 0.0272 | 0.1016 | <0.0001 |
| LHem GM | 0.71 | (0.71, 0.92) | 0.15 | (0.00, 0.49) | 0.13 | (0.08, 0.24) | 0.0002 | 0.4839 | <0.0001 |
| RHem WM | 0.82 | (0.75, 0.94) | 0.08 | (0.00, 0.42) | 0.10 | (0.06, 0.17) | <0.0001 | 0.7083 | <0.0001 |
| LHem WM | 0.79 | (0.79, 0.91) | 0.07 | (0.00, 0.43) | 0.15 | (0.09, 0.27) | 0.0003 | 0.7642 | <0.0001 |
| RHem Tot | 0.64 | (0.61, 0.92) | 0.25 | (0.00, 0.55) | 0.12 | (0.07, 0.21) | 0.0002 | 0.2384 | <0.0001 |
| LHem Tot | 0.74 | (0.74, 0.93) | 0.15 | (0.00, 0.48) | 0.11 | (0.06, 0.19) | <0.0001 | 0.4751 | <0.0001 |
| Corpus callosum | 0.04 | (0.04, 0.66) | 0.54 | (0.00, 0.70) | 0.42 | (0.27, 0.60) | 0.8875 | 0.0693 | <0.0001 |
Adjusted for gender, gestational age at birth, and gestational age at MRI.
Abbreviations: CSF, cerebrospinal fluid; EM, early myelinated white matter; GM, gray matter; ICV, intracranial volume; LHem, left hemisphere; RHem, right hemisphere; WM, unmyelinated white matter.
Of note, there was high heritability of lateral ventricle volume in the neonatal period (0.71) and low heritability of total cerebellum volume (0.17). The cerebellum had a significant common environment contribution to volume (c 2 = 0.62).
We previously found a posterior to anterior gradient in cortical gray matter growth in the neonatal period, with robust growth of gray matter in the occipital and parietal regions and much slower growth in the frontal and prefrontal regions [Gilmore et al.,2007]. By combining the left and right superior and inferior parcellations of a region into one overall regional parcellation (Table IV; Fig. 1), there was a regional difference in heritability of gray matter growth with posterior regions more heritable than frontal regions: occipital gray matter (0.57), parietal gray matter (0.65), frontal gray matter (0.31), and prefrontal gray matter (0.30). Heritability of unmyelinated white matter was similar across the cortical regions.
Figure 1.
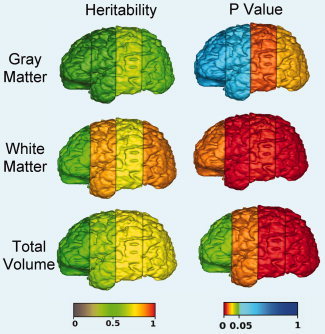
Heritability (a 2 from univariate ACE model maximum likelihood parameter estimates) and P values for combined cortical regions. Left and right superior and inferior subregions of the prefrontal, frontal, parietal, and occipital regions were combined. Note higher heritability of gray matter in posterior regions compared to anterior regions. Heritability of white matter was similar across regions.
Heritability of gray and unmyelinated white matter within the cortical box parcellation is depicted in Supporting Information Figure 2. Parameter estimates and tests of the statistical significance are presented in Supporting Information Table I. For gray matter, heritability ranges from 0.10 to 0.68, with generally higher heritabilities in the left hemisphere. This is consistent with the higher heritability of left (0.71) compared to right (0.44) total cortical gray matter. For unmyelinated white matter, heritabilities were generally higher than for gray matter and ranged from 0.28 to 0.79. There was no clear hemispheric difference, consistent with the similar heritablities of left (0.79) and right (0.82) total cortical unmyelinated white matter.
DISCUSSION
To our knowledge, this is the first twin study of neonatal brain structure and offers many interesting findings with respect to the genetic and environmental contributions to very early brain development. By comparing our results to previous studies in older children and adults, this study also offers insights into how genetic and environmental factors act over the course human brain development. Changes in heritability, the ratio of genetic variance to total variance, can be the result of a change in genetic or environmental variance.
Overall, we observed high heritabilities of ICV (0.73) and total white matter (0.85) in the neonate, similar to that observed in older children (0.89 and 0.85; Wallace et al. [2006]) and adults (0.90 and 0.88; Baare et al. [2001]). Studies in older children and adults find that gray matter has somewhat less heritability than white matter, a similar pattern is observed in neonates. We found that the heritability of total gray matter in the neonatal period (0.56) is somewhat less than has been observed in older children (0.82; Wallace et al. [2006]) and adults (0.82; Baare et al. [2001]). In the first 2 years of life, cortical gray matter volume increases 149% [Knickmeyer et al.,2008], consistent with rapid development of synapses in this period [Glantz et al.,2007; Huttenlocher and Dabholkar,1997]. It appears that the gray matter heritability increases between the neonatal period and older childhood. Intriguingly, the heritability of general cognitive ability also increases from childhood to young adulthood [Haworth et al., in press]. As it has been demonstrated that general cognitive ability and gray matter volume are correlated in adult twins [Thompson et al.,2001] and that this correlation is due to genetic factors [Posthuma et al.,2002], the increasing heritability of cognitive ability may coincide with increasing heritability of gray matter volumes with age.
There was a posterior to anterior gradient of gray matter heritability with posterior regions more heritable than frontal regions: occipital GM (0.57), parietal GM (0.65), frontal GM (0.31), and prefrontal gray matter (0.30). In the neonatal period, cortical gray matter volumes increase rapidly in the parietal and occipital regions, with much less growth in the frontal and prefrontal regions [Gilmore et al.,2007]. This is consistent with regional variation in synapse development [Huttenlocher and Dabholkar,1997] and the development of functional sensory and motor systems before executive function systems in the infant. The higher heritabilities of gray matter in the rapidly growing occipital and parietal regions compared to the frontal and prefrontal regions as well as the overall increase of gray matter heritability from the neonatal period to late childhood indicates that genetic influence increases as gray matter develops. This is consistent with the study of Lenroot et al. [2009] that found heritabilities of cortical thickness increased from late childhood through adolescence and that earlier maturing primary sensory regions had higher heritabilities than later maturing regions associated with complex cognitive processes. However, Wallace et al. [2006] found that the heritability of overall cortical gray matter volume decreased with increasing age in this cohort.
We also found evidence of greater heritability of gray matter in the left hemisphere (0.71) compared to the right hemisphere (0.44); there was little difference in heritability of white matter between the left (0.79) and right (0.82) hemispheres. In the neonatal period, left hemisphere gray matter volume is about 4.6% larger than the right, whereas white matter is only 1.2% greater in the left hemisphere [Gilmore et al.,2007]. In older children, the right hemisphere is larger than the left, and in adults, the right is only modestly larger (0.19%; Gur et al. [1999]). In adults, there is little difference in the heritability of hemispheric volume (left/right: 0.67/0.64; Geschwind et al. [2002]). This also suggests that for gray matter, higher heritability is associated with growth and maturation.
In contrast to gray matter, white matter volumes exhibit similar heritability in neonates, older children, and adults. We also observed no overall regional differences in heritability of unmyelinated white matter volume. Most white matter in the neonatal period is unmyelinated [Gilmore et al.,2007]. Although Wallace et al. [2006] found white matter volume heritability increased with age in childhood and adolescence, overall heritability appears to be high‐throughout postnatal development into adulthood. Overall, white matter volume may be less moderated by environmental influences through development; the “hard wiring” of the brain (as much as this reflected in white matter volumes) may be genetically determined from a very early age. Total volume is a crude measure of maturation; therefore, we have obtained diffusion tensor imaging in this cohort and will be able to study genetic and environmental contributions to white matter tract maturation and myelination.
The high heritability of lateral ventricle volume in the neonatal period is of interest and contrasts with the low heritability found in older children and adults (reviewed in Schmitt et al. [2007a]). The volume of the lateral ventricle increases a remarkable 280% in the first year of life while total brain volume increases about 100% [Knickmeyer et al.,2008]. Our findings suggest that the volume of the lateral ventricle is genetically determined in early development, but becomes more influenced by environmental factors through postnatal development. Interestingly, recently, a twin study of ventricle shape in young adults found low heritability of overall lateral ventricle volume, but more genetic influence in earlier‐maturing occipital horns compared to the later‐maturing frontal horns [Chou et al.,2009]. It has been suggested that candidate genes might be best detected in periods where heritability for the phenotype of interest is high [Haworth et al., in press]. This would suggest that the neonatal period would be a particularly powerful period in which to look for genetic effects on ventricle volume.
Prenatal ventricle size on ultrasound is predictive of neonatal ventricle volume [Gilmore et al.,2008], and we have argued that prenatal ventricle volume is a early marker of risk for neurodevelopmental disorders [Gilmore et al.,1998]. Our finding of high heritability of neonatal lateral ventricle volume supports this notion. Although heritability of lateral ventricle volume is low in adults, the shape is more similar in MZ compared to DZ twins [Styner et al.,2005]. We are currently studying the heritability of ventricle shape in this cohort as well.
We also found very low heritability (0.17) of cerebellum volume in the neonatal period. Studies in adults find high heritability (>0.60) of cerebellar volume [Posthuma et al.,2000; Wright et al.,2002]. In late childhood and adolescence, the heritability estimate is somewhat lower at 0.49 [Wallace et al.,2006], though in a recent study of 9‐year‐olds heritability of the cerebellum was estimated to be higher at 0.88 [Peper et al.,2009]. The volume of the cerebellum increases dramatically in the first year of life—240%, by 15% more in the second‐year old life [Knickmeyer et al.,2008] and does not change during later childhood and adolescence [Giedd et al.,1996]. It appears that heritability increases with growth of volume in the cerebellum. The cerebellum was also the only major brain region to show a significant influence of the common environment. This could reflect this structure's heightened susceptibility to environmental insults such as alcohol, lead, and anoxia [Welsh et al.,2002].
In this cohort, we previously found that in the prenatal period, overall brain size determined by ultrasound was similarly discordant in MZ and DZ twin pairs, suggesting that variation in placental blood flow and other prenatal factors had a significant effect on MZ twin brain sizes [Mukherjee et al.,2009]. Interestingly, in the first weeks of life after birth, MZ twin brain sizes become much less discordant, indicating a “normalization” of genetically controlled developmental trajectories in the absence of the constraints of the prenatal environment.
The increase of heritability of gray matter volume and cerebellum volume over the course of postnatal brain development may be examples of “canalization,” recently discussed in relation to twin studies of brain structure by Lenroot and Giedd [2008]. The heritability of a structure will increase as various genetic programs act over development. The mature phenotype is relatively resistant to typical environmental influences as long as necessary environmental factors are present. Under normal conditions, environment influences may shape the trajectory, though the endpoint of the mature phenotype remains highly heritable.
CONCLUSION
This study provides new information about genetic and environmental contributions to brain structure in the neonatal period. In addition, inferences about how these contributions change over the course of development can be made by comparison of these results with twin studies of brain structure in older children and adults. Three general patterns are observed depending on the brain structure. The heritability of white matter volume is high in the neonatal period and is similar across development. For cortical gray matter volume and especially cerebellum volume, heritability increases with development. In contrast, the heritability of lateral ventricle volume is high at birth and decreases substantially through adulthood.
Future studies are needed to more carefully define the evolution of genetic and environmental effects over the course of childhood brain development. We are currently conducting longitudinal follow‐up of this cohort at ages 1 and 2 years and beyond.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplementary Figure 1
Supplementary Figure 2
Supplementary Table 1
Acknowledgements
We thank Dianne Evans and Mary Norton for study coordination, Barbara Hertzberg MD for subject recruitment at DUMC, Joseph Blocher and Meghan Casey for technical assistance with the image analysis, Cassian Marc for preparing the 3D visualizations, and Abby Scheer and Robert Hamer PhD for database support.
This article was published online on 8 Jan 2010. An error was subsequently identified. This notice is included in the online and print versions to indicate that both have been corrected 12 May 2010.
Footnotes
Panizzon M, Neale MC, Fennema‐Notestine C, Prom‐Wormley E, Eyler L, Stevens A, Franz C, Lyons M, Grant M, Jak A, Jernigan T, Xian H, Fischl B, Thermenos H, Seidman L, Tsuang M, Dale A, Kremen W. Heritability of brain ventricle size: Converging evidence from inconsistent results. Neurobiology of Aging (in revision).
REFERENCES
- Baaré WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJ, Schnack HG, van Haren NE, van Oel CJ, Kahn RS ( 2001): Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex 11: 816–824. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM ( 2009): Genetics of brain fiber architecture and intellectual performance. J Neurosci 29: 2212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YY, Lepore N, Chaing MC, Avedissian C, Barysheva M, McMahon KL, de Zubicaray GI, Meredith M, Wright MJ, Toga AW, Thompson PM ( 2009): Mapping genetic influences on ventricular structure in twins. NeuroImage 44: 1312–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AWF ( 1972): Likelihood. Cambridge, London, UK: Cambridge University Press. [Google Scholar]
- Falconer DS, Mackay TFC ( 1996): Introduction to Quantitative Genetics. Harlow, Essex, UK: Longmans Green. [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D ( 2002): Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci USA 99: 3176–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL ( 1996): Quantitative magnetic resonance imaging of human brain development: ages 4‐18. Cereb Cortex 6: 551–560. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, van Tol J, Kliewer MA, Silva SG, Cohen SB, Hertzberg BS, Chescheir NC ( 1998): Mild ventriculomegaly detected in utero with ultrasound: clinical associations and implications for schizophrenia. Schizophr Res 33: 133–140. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, et al. ( 2007): Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci 27: 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Smith LC, Wolfe HM, Hertzberg BS, Smith JK, Chescheir NC, Evans DD, Kang C, Hamer RM, Lin W, et al. ( 2008): Prenatal mild ventriculomegaly predicts abnormal development of the neonatal brain. Biol Psychiatry 64: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF ( 2007): Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid‐gestation into early adulthood. Neuroscience 149: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE ( 1999): Sex differences in brain gray and white matter in healthy young adults: Correlations with cognitive performance. J Neurosci 19: 4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, Bartels M, Posthuma D, Boomsma DI, Davis OS, Kovas Y, Corley RP, Defries JC, Hewitt JK, Olson RK, Rhea SA, Wadsworth SJ, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, Plomin R ( 2009): The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry June 2 epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J ( 2005): Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Arch Gen Psychiatry 62: 1366–1376. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, van Haren NE, Collins DL, Evans AC, Amunts K, Bürgel U, Zilles K, de Geus E, Boomsma DI, Kahn RS ( 2006): Genetic contributions to human brain morphology and intelligence. J Neurosci 26: 10235–10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS ( 1997): Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387: 167–178. [DOI] [PubMed] [Google Scholar]
- Kagan J, Herschkowitz N ( 2005): A Young Mind in a Growing Brain. Mahwah, New Jersey: Lawrence Erlbaum Associates. [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH ( 2008): A structural MRI study of human brain development from birth to 2 years. J Neurosci 28: 12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN ( 2008): The changing impact of genes and environment on brain development during childhood and adolescence: Initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol 20: 1161–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN ( 2009): Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp 30: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P ( 2002): Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25: 409–432. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Kang C, Wolfe HM, Hertzberg BS, Smith KS, Lin W, Gerig G, Hamer R, Gilmore JH ( 2009): Discordance of prenatal and neonatal brain development in twins. Early Hum Dev 85: 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Cardon LR ( 1992): Methodology for Genetic Studies of Twins and Families. NATO ASI Ser. D, Vol. 67 Dordrecht, The Netherlands: Kluwer. [Google Scholar]
- Neale MC, Miller MB ( 1997): The use of likelihood‐based confidence intervals in genetic models. Behav Genet 27: 113–120. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH ( 1999): Mx: Statistical Modeling [Computer Manual, 5th ed]. Richmond, VA: Virginia Commonwealth University, Department of Psychiatry. [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE ( 2007): Genetic influences on human brain structure: A review of brain imaging studies in twins. Hum Brain Mapp 28: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Schnack HG, Brouwer RM, Van Baal GC, Pjetri E, Szekely E, van Leeuwen M, van den Berg SM, Collins DL, Evans AC, Boomsma DI, Kahn RS, Hulshoff Pol HE ( 2009): Heritability of regional and global brain structure at the onset of puberty: a magnetic resonance imaging study in 9‐year‐old twin pairs. Hum Brain Mapp 30: 2184–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Swan GE, Carmelli D ( 2000): Brain structure in men remains highly heritable in the seventh and eighth decades of life. Neurobiol Aging 21: 63–74. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Neale MC, Hulshoff Pol HE, Baare WEC, Kahn RS, Boomsma D ( 2000): Multivariate genetic analysis of brain structure in an extended twin design. Behav Genet 30: 311–319. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Baare WEC, Hulshoff Pol HE, Kahn RS, Boomsma DI ( 2002): The association between brain volume and intelligence is of genetic origin. Nat Neurosci 5: 83–84. [DOI] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin W, Gerig G ( 2005): Automatic segmentation of MR images of the developing newborn brain. Med Image Anal 9: 457–466. [DOI] [PubMed] [Google Scholar]
- R Development Core Team ( 2005): A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC ( 2007a): Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res Hum Genet 10: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Wallace GL, Rosenthal MA, Molloy EA, Ordaz S, Lenroot R, Clasen LS, Blumenthal JD, Kendler KS, Neale MC, Giedd JN ( 2007b): A multivariate analysis of neuroanatomic relationships in a genetically informative pediatric sample. Neuroimage 35: 70–82. [DOI] [PubMed] [Google Scholar]
- Styner M, Lieberman JA, McClure RK, Weinberger DR, Jones DW, Gerig G ( 2005): Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease‐specific factors. Proc Natl Acad Sci USA 102: 4872–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold‐Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW ( 2001): Genetic influences on brain structure. Nature Neuroscience 4: 1253–1258. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Schmitt JE, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN ( 2006): A pediatric twin study of brain morphometry. Journal of Child Psychology and Psychiatry 47: 987–993. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O'Hearn E, Molliver ME, Aicher SA ( 2002): Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol 89: 331–359. [PubMed] [Google Scholar]
- Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET ( 2002): Genetic contributions to regional variability in human brain structure: Methods and preliminary results. Neuroimage 17: 256–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplementary Figure 1
Supplementary Figure 2
Supplementary Table 1


