Abstract
The ability of Toxoplasma gondii to cycle between the tachyzoite and bradyzoite life stages in intermediate hosts is key to parasite survival and the pathogenesis of toxoplasmosis. Studies from a number of laboratories indicate that differentiation in T. gondii is a stress-induced phenomenon. The signalling pathways or molecular mechanisms that control formation of the latent bradyzoite stage are unknown and specific effectors of differentiation have not been identified. We engineered a reporter parasite to facilitate simultaneous comparison of differentiation and replication after various treatments. Chloramphenicol acetyltransferase (CAT), expressed constitutively from the α-tubulin promoter (TUB1), was used to quantitate parasite number. β-galactosidase (β-GAL), expressed from a bradyzoite specific promoter (BAG1), was used as a measure of bradyzoite gene expression. Sodium nitroprusside, a well-known inducer of bradyzoite differentiation, reduced reporter parasite replication and caused bradyzoite differentiation. Stress-induced differentiation in many other pathogens is regulated by cyclic nucleotide kinases. Specific inhibitors of the cAMP dependent protein kinase and apicomplexan cGMP dependent protein kinase inhibited replication and induced differentiation. The β-GAL/CAT reporter parasite provides a method to quantify and compare agents that cause differentiation in T. gondii.
Keywords: Differentiation, Bradyzoite, Kinase, Toxoplasma, Reporter parasite, Cyclic nucleotide, cAMP, cGMP
1. Introduction
Toxoplasma gondii is an obligate intracellular parasite that is able to infect all nucleated mammalian and avian cells (Luft et al., 1984; McLeod et al., 1991; Wong and Remington, 1993). Humans are normally infected after consumption of contaminated foods. The immune system in normal individuals is able to control the infection and most infected persons have no clinical disease due to infection. However, even in healthy individuals, the immune system is not able to eradicate the infection and T. gondii persists as latent bradyzoites in the host (Yap and Sher, 1999). Disease due to T. gondii is most often seen in immunocompromised persons and congenitally infected fetuses. Toxoplasma-induced encephalitis occurs in immunocompromised patients and progressive encephalitis and retinitis occurs in children infected in utero (Luft et al., 1984; Wong and Remington, 1993). The recrudescence of bradyzoites as the immune system wanes is thought to be the cause of Toxoplasma-induced encephalitis in most immunocompromised individuals (Luft and Remington, 1988; Weiss et al., 1988). The ability of T. gondii to cycle between the tachyzoite and bradyzoite forms in intermediate hosts is key to its survival and a major factor in pathogenesis of toxoplasmosis.
Recent research has focused upon defining the differences between bradyzoites and tachyzoites. Differences in replication rate, gene expression and metabolism exist and have been well characterized between these two forms of the parasite (Reviewed in Weiss and Kim, 2000). Tachyzoites replicate faster than bradyzoites. Tachyzoites express unique stage-specific genes such as SAG1 and LDH1 while bradyzoites express other stage-specific genes such as CST1, BAG1, and LDH2 (reviewed in Weiss and Kim, 2000). Using antibodies to stage-specific proteins, several investigators have established that stress conditions including pH shock, heat shock, chemical stress, mitochondrial inhibitors and nitric oxide induce bradyzoite formation (Bohne et al., 1994; Soete et al., 1994; Tomavo and Boothroyd, 1995; Weiss et al., 1995; Soete and Dubremetz, 1996). The mechanism of action by which these conditions induce differentiation is not known.
Cyclic nucleotide signalling pathways are conserved and have been implicated in stress-induced differentiation in other organisms. For instance, cAMP signaling via the cAMP-dependent kinase (Protein Kinase A or PKA) is critical for stress-induced pseudohyphal differentiation in Saccharomyces cerevisiae and expression of virulence traits in pathogenic fungi (D'Souza and Heitman, 2001). Both cAMP and PKA are essential for differentiation of Dictyostelium discoideum (Aubry and Firtel, 1999). In addition, cAMP is reported to induce gametocyte formation in malaria (Kaushal et al., 1980) and PKA activity has been correlated with the ability of Plasmodium falciparum strains to transform into gametocytes (Read and Mikkelsen, 1991).
The methods used to establish which agents induce bradyzoite differentiation in T. gondii are not well adapted for quantitative assays. As tachyzoites differentiate into bradyzoites two measurable events occur: (i) parasite replication slows; and (ii) bradyzoite-specific proteins are expressed. T. gondii replication can be quantified by counting parasites microscopically, measuring uracil uptake, enzymatic quantitation of stably transfected β-galactosidase and quantitation of yellow fluorescent protein or green fluorescent protein (GFP) fluorescence (McFadden et al., 1997; Weiss et al., 1998; Kirkman et al., 2001; Gubbels et al., 2003).
Bradyzoite-specific gene expression has been studied using bradyzoite-specific antibodies in immunofluorescence assays (IFA) or Western blots. Studies to elucidate the possible molecular mechanisms used by T. gondii to differentiate have typically relied upon microscopic IFA methods to measure differentiation (Weiss et al., 1998; Kirkman et al., 2001). This method of measuring differentiation is laborious and has numerous steps in which experimental variation could mask significant but subtle results. Flow cytometry can also be used to assess bradyzoite differentiation in parasites expressing GFP driven by a bradyzoite-specific promoter such as LDH2 but this method does not quantitate growth in parallel (Singh et al., 2002). Currently, there is no parasite that allows enzymatic quantitation of both growth and bradyzoite gene expression.
To elucidate signalling pathways that may be involved in signalling the tachyzoite to bradyzoite transition, we created a new reporter parasite to measure differentiation in T. gondii. This reporter parasite was used to enzymatically quantitate replication and bradyzoite gene expression using a known differentiation inducer sodium nitroprusside (SNP) as well as agents that affect cyclic nucleotide signalling. These studies implicate both PKA and the cGMP-dependent kinase (PKG) in signalling differentiation in T. gondii.
2. Materials and methods
2.1. Parasite and tissue culture
PLK, a clonal derivative of the Type II strain ME49, was used for creation of the reporter parasite. These PLK parasites had been previously passed through mice and were known to generate cysts efficiently in vivo (Zhang et al., 1999). Parasites were maintained by serial passage in confluent monolayers of human foreskin fibroblasts (HFF) grown in Dulbecco's modified Eagle's medium (Gibco BRL) supplemented with 10% FCS (Gibco BRL) and 1% penicillin–streptomycin (Gibco BRL).
2.2. Compounds and reagents
Forskolin, 1-methyl-3-isobutylxanthine (IBMX), 8-(4-chlorophenolthio)-cAMP (CPT-cAMP), 8-(4-chlorophenolthio)-cGMP (CPT-cGMP), SNP, N-(2-[p-Bromocinnamylamino]ethyl)-5-isoquinolinesulfonamide hydrochloride (H89), acetyl-CoA, and 4-methyumbelliferyl-β-d-galactoside (4MUG) were obtained from Sigma. Pyrrole 4-[2-(4-fluorophenyl)-5-(1-methylpiperidine-4-yl)-1H-pyrrol-3-yl]pyridine (Compound 1) was a gift from Merck Research Laboratories.
Forskolin, CPT-cAMP, CPT-cGMP, and IBMX were dissolved in dimethyl sulfoxide; SNP was dissolved in Dulbecco's modified Eagle's medium. H89, Compound 1, Acetyl-CoA and 4MUG were dissolved in H2O.
2.3. Creation of reporter parasite
PLK strain parasites were co-transfected with 25 μg TUB-CAT plasmid (Soldati and Boothroyd, 1993) mixed with 100 μg BAG1 β-galactosidase (β-GAL) plasmid (Ma et al., 2004) using standard electroporation conditions with a BTX electroporater (Kim et al., 1993). Stable T. gondii transfectants bearing the chloramphenicol acetyltransferase (CAT) marker driven by the α-tubulin promoter (contained in the TUB-CAT plasmid) were selected for using 20 μM chloramphenicol as previously described (Kim et al., 1993). Parasites emerging from drug selection were cloned by limiting dilution. As expected, all clones expressed CAT activity. Single clones were then screened for β-GAL activity (Seeber and Boothroyd, 1996) in the presence of 100 μM SNP at 48 h. A single clonal line was used for subsequent experiments.
2.4. Culture conditions
Transgenic PLK parasites were grown in T25 tissue culture flasks at 37 8C and 5% CO2. Parasites (2.6×106) were inoculated onto a confluent T25 flask of HFF cells and allowed to invade host cells. Uninvaded parasites were removed and fresh media containing the desired agent being tested was added after 2 h. The following final concentrations of compounds were used: 100 μM SNP, 500 μM IBMX, 10 μM forskolin, 250 μM CPT-cAMP, 250 μM CPT-cGMP, 3 μM H89, 2 μM Compound 1 (Nare et al., 2002).
2.5. CAT and β-GAL reporter assays
Parasites grown in T25 tissue culture flasks were used for both CAT assay and β-GAL assay. T25 tissue culture plates were scraped with a plastic policeman (in a volume of 5 ml). One millilitre of parasite/HFF cell suspension was used in FAST CAT (Molecule Probes) fluorescent CAT assay. Reactions were analysed by thin layer chromatography (TLC) using chloroform:methanol (9:1 v/v). The TLC was visualized using a phosphoimager (absorption 505 nm, excitation 512 nm). The percent of chloramphenicol acetylated was determined using ImageQuant software (Molecular Dynamics).
The remaining 4 ml of cells were used in the β-GAL assay. The cells were lysed by three cycles of freezing (−80 °C) and thawing (37 °C). The β-GAL assay was performed using as previously described (Seeber and Boothroyd, 1996). The β-GAL activity was determined by using DyNA Quant 2000 (Hoefer Pharmacia Biotech). Both CAT activity and β-GAL activity were measured at 24, 48 and 72 h.
For standardization of growth assays, parasites were counted using a hemocytometer and serially diluted to establish a dynamic range where the corresponding CAT activity did not saturate the measuring method. Parasites (104–106) encompassed the range of 0–100% CAT activity. Seven quantities of parasites (104, 2.5 × 104, 5.0 × 104, 105, 2.5 × 105, 5.0 × 105, 106) were tested for CAT activity with at least 15 samples taken for each parasite concentration.
2.6. Derivation of equations used to calculate parasite number and doubling time
Parasite doubling was assumed to be exponential with each parasite within a vacuole doubling by endodyogeny. Therefore:
Parasite number = C2Time/Doubling rate,
with C initial parasite number.
Transforming this equation derived from the exponential growth model with the natural log creates a linear equation (y = Mx + B) with time as the independent variable:
ln(Parasite number) = ln(C2Time/Doubling rate)
ln(Parasite number) = ln(C) + ln(2Time/Doubling rate)
ln(Parasite number) = [Time/Doubling rate]ln(2) + ln(C)
The doubling rate was determined by plotting the exponential curve on a semi-log plot to linearise the curve. A trend line was fit to the linear data using the least squares method. The slope of the trend line is the doubling rate for the parasite population.
3. Results
3.1. Creation and characterization of the bradyzoite reporter parasite
The ME49/PLK genetic background was used to create a stable reporter parasite to measure bradyzoite differentiation. Stable transformants bearing both CAT (constitutive) and β-GAL (bradyzoite) reporters were obtained and characterized (Kim et al., 1993; Seeber and Boothroyd, 1996). A single cloned line was used for subsequent experiments.
We first verified that CAT activity of the reporter parasite was proportional to parasite number and could be used to measure replication (Fig. 1). The CAT reporter is widely used because transcription levels of CAT correspond well with enzymatic activity (Hruby et al., 1990). The constitutive TUB1 promoter (Knoll and Boothroyd, 1998) was used to control cat expression (Soldati and Boothroyd, 1993). Since the parasite population is derived from a single cloned cell line, we assumed each parasite expressed the same amount of CAT. This was verified by empirically testing the relationship between CAT activity and parasite number (Fig. 1). There was a linear relationship between the average percent of chloramphenicol acetylated and the log of parasite number. Linear regression using the least squares method was used to create an equation to represent the relationship between CAT activity and parasite number:
Fig. 1.
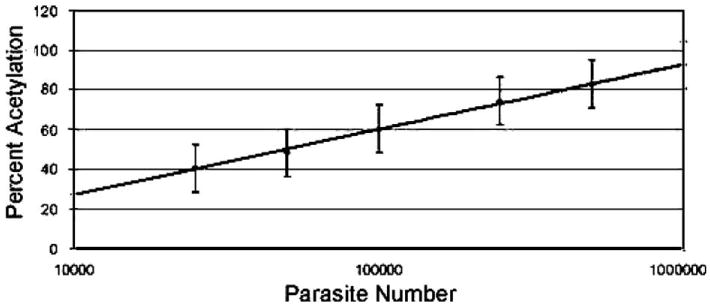
Reporter activity correlates with parasite number. The relationship between chloramphenicol acetyltransferase (CAT) activity (acetylation) and parasite number was derived mathematically. At least 15 independent samples were tested for CAT activity at seven different parasite concentrations. A linear relationship was found on a semi-log plot and the linear trend line was fitted to the empirical data yielding the equation: percent acetylation = 32.92 × log10(Parasite number) − 104.54. The standard deviation for CAT activity was 12%.
Percent acetylation = 32.92 × log10(Parasite number) − 104.54
Growth curves created by measuring CAT activity at 24, 48 and 72 h were used to determine replication rates. Parasite number was plotted versus time and fit to a trend line using an exponential growth model. The mean of five independent samples at each time point (with 95% confidence interval) was used. We assumed the population doubles after each round of replication since T. gondii undergoes a unique form of binary fission (endodygeny) where each mother parasite gives rise to two daughter parasites. Previous studies have demonstrated growth of T. gondii in vitro to be exponential (McFadden et al., 1997; Gubbels et al., 2003).
The predicted growth curve of ME49 strain was plotted using the published replication rate of 9 h (Radke et al., 2001) (Fig. 2(A)). The growth curve of the reporter parasite was plotted in parallel using CAT activity to estimate parasite number (PLK reporter parasite, Fig. 2(A)). The doubling time for the reporter parasite was 9.4 h. Doubling time of the reporter parasite increased to 14.2 h when the parasite was exposed to the differentiating agent SNP. This is consistent with the slower replication rate associated with transition to bradyzoites (Bohne et al., 1994).
Fig. 2.
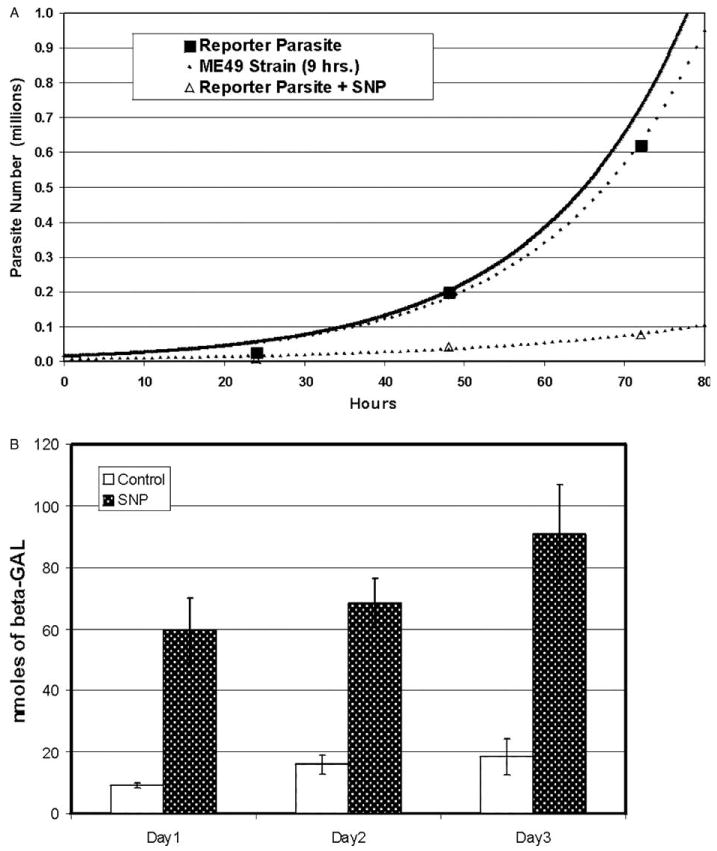
Growth curves and bradyzoite reporter activity of the reporter parasite. (A) The growth curves for the reporter parasite were derived using chloramphenicol acetyltransferase (CAT) activity. The CAT activity was measured at 24, 48 and 72 h under normal growth conditions (black squares) or with 100 μM sodium nitroprusside (SNP), a bradyzoite inducing agent (triangles). A minimum of five independent samples was used to determine CAT activity at each time point and the average deduced parasite number is plotted. The experimentally derived growth curves for the reporter parasite (dotted line with boxes) and reporter parasites exposed to SNP (dotted line with triangles) are shown. The predicted growth curve of ME49 parasites with a doubling rate of 9 h (Radke et al., 2001) is shown for comparison (solid black line). (B) The β-galactosidase (β-GAL)/chloramphenicol acetyltransferase (CAT) reporter parasite has inducible β-GAL expression in response to sodium nitroprusside (SNP). Expression of β-GAL, driven by the bradyzoite specific promoter BAG1, was measured in parallel with CAT activity as discussed in the materials and methods. The β-GAL activity is minimal during normal culture conditions at all measured time points. The addition of a known bradyzoite inducer, 100 μM SNP, causes an increase in β-GAL activity as early as 24 h. The β-GAL activity stays elevated in comparison with controls at all time points.
The bradyzoite-specific promoter BAG1 was used to drive β-GAL gene expression (Ma et al., 2004) (Fig. 2(B)). BAG1 expression is minimal when the parasites are grown in vitro as tachyzoites and increases dramatically after induction to bradyzoites (Weiss and Kim, 2000). The increase in BAG1 transcript levels has been reported to occur as early as 24 h (Bohne et al., 1997). As expected, the reporter parasite has minimal β-GAL activity when grown in standard cell culture conditions and β-GAL expression is low no matter the duration of culture. Addition of 100 μM SNP increased β-GAL activity approximately four-fold over the control (Fig. 2(B)). This increase in β-GAL activity is seen as early as 24 h. The increase in β-GAL activity is sustained up to 72 h, our last measured time point.
3.2. Effects of the PKA pathway on differentiation
Compounds that target the cAMP pathway were tested on the reporter parasite. Forskolin, IBMX and CPT-cAMP were used to stimulate the pathway. H89 was used to inhibit PKA, the main downstream effector of cAMP. Concentrations were chosen after preliminary experiments to identify concentrations of compounds that did not have toxic effects upon host cells but had effects upon bradyzoite differentiation.
Because the PKG inhibitor Compound 1 affected parasite invasion ((Wiersma et al., 2004); Eaton, Weiss and Kim, unpublished data), compounds were added to parasite cultures 2 h after parasite inoculation. In previous studies, we used a protocol in which inducing agents were added simultaneously to HFF with the parasite inoculum (Weiss et al., 1995, 1998; Kirkman et al., 2001). We have consistently observed that differentiation occurs more reliably with less damage to host cells if parasites are exposed to inducers prior to invasion of host cells (Weiss and Kim, unpublished data) consistent with the parasites being more vulnerable to environmental stresses (Weiss et al., 1998; Yahiaoui et al., 1999).
Fig. 3(A) shows the growth curves for agents affecting the cAMP pathway. For each treatment, CAT activity was used to create growth curves and to determine replication rates. The β-GAL activity was used as a surrogate for BAG1 expression. The average of at least five independent samples was used to determine CAT activity and β-GAL activity. Table 1 gives the doubling rate for each treatment. Fig. 3(B) shows the β-GAL activity of treated cultures normalized to β-GAL activity for the untreated control.
Fig. 3.
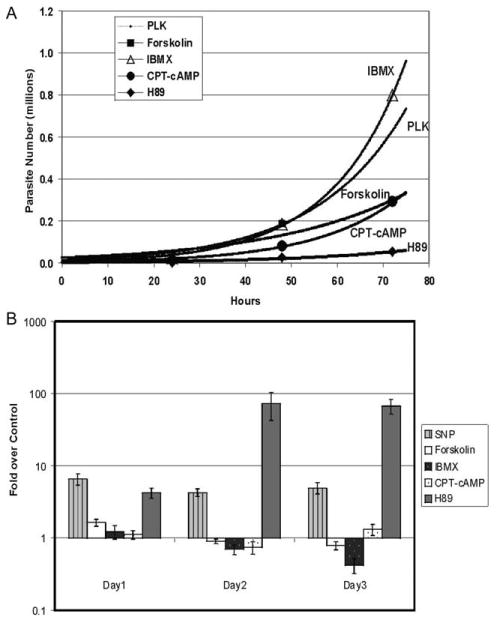
Effect of cAMP modulating agents on parasite replication and bradyzoite reporter activity. (A) The reporter parasite was used to determine the effect of cAMP modulating agents on parasite replication. Forskolin (10 μM; black squares) activates adenylyl cyclase, 1-methyl-3-isobutylxanthine (IBMX) (500 μM; grey triangles) inhibits phosphodiesterases and 8-(4-chlorophenolthio)-cAMP (CPT-cAMP) (250 μM; grey circles) is a non-hydrolyzable form of cAMP. The cAMP dependent kinase (PKA)-specific inhibitor H89 (2 μM; black diamonds) was used to determine the effect of inhibition of the cAMP pathway. Parasites were cultured in media with each agent for up to 72 h. The chloramphenicol acetyltransferase (CAT) activity was measured at 24, 48 and 72 h. At least five independent samples for CAT activity were taken for each time point and the average CAT activity was used to determine parasite number. Growth curves were plotted and lines are indicated on the graph (PLK strain reporter parasite control points are identical to Fig. 2(A) and are not shown). (B) The reporter parasite was cultured in each modulating agent for up to 72 h. β-GAL activity was measured at 24, 48 and 72 h. At least five independent samples were taken for each time point and average β-GAL activity for each time point was determined. The ratio of β-GAL activity for each drug versus the β-GAL activity for untreated control cultures is plotted. The 95% confidence interval is shown with the error bars. Stimulators of the cAMP did not increase β-GAL activity compared with untreated control cultures. H89 increased β-GAL activity at all time points.
Table 1.
Doubling rate of the reporter parasite treated with cAMP and cGMP pathway modulators
| Doubling rate (h) | Slope | Coefficient of determinant | |
|---|---|---|---|
| ME49 Strain | 9 | ||
| Untreated | 9.4 | 0.074 | 0.994 |
| SNP | 14.2 | 0.049 | 0.950 |
| Forskolin | 9.7 | 0.073 | 0.75 |
| IBMX | 7.7 | 0.090 | 0.999 |
| CPT-cAMP | 8.8 | 0.078 | 0.999 |
| H89 | 16.4 | 0.042 | 0.900 |
| CPT-cGMP | 8.7 | 0.079 | 0.852 |
| Compound 1 | 24.4 | 0.028 | 0.983 |
The doubling rate of PLK reporter parasite after treatment with cAMP or cGMP pathway modulators was determined as described in the materials and methods. Growth curves are shown in Figs. 2–4. The slope corresponds to the exponent of the linear trend line for the growth curve plotted on a semi-log plot. The coefficient of determinant indicates how well the trend line fits the actual data (coefficient of determinant = 1 means the trend line fits the experimental data exactly). The ME49 value is that reported by Radke et al. (2001). SNP, sodium nitroprusside; IBMX, 1-methyl-3-isobutylxanthine; CPT-cAMP, 8-(4-chlorophenolthio)-cAMP; CPT-cGMP, 8-(4-chlorophenolthio)-cGMP; Compound 1, pyrrole 4-[2-(4-fluorophenyl)-5-(1-methylpiperidine-4-yl)-1H-pyrrol-3-yl]pyridine.
Three agents tested are known to stimulate the cAMP pathway: forskolin, IBMX and CPT-cAMP. Forskolin is an activator of adenylyl cyclase. IBMX inhibits phosphodiesterases (both cAMP and cGMP specific). CPT-cAMP is a membrane permeable non-hydrolyzable form of cAMP. The activators of the cAMP pathway did not slow replication in comparison with the control. Forskolin, IBMX and CPT-cAMP gave doubling rates of 9.7, 7.7 and 8.8 h. These doubling rates suggest that activation of the cAMP pathway does not slow replication and may even increase parasite replication.
Parasites treated with these agents had increased amounts of β-GAL activity in comparison with the control at 24 h but the increases were less than two-fold. Only the increase in β-GAL activity induced by forskolin was statistically significant. At 48 h all agents had less β-GAL activity than the controls. CPT-cAMP treatment elicited a slight increase in β-GAL activity versus the control at 72 h (Fig. 3(B)).
We also tested the effect of inhibiting PKA using H89, a membrane permeable inhibitor of PKA. The doubling time of 16.4 h for H89-treated parasites was significantly longer than controls. H89-treated parasites also had increased β-GAL expression at all tested time points. The β-GAL activity increased from four-fold greater than controls at 24 h to over 70-fold greater at 48 and 72 h.
3.3. Effects of the PKG pathway on differentiation
Signaling pathways for the cyclic nucleotides cGMP and cAMP typically intersect. We tested a non-hydrolyzable form of cGMP (CPT-cGMP) and Compound 1, a specific inhibitor of PKG. Compound 1 is a cytostatic agent against T. gondii suspected to induce bradyzoite formation (Nare et al., 2002). Fig. 4(A) shows the growth curves for the cGMP modulating agents with calculated doubling times shown in Table 1. CPT-cGMP was similar to the activators of cAMP pathway in that it did not slow replication. Compound 1, the inhibitor of PKG, increased the replication rate to 24.4 h. The expression of β-GAL correlated with decreased replication rate (Fig. 4(B)). Thus inhibition of both PKA and PKG using specific inhibitors caused differentiation.
Fig. 4.
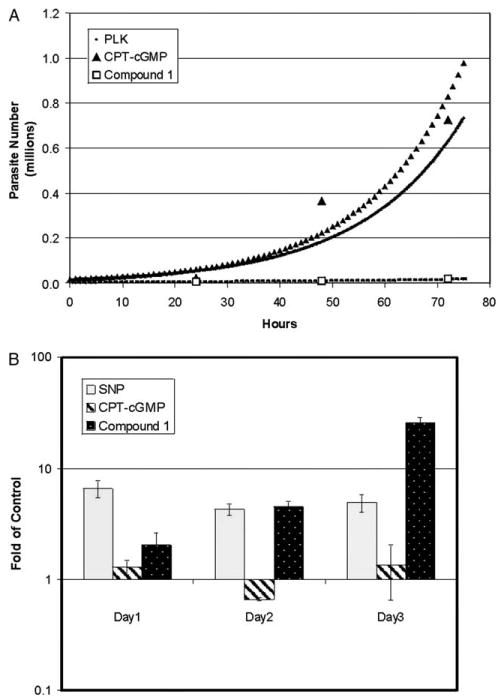
Effect of cGMP modulating agents on parasite replication and bradyzoite reporter activity. (A) The cGMP modulating agents 8-(4-chlorophenolthio)-cGMP (CPT-cGMP) (250 μM; triangles) and pyrrole 4-[2-(4-fluorophenyl)-5-(1-methylpiperidine-4-yl)-1H-pyrrol-3-yl]pyridine (Compound 1) (2 μM; open squares), a cGMP dependent kinase (PKG)-specific inhibitor, were tested for effects upon parasite replication. Parasites were cultured in media with each agent for up to 72 h. Chloramphenicol acetyltransferase (CAT) activity was measured at 24, 48, and 72 h. At least five independent samples for CAT activity were taken for each time point and used to determine the parasite number. The derived growth curves for treated cultures (line with grey triangles, CPT-cGMP; line with open squares, Compound 1) and the untreated reporter parasite control (black line) are shown. Points for PLK reporter parasite are not shown but are identical to Fig. 2(A). (B) The effect of cGMP modulating agents on bradyzoite reporter activity. The reporter parasite was cultured with each modulating agent for up to 72 h. β-Galactosidase (β-GAL) activity was measured at 24, 48 and 72 h. At least five independent samples were taken for each time point and the average β-GAL activity was determined. The ratio of β-GAL activity for each drug treatment versus the β-GAL activity for the untreated control is shown with the 95% confidence interval.
4. Discussion
In T. gondii the differentiation of tachyzoites into bradyzoites is stress-induced. It is hypothesized that the parasite is able to sense changes in its environment and responds accordingly. Numerous stress conditions such as pH shock, temperature stress, chemical stress and inhibition of metabolism induce differentiation in vitro. Although the molecular mechanisms T. gondii uses to differentiate remain unknown, work in other systems has provided clues to some signalling pathways that may be important in transducing environmental signals that trigger differentiation to latent bradyzoites. The cAMP signalling pathway is important in responding to changes in environment for nearly all eukaryotes.
The tachyzoite–bradyzoite transition is reminiscent of the dimorphic pattern of growth common in many species of yeast (Ustilago maydis, Cryptococcus neoformans and Candida albicans). This is a stress-induced response to non-ideal environmental conditions such as nitrogen starvation, poor carbon sources, temperature stress or osmotic stress (Borges-Walmsley and Walmsley, 2000; Zaragoza and Gancedo, 2000; D'Souza and Heitman, 2001). In pathogenic fungi dimorphic growth is linked to pathogenicity and induction of virulence. The ability of S. cerevisiae (and other fungi) to sense the external environment and change its growth pattern accordingly serves as an important model in understanding how external environmental signals are transduced into distinct cellular phenotypes.
To create a quantitative assay for differentiating agents, we developed an enzymatic assay for differentiation. Our reporter parasite, a transgenic parasite expressing CAT constitutively and β-GAL from a bradyzoite specific promoter, allows for enzymatic quantification of differentiation. Enzymatic quantification is less laborious and is subject to less experimental bias compared with previous methods used to measure differentiation. CAT activity correlates with parasite number and growth curves can be created to determine replication rates. β-GAL activity reflects gene expression from the BAG1 promoter. BAG1 expression is an early event in bradyzoite differentiation (Weiss and Kim, 2000). This reporter parasite conveniently allows quantification of two bradyzoite hallmarks: reduced replication and bradyzoite-specific gene expression.
Using this reporter parasite, we tested agents that affect the cAMP and cGMP pathways. Previous studies by Choi et al. suggest that cAMP and chemicals that increased cAMP levels regulate parasite replication with effects that are both dose and time dependent (Choi et al., 1990). Our laboratory tested agents that modulate cyclic nucleotide signalling on bradyzoite differentiation by counting cyst formation using an immunofluorescence assay. Bradyzoite formation was monitored with dolichos biflorus agglutinin (DBA), which labels the cyst wall glycoprotein CST1 (Kirkman et al., 2001; Zhang et al., 2001). All tested agents (CPT-cGMP, forskokin, IBMX) increased cAMP levels in the parasite. Treatment with non-hydrolyzable form of cAMP (CPT-cAMP) and IBMX resulted in less cyst formation and IBMX plus forskolin also inhibited cyst formation (Kirkman et al., 2001) but forskolin alone and CPT-cGMP induced bradyzoite formation. Those studies suggested that cyclic nucleotide signalling has both inhibitory and inducing effects upon bradyzoite differentiation. The current studies indicate PKG and PKA, down stream effectors of cyclic nucleotide signalling, are involved in these signalling pathways.
Although both forskolin and CPT-cGMP induced bradyzoite reporter activity, their effects were less pronounced in comparison with our earlier IFA studies (Kirkman et al., 2001). We observed a transient increase in β-GAL with forskolin but this increase is not sustained. The CPT-cGMP treatment, which also induced bradyzoite formation in the IFA assay, elicited a transient increase in β-GAL activity, which was reproducible but not statistically significant (Figs. 3 and 4). Interestingly, the forskolin growth curve did not fit the predicted trend line for exponential growth as well as the curves for other conditions (correlation coefficient 0.75; Table 1). In prior IFA experiments, forskolin induced differentiation but bradyzoite vacuoles were consistently larger than those seen with other SNP or other inducing agents (Kirkman et al., 2001), suggesting that forskolin stimulation of differentiation is qualitatively different from SNP induction and that the response to forskolin treatment is heterogeneous.
The modest effects seen with forskolin and CPT-cGMP in our reporter parasite are likely to be due to differences in how the experiments were performed. The differentiating agents were added to cultures after host cell invasion in this study but added with parasites in our prior study. We have consistently observed more reliable differentiation when parasites are exposed extracellularly to inducing agents prior to invasion of host cells (Weiss et al., 1998; Weiss and Kim, 2000). Being established within a vacuole in the host cell is protective, making parasites relatively resistant to weak bradyzoite inducers like cGMP (Kirkman et al., 2001). Experiments could not be performed as done earlier because Compound 1 affects invasion (Wiersma et al., 2004; Eaton, Weiss and Kim, unpublished data).
In conclusion, the cAMP and cGMP pathways are implicated in regulating multiple events in T. gondii including host cell invasion, cell proliferation and differentiation. Using quantitative assays with a reporter parasite, we have shown inhibition of PKA and PKG induces bradyzoite gene expression and slows replication.
H-89 and Compound 1 are the first specific agents described to induce differentiation and provide tools to explore this phenomenon. The exact signalling cascades involved require further elucidation. Cyclic nucleotides regulate the activities of phosphodiesterases and downstream effectors such as PKA and PKG. Both adenylyl cyclases and guanylyl cyclases have been identified in malaria species and orthologues are also present in T. gondii (Muhia et al., 2003; Baker and Kelly, 2004). Localization and activity of cyclases, kinases and transcription factors are affected by stimulation of these pathways. The final phenotype is a result of the balance of actions of each of these molecular effectors of cyclic nucleotide signalling. Further, the effect of agents that affect cyclic nucleotide signalling can be host-dependent, parasite-dependent or a combination of both. The bradyzoite reporter parasite provides a tool to dissect the role of cyclic nucleotide signalling in parasite proliferation and differentiation using quantitative methods.
Acknowledgments
Supported by grants from the NIH (to KK and to LMW), a Burroughs Wellcome Fund New Investigator Award in Parasitology (KK). Data from this manuscript were included in a thesis submitted in partial fulfillment of the requirements for a Doctor of Philosophy from the Sue Golding Graduate Division of the Albert Einstein College of Medicine.
References
- Aubry L, Firtel R. Integration of signaling networks that regulate Dictyostelium differentiation. Annu Rev Cell Dev Biol. 1999;15:469–517. doi: 10.1146/annurev.cellbio.15.1.469. [DOI] [PubMed] [Google Scholar]
- Baker DA, Kelly JM. Purine nucleotide cyclases in the malaria parasite. Trends Parasitol. 2004;20:227–232. doi: 10.1016/j.pt.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne W, Wirsing A, Gross U. Bradyzoite-specific gene expression in Toxoplasma gondii requires minimal genomic elements. Mol Biochem Parasitol. 1997;85:89–98. doi: 10.1016/s0166-6851(96)02814-9. [DOI] [PubMed] [Google Scholar]
- Borges-Walmsley MI, Walmsley AR. cAMP signalling in pathogenic fungi: control of dimorphic switching and pathogenicity. Trends Microbiol. 2000;8:133–141. doi: 10.1016/s0966-842x(00)01698-x. [DOI] [PubMed] [Google Scholar]
- Choi WY, Nam HW, Youn JH, Kim DJ, Kim WK, Kim WS. The effect of cyclic AMP on the growth of Toxoplasma gondii in vitro. Kisaengchunghak Chapchi. 1990;28:71–78. doi: 10.3347/kjp.1990.28.2.71. [DOI] [PubMed] [Google Scholar]
- D'Souza CA, Heitman J. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol Rev. 2001;25:349–364. doi: 10.1111/j.1574-6976.2001.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, Li C, Striepen B. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob Agents Chemother. 2003;47:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby DE, Brinkley JM, Kang HC, Haugland RP, Young SL, Melner MH. Use of a fluorescent chloramphenicol derivative as a substrate for CAT assays. Biotechniques. 1990;8:170–171. [PubMed] [Google Scholar]
- Kaushal DC, Carter R, Miller LH, Krishna G. Gametocytogenesis by malaria parasites in continuous culture. Nature. 1980;286:490–492. doi: 10.1038/286490a0. [DOI] [PubMed] [Google Scholar]
- Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- Kirkman LA, Weiss LM, Kim K. Cyclic nucleotide signaling in Toxoplasma gondii bradyzoite differentiation. Infect Immun. 2001;69:148–153. doi: 10.1128/IAI.69.1.148-153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll LJ, Boothroyd JC. Isolation of developmentally regulated genes from Toxoplasma gondii by a gene trap with the positive and negative selectable marker hypoxanthine–xanthine–guanine phosphoribosyltransferase. Mol Cell Biol. 1998;18:807–814. doi: 10.1128/mcb.18.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft BJ, Remington JS. AIDS commentary. Toxoplasmic encephalitis. J Infect Dis. 1988;157:1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. Jama. 1984;252:913–917. [PubMed] [Google Scholar]
- Ma YF, Zhang YW, Kim K, Weiss LM. Identification and characterization of a regulatory region in the Toxoplasma gondii hsp70 genomic locus. Int J Parasitol. 2004;34:333–346. doi: 10.1016/j.ijpara.2003.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DC, Seeber F, Boothroyd JC. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob Agents Chemother. 1997;41:1849–1853. doi: 10.1128/aac.41.9.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R, Mack D, Brown C. Toxoplasma gondii—new advances in cellular and molecular biology. Exp Parasitol. 1991;72:109–121. doi: 10.1016/0014-4894(91)90129-k. [DOI] [PubMed] [Google Scholar]
- Muhia DK, Swales CA, Eckstein-Ludwig U, Saran S, Polley SD, Kelly JM, Schaap P, Krishna S, Baker DA. Multiple splice variants encode a novel adenylyl cyclase of possible plastid origin expressed in the sexual stage of the malaria parasite Plasmodium falciparum. J Biol Chem. 2003;278:22014–22022. doi: 10.1074/jbc.M301639200. [DOI] [PubMed] [Google Scholar]
- Nare B, Allocco JJ, Liberator PA, Donald RG. Evaluation of a cyclic GMP-dependent protein kinase inhibitor in treatment of murine toxoplasmosis: gamma interferon is required for efficacy. Antimicrob Agents Chemother. 2002;46:300–307. doi: 10.1128/AAC.46.2.300-307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke JR, Striepen B, Guerini MN, Jerome ME, Roos DS, White MW. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:165–175. doi: 10.1016/s0166-6851(01)00284-5. [DOI] [PubMed] [Google Scholar]
- Read LK, Mikkelsen RB. Comparison of adenylate cyclase and cAMP-dependent protein kinase in gametocytogenic and nongametocytogenic clones of Plasmodium falciparum. J Parasitol. 1991;77:346–352. [PubMed] [Google Scholar]
- Seeber F, Boothroyd JC. Escherichia coli beta-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene. 1996;169:39–45. doi: 10.1016/0378-1119(95)00786-5. [DOI] [PubMed] [Google Scholar]
- Singh U, Brewer JL, Boothroyd JC. Genetic analysis of tachyzoite to bradyzoite differentiation mutants in Toxoplasma gondii reveals a hierarchy of gene induction. Mol Microbiol. 2002;44:721–733. doi: 10.1046/j.1365-2958.2002.02903.x. [DOI] [PubMed] [Google Scholar]
- Soete M, Dubremetz JF. Toxoplasma gondii: kinetics of stage-specific protein expression during tachyzoite-bradyzoite conversion in vitro. Curr Top Microbiol Immunol. 1996;219:76–80. [PubMed] [Google Scholar]
- Soete M, Camus D, Dubremetz JF. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- Soldati D, Boothroyd JC. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- Tomavo S, Boothroyd JC. Interconnection between organellar functions, development and drug resistance in the protozoan parasite, Toxoplasma gondii. Int J Parasitol. 1995;25:1293–1299. doi: 10.1016/0020-7519(95)00066-b. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:D391–D405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Laplace D, Takvorian PM, Tanowitz HB, Cali A, Wittner M. A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J Eukaryot Microbiol. 1995;42:150–157. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Ma YF, Takvorian PM, Tanowitz HB, Wittner M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect Immun. 1998;66:3295–3302. doi: 10.1128/iai.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Udem SA, Tanowitz H, Wittner M. Western blot analysis of the antibody response of patients with AIDS and toxoplasma encephalitis: antigenic diversity among Toxoplasma strains. J Infect Dis. 1988;157:7–13. doi: 10.1093/infdis/157.1.7. [DOI] [PubMed] [Google Scholar]
- Wiersma HI, Galuska SS, Tomley FM, Sibley LD, Liberator PA, Donald RGK. A role for coccidian parasite cGMP-dependent protein kinase in parasite motility and invasion. Int J Parasitol. 2004;34:369–380. doi: 10.1016/j.ijpara.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Wong SY, Remington JS. Biology of Toxoplasma gondii. Aids. 1993;7:299–316. doi: 10.1097/00002030-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Yahiaoui B, Dzierszinski F, Bernigaud A, Slomianny C, Camus D, Tomavo S. Isolation and characterization of a subtractive library enriched for developmentally regulated transcripts expressed during encystation of Toxoplasma gondii. Mol Biochem Parasitol. 1999;99:223–235. doi: 10.1016/s0166-6851(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Yap GS, Sher A. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology. 1999;201:240–247. doi: 10.1016/S0171-2985(99)80064-3. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Gancedo JM. Pseudohyphal growth is induced in Saccharomyces cerevisiae by a combination of stress and cAMP signalling. Antonie Van Leeuwenhoek. 2000;78:187–194. doi: 10.1023/a:1026594407609. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Halonen SK, Ma YF, Wittner M, Weiss LM. Initial characterization of CST1, a Toxoplasma gondii cyst wall glycoprotein. Infect Immun. 2001;69:501–507. doi: 10.1128/IAI.69.1.501-507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Kim K, Ma YF, Wittner M, Tanowitz HB, Weiss LM. Disruption of the Toxoplasma gondii bradyzoite-specific gene BAG1 decreases in vivo cyst formation. Mol Microbiol. 1999;31:691–701. doi: 10.1046/j.1365-2958.1999.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


