Abstract
Background:
Tobacco smoking is a principal cause of COPD-emphysema (COPD-E). Whether discontinuing smoking for at least 4 years halts airway inflammation and progression of COPD-E in prior smokers is unknown. In this study we investigated whether discontinuing smoking for approximately 4 years in ex-smokers with GOLD (Global Initiative for Chronic Lung Disease) stage IIb (moderately severe) COPD-E stopped airway inflammation (ie, sputum biomarkers) and halted the progression of COPD-E on chest CT scan.
Methods:
Ten ex-smokers with COPD-E who had quit smoking underwent chest CT scans to document the extent of COPD-E, assessment of lung function (FEV1 and diffusing capacity of lung for carbon monoxide), sputum induction for biomarkers of inflammation (measured by enzyme-linked immunosorbent assay), and blood cotinine levels at baseline and approximately 4 years later. Normal healthy subjects (n = 7) and normal current smokers with no CT scan evidence of COPD-E (n = 8) served as sputum biomarker comparison groups.
Results:
After approximately 4 years of not smoking (documented by cotinine levels), ex-smokers with COPD-E had persistent increased levels of mediators of inflammation in sputum (myeloperoxidase, leukotriene B4, IL-8, monocyte chemoattractant protein-1, matrix metalloprotease-9), which was associated with significant progression of COPD-E on chest CT scan.
Conclusions:
Cessation of tobacco smoking in heavy smokers with moderately severe COPD-E is associated with evidence of persistent airway inflammation and progression of COPD-E on CT scan 4 years later. Discontinuing smoking may slow the rate of progression of moderate severity COPD-E, but it does not prevent persistent airway inflammation and significant progression of COPD-E on CT scan.
COPD is characterized by increased numbers of inflammatory cells (eg, neutrophils, macrophages, CD81 T lymphocytes) in the airway and parenchyma, increased expression of lung cytokines and chemokines, and increased extracellular matrix components.1,2 Tobacco smoking is the most important risk factor for developing COPD.3 Cessation of tobacco smoking is the only intervention in COPD that slows the accelerated decline in FEV1.4‐7 Although tobacco smoke is a very important precipitant of COPD, there is evidence that the inflammation in COPD persists even after smoking cessation in cross-sectional studies using either resected lungs,8,9 bronchial biopsies,10‐12 or induced sputum.13‐15 For example, there was no difference in the intensity of inflammation in central and peripheral airways in studies of the resected lungs of smokers and ex-smokers undergoing surgery for a lung tumor.8,9 Studies of bronchial biopsy specimens of smokers and ex-smokers with moderate to severe COPD have also demonstrated no difference in mediators of inflammation associated with neutrophil and mononuclear cell recruitment, such as IL-8 and monocyte chemoattractant protein (MCP)-1, as well as the MCP-1 chemokine receptor 2, in the lung tissue.10 Although cross-sectional studies of lung tissue in COPD demonstrate more consistent findings of persistent inflammation in ex-smokers,3,8‐11 cross-sectional studies of sputum, blood, or BAL generally demonstrate reductions in biomarkers of inflammation3 or no difference in ex-smokers vs smokers.3,13‐15 In the only longitudinal study of the effect of smoking cessation on airway inflammation in COPD, 12 subjects with COPD (defined by pulmonary function and not by CT scan) were found to have persistent airway inflammation in bronchial biopsy specimens and a significant increase in the number of sputum neutrophils and IL-8 levels at 1 year.6
Although these studies3,8‐15 have provided important information regarding the potential for continued airway inflammation following smoking cessation in COPD, the studies have mainly been cross-sectional and have focused on subjects with chronic bronchitis rather than subjects with chest CT scan-diagnosed COPD-emphysema (COPD-E). In addition, most studies have not used CT scan to define subjects as having emphysema as an entry criterion and have not used blood cotinine levels to verify smoking status. In this study we have performed a longitudinal assessment (at baseline and after 4 years of observation) of continued airway inflammation in a cohort of ex-smokers who had the diagnosis of COPD-E established based on the presence of significant emphysema on chest CT scan at the baseline visit. Our study demonstrates persistent increased levels of mediators of inflammation in sputum (myeloperoxidase [MPO], leukotriene B4 [LTB4], IL-8, MCP-1, matrix metalloprotease-9 [MMP-9]), which was associated with significant progression of COPD-E on chest CT scan in subjects with COPD-E who discontinued tobacco smoking for at least 4 years, as verified by blood cotinine levels.
Materials and Methods
COPD and Control Subjects
Baseline Visits:
Study and control subjects were recruited as part of a National Institutes of Health Biomarker study, and their clinical characteristics, pulmonary function, and selected biomarkers have previously been described in detail.16,17 In brief, subjects with COPD-E were recruited based on clinical evaluation and chest CT scan evidence of emphysema, which had to be present to be included in the study, whereas control subjects were required to have no clinical evidence of COPD and a normal chest CT scan.16 Subjects also completed pulmonary function studies, clinical evaluation (history of chronic cough, sputum production, dyspnea), and a standardized COPD questionnaire (ie, St. George Questionnaire).16 The COPD-E cohort (n = 10) we enrolled was composed of subjects with GOLD (Global Initiative for Chronic Lung Disease) stage IIb moderately severe COPD (FEV1 30%-50%), with chest CT scan evidence of significant emphysema.16 At a later date we enrolled normal individuals who were nonsmokers (n = 7) and normal current smokers (> 20 pack-years) (n = 8). The normal nonsmokers and the normal current smokers had no evidence of disease on history and physical examination, a normal chest CT scan, and normal pulmonary function.16 All the study subjects had blood cotinine levels to verify their smoking status. Additional details are provided in e-Appendix 1.
Repeat Visit 4 Years Later:
The 10 subjects with COPD-E underwent sputum induction, blood cotinine level measurement, pulmonary function, and chest CT scan approximately 4 years after their initial baseline assessments. The normal individuals and current normal smokers were not followed for 4 years. A select number of current normal smokers (n = 6/8 consented to return for repeat CT scan) had a repeat chest CT scan approximately 2 years later. This protocol was approved by the University of California San Diego Human Subjects Protection Committee (# 040962). Additional details are provided in e-Appendix 1.
Chest CT Scan
Subjects had a full-chest noncontrast helical CT scan during a single held maximal inspiration, with 10 mm collimation (baseline visit) and 2.5 mm collimation (follow-up visit), using the General Electric standard algorithm for reconstruction as previously described.16,18 Equivalent measurements were created by reconstructing the follow-up 2.5-mm scan to 10 mm. The percent of voxels with density < −910 Hounsfield units (HU) was termed the CT scan score. Additional details are provided in e-Appendix 1.
Pulmonary Function Studies
All the study subjects had pulmonary function studies including FEV1, diffusing capacity of the lung for carbon monoxide (Dlco), and volume-adjusted Dlco according to American Thoracic Society guidelines.19 Additional details are provided in e-Appendix 1.
Cotinine Levels
Serum cotinine levels were assayed by gas chromatography as described.16,20 Additional details are provided in e-Appendix 1.
Sputum Biomarkers
Selected sputum biomarkers (MPO, IL-8, LTB4, MCP-1, MMP-9) were quantitated by enzyme-linked immunosorbent assay (ELISA). Additional details are provided in e-Table 1.
Statistical Analysis
Results in the different groups were compared by analysis of variance using the nonparametric Kruskal-Wallis test followed by post testing using Dunn multiple comparison of means. A P value < .05 was considered statistically significant.
Results
Baseline COPD-E Study Subject CT Scan, Pulmonary Function
At the baseline visit, the subjects with COPD-E (n = 10) had a mean age of 69 ± 2 years, were clinically stable, and had a St. George Questionnaire mean score of 37 ± 10. Based on the GOLD criteria, the subjects with COPD-E had GOLD stage IIb moderate severity COPD (GOLD stage IIb; FEV1/FVC ratio < 0.70, and FEV1 ≤ 50% and ≥ 30% predicted).21
The subjects with COPD-E at the baseline visit all had evidence of significant emphysema based on their chest CT scan (Table 1). Subjects with COPD-E had significantly higher chest CT scan scores (34.2% ± 4.5% voxels < −910 HU) compared with either the normal nonsmoking group (5.6% ± 1.7%) (P = .001) or the normal current smoker group (2.3% ± 0.4%) (P = .0001) (Table 1).
Table 1.
—Subject Demographics
| COPD-E/Ex-Smoker |
||||
| Demographic | Nonsmokers at Year 0 | Smokers at Year 0 | at Year 0 | at Year 4 |
| Subjects, No. | 7 | 8 | 10 | 10 |
| Sex, male (female) | 1 (6) | 4 (4) | 9 (1) | 9 (1) |
| Age, y | 50 ± 4 | 52 ± 3 | 69 ± 2 | 74 ± 1 |
| Smoking, median (range), pack-years | 0 | 41 (25-264) | 61 (34-112) | 61 (34-112) |
| Years of not smoking | NA | NA | 15.1 ± 7.6 | 20.3 ± 2.1 |
| Cotinine, ng/mL | < 10 | 224 ± 51 | < 10 | < 10 |
| Chest CT scan, %a | 5.6 ± 1.7 | 2.3 ± 0.4 | 34.2 ± 4.5 | 51.0 ± 1.0 |
| FEV1, % predicted | 98 ± 5 | 97 ± 5 | 44 ± 4 | 38 ± 3 |
| Dlco, % | 76.7 ± 3.8 | 82.1 ± 5.4 | 42.7 ± 2.9 | 36.5 ± 2.6 |
Data are presented as mean ± SD unless otherwise noted. Subjects with COPD-E returned approximately 4 y after year 0 for follow-up. The mean duration between visits year 0 and year 4 was 4 y and 5 mo. Nine out of 10 subjects had their follow-up visits 4 to 5 y after their year 0 visit. One out 10 subjects had their return visit 3 y and 11 mo after the baseline year 0 visit. COPD-E = COPD-emphysema; Dlco = diffusing capacity of the lung for carbon monoxide; HU = Hounsfield unit; NA = not applicable.
Chest CT score: The % of voxels with density < −910 HU was termed the chest CT scan %. Nine out of 10 subjects with COPD-E had a year 0 chest CT scan > 10%. One out of 10 subjects had a year 0 chest CT scan of 7.1%.
Four-Year Follow-up: COPD-E Study Subject Chest CT Scan
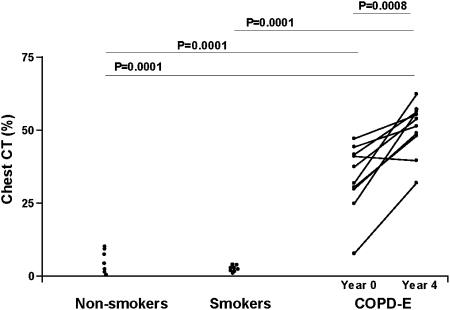
Subjects with COPD-E had significantly higher chest CT scan scores at their 4-year visit (51.0 ± 1.0% voxels < −910 HU) compared with the baseline visit (P = .008) (Fig 1). Nine of the 10 subjects with COPD-E demonstrated progression of their emphysema on chest CT scan, and one subject did not (Fig 1).
Figure 1.
Subjects with COPD-E (n = 10) had baseline (year 0) and repeat chest CT scans (year 4). Normal current smokers without COPD-E (n = 8), and healthy nonsmokers (n = 7) had baseline chest CT scans. The percent of voxels with density < −910 Hounsfield unit (HU) was recorded in each subject and reported as the chest CT scan %. COPD-E = COPD-emphysema.
We also compared baseline and repeat chest CT scans obtained approximately 2 years later in several normal current smokers (n = 6/8 subjects enrolled). There was no significant change in CT scan scores at their 2-year visit (2.3% ± 0.4% voxels < −910 HU) compared with the baseline visit (3.7% ± 1.2%) (P = not significant).
Four-Year Follow-up: COPD-E Study Subject, Pulmonary Function
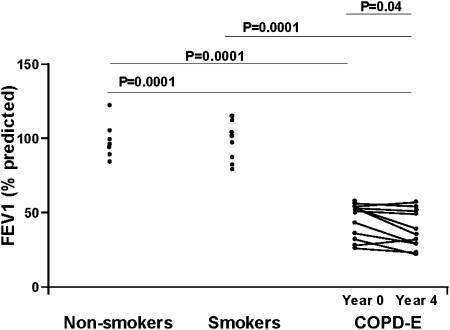
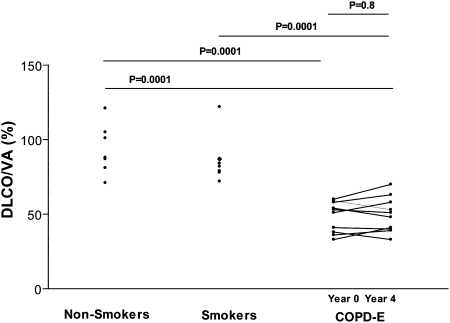
Repeat pulmonary function performed 4 years after the initial visit demonstrated that there was a statistically significant reduction in FEV1 (P = .04) (Fig 2), but no significant change in Dlco (Table 1) or volume-adjusted Dlco (Fig 3). Eight of the 10 subjects with COPD-E demonstrated reductions in FEV1 over the 4-year study, and two subjects did not (Fig 2).
Figure 2.
Subjects with COPD-E (n = 10) had baseline (year 0) and repeat FEV1 (year 4) measurements. Normal current smokers without COPD-E (n = 8) and healthy nonsmokers (n = 7) had baseline FEV1 measurements. See Figure 1 legend for expansion of abbreviation.
Figure 3.
Subjects with COPD-E (n = 10) had baseline (year 0) and repeat Dlco/VA measurements (year 4). Normal current smokers without COPD-E (n = 8) and healthy nonsmokers (n = 7) had baseline Dlco/VA measurements. Dlco/VA = volume-adjusted diffusing capacity of the lung for carbon monoxide. See Figure 1 legend for expansion of other abbreviation.
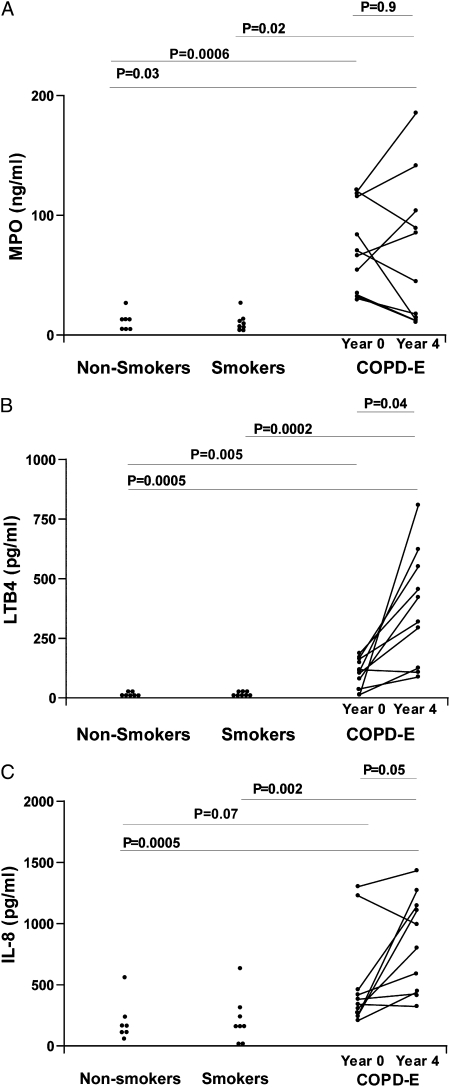
Persistent Sputum Neutrophil Inflammation (MPO), and Neutrophil Chemoattractants (LTB4, IL-8) in Subjects with COPD-E Who Quit Smoking for 4 Years: Comparison of COPD-E and Normal Control Subjects
Subjects with COPD-E had statistically significantly higher levels of sputum MPO (Fig 4A), LTB4 (Fig 4B), and IL-8 (Fig 4C) at baseline and at year 4 compared with either the normal nonsmoking group or the normal current smoker group. There was no significant difference in levels of sputum MPO in comparing baseline sputum MPO and sputum MPO levels at year 4 (P = .90) (Fig 4A), but, interestingly, there was an increase in levels of sputum LTB4 (P = .04) (Fig 4B) and IL-8 (P = .05) (Fig 4C) in comparing baseline and year 4 sputum levels.
Figure 4.
A, Subjects with COPD-E (n = 10) had baseline (year 0) and repeat (year 4) MPO assessed by ELISA. B, Subjects with COPD-E (n = 10) had baseline (year 0) and repeat (year 4) LTB4 levels assessed by ELISA. C, Subjects with COPD-E (n = 10) had baseline (year 0) and repeat (year 4) sputum IL-8 levels assessed by ELISA. Normal current smokers without COPD-E (n = 8) and healthy nonsmokers (n = 7) had baseline sputum MPO, LTB4, and IL-8 measurements. ELISA = enzyme-linked immunosorbent assay; LTB4 = leukotriene B4; MPO = myeloperoxidase. See Figure 1 legend for expansion of other abbreviation.
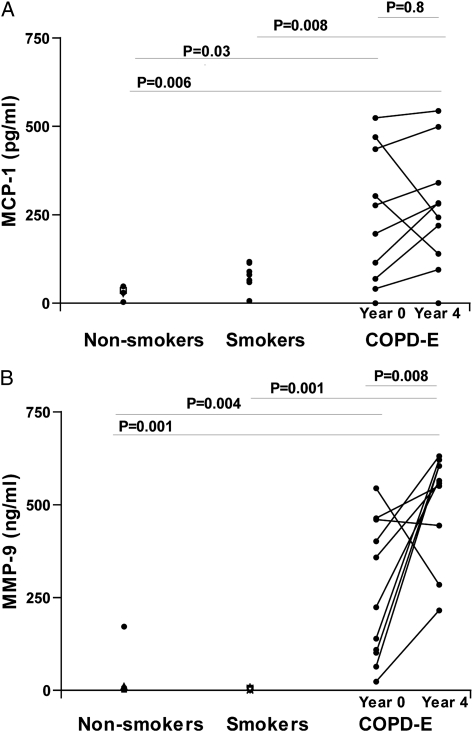
Persistent Sputum Mononuclear Cell Chemoattractants (MCP-1) and Metalloproteases (MMP-9) in Subjects With COPD-E Who Quit Smoking for 4 Years
Subjects with COPD-E had statistically significantly higher levels of the mononuclear cell chemoattractant MCP-1 (Fig 5A) and the metalloprotease MMP-9 (Fig 5B) in sputum at baseline and at year 4 compared with either the normal nonsmoking group or the normal current smoker group. There was no significant difference in levels of sputum MCP-1 in comparing baseline sputum MCP-1 and year 4 sputum MCP-1 levels (P = .80) (Fig 5A), but there was a significant increase in levels of sputum MMP-9 in comparing baseline sputum MMP-9 and year 4 sputum MMP-9 levels (P = .008) (Fig 5B).
Figure 5.
A, Subjects with COPD-E (n = 10) had baseline (year 0) and repeat (year 4) sputum MCP-1 levels assessed by ELISA. B, Subjects with COPD-E (n = 10) had baseline (year 0) and repeat (year 4) sputum MMP-9 levels assessed by ELISA. Normal current smokers without COPD-E (n = 8) and healthy nonsmokers (n = 7) had baseline sputum MCP-1 and MMP-9 measurements. MCP-1 = monocyte chemoattractant protein-1; MMP-9 = matrix metalloprotease-9. See Figure 1 and 4 legends for expansion of other abbreviations.
Cotinine Assay and History of Quitting Cigarette Smoking
Each of the subjects in the normal current smoking group (median 41 pack-years; range 25-264 pack-years) and in the COPD-E group (median 61 pack-years; range 34-112 pack-years) had ≥ 20 pack-year history of smoking at entry into the study. At the baseline visit the cotinine assay confirmed that the normal current smoking group had high levels of serum cotinine (224 ± 51 ng/mL), whereas this was not the case in either the normal nonsmoking group (< 10 ng/mL) or the subjects with COPD-E (< 10 ng/mL) who were all former smokers who had quit > 2 years ago (Table 1). The subjects with COPD-E had a mean duration of having quit smoking at entry into the study of 15.1 ± 7.7 years (by history at entry into study not verified by cotinine assay). Repeat visit cotinine assays confirmed that all the subjects with COPD-E were not smoking (cotinine levels at year 4 visit < 10 ng/mL), and that the current smokers without COPD-E continued to be smokers (cotinine level at 2 years, 315 ± 84 ng/mL vs cotinine level at baseline, 224 ± 51 ng/mL).
Discussion
This study demonstrated that ex-smokers with GOLD Stage IIb COPD-E who were followed for 4 years while not smoking have persistent airway inflammation detectable in sputum and progression of emphysema on chest CT scan. The mediators of inflammation that persisted at elevated levels in sputum included mediators associated with neutrophil-mediated inflammation (MPO, LTB4, IL-8), mediators associated with recruitment of mononuclear cells (MCP-1), and mediators associated with extracellular matrix remodeling (MMP-9), all of which have been implicated in the pathogenesis of COPD.1,2 Interestingly, the subjects with COPD-E entered into this study were all ex-smokers who by history had not smoked for at least 2 years and had a history of not smoking for 15 ± 7 years. Although their past history of quitting smoking prior to entering the study was not verified by cotinine levels, we were able to verify by cotinine levels that they were not smoking at the baseline and 4-year follow-up visit. Thus, the persistent inflammation in sputum of subjects with moderate to severe COPD-E who have quit smoking is likely to be of even longer duration than the 4 years we have followed these subjects.
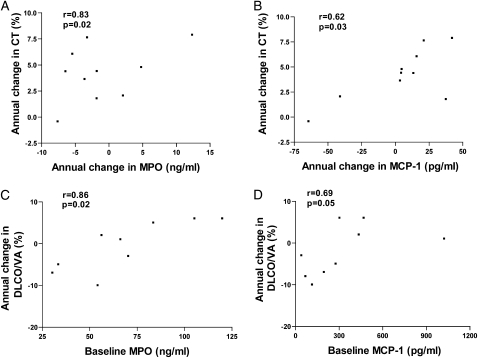
Although these mediators of inflammation have been detected at increased levels in sputum in several past cross-sectional studies of COPD,3,22,23 the majority of cross-sectional studies have not examined levels of these mediators in subjects phenotyped as having emphysema based on chest CT scan as was done in this study. In addition, previous studies have not examined whether levels of mediators of inflammation persist in subjects with COPD-E phenotyped by repeat CT scans over a 4-year period. We are aware of a limited number of cross-sectional studies of biomarkers in BAL or sputum in subjects with COPD-E phenotyped by CT scan, including studies from our group16,17 and others,24‐26 but none of these studies has evaluated changes in biomarkers of inflammation over time in relation to changes in the extent of COPD-E on chest CT scan in subjects with COPD-E who have quit smoking. A longitudinal study by Parr et al27 of subjects with chronic bronchitis has demonstrated in a subgroup analysis that sputum MPO correlated with decline in FEV1, sputum LTB4 with Dlco decline, and IL-8 with progression of lung densitometric changes. In our study, no correlation of baseline inflammatory markers with progression of CT scan densitometry was observed. This difference from the study reported by Parr et al27 may reflect differences in patient phenotype or the number of subjects studied. However, in the current study there was a significant correlation between the change of sputum biomarker level and change in CT scan score for MPO and MCP-1 (e-Appendix 1, Figure 6). There was also a significant correlation between the baseline sputum mediator levels of either MPO or MCP-1 and change in volume-adjusted Dlco from baseline to year 4, as well as a correlation between the baseline sputum mediator level of MMP-9 and change in FEV1 from baseline to year 4 (e-Appendix 1, Fig 6). The volume-adjusted Dlco correlated better than the non-volume-adjusted Dlco with the biomarkers measured (e-Appendix 1). The importance of using CT scans to phenotype subjects in studies of COPD-E and control subjects (such as current smokers without COPD-E) is the demonstration that, unlike the normal current smokers in this study, control “healthy” smoking subjects with near-normal FEV1 may have emphysematous lesions on CT scan28 and, thus, be misclassified if only pulmonary function studies and not CT scans are performed. Chest CT scans are providing an important method of documenting the extent of emphysema and have demonstrated that the area of the lung with chest CT scan attenuation values below −910 HU and −950 HU correlates significantly with macroscopic29 and microscopic30 pathologic features of emphysema. Although there are many cross-sectional studies demonstrating the association of low attenuation on chest CT scan and emphysema,31 more recent longitudinal studies have investigated the progression of emphysema on chest CT scan over time periods ranging from 6 months to 5 years32‐34 but have not examined longitudinal changes in levels of biomarkers of inflammation in relation to longitudinal changes in CT scans in ex-smokers.
Figure 6.
A, The annual change in CT scan in COPD-E (n = 10) correlated with the annual change in sputum MPO level. B, The annual change in CT scan in COPD-E (n = 10) correlated with the annual change in sputum MCP-1 level. C, The annual change in volume-adjusted Dlco correlated with the baseline sputum MPO level. D, The annual change in volume-adjusted Dlco correlated with the baseline sputum MCP-1 level. See Figures 1, 3, 4, and 5 legends for expansion of abbreviations.
Although our study demonstrates that airway inflammation persists in ex-smokers and is associated with increased extent of COPD-E 4 years later, there are several limitations in the study design, including the small number of subjects studied, the duration of having quit smoking before entering the study, the limited number of time-points studied, and the absence of a control group of study subjects with COPD-E who continued to smoke to compare the extent of airway inflammation and rate of decline in COPD-E in persistent vs ex-smokers. Although no sputum bacterial counts were assessed, subjects had no history of recent infection. Levels of mediators of inflammation may also be influenced by whether the sputum was spontaneous in subjects with chronic sputum production or induced as in this study, as well as by day to day variability in sputum mediator levels and the stability of mediators stored in a −80°C freezer. Because some mediator levels were lower at the first visit compared with the second visit, it is possible that either the stability of mediators in the freezer or bacterial colonization of sputum were the cause of changes in sputum mediator levels with time. An alternative explanation is that the day-to-day variability of sputum mediator levels in an individual subject contributed to the change in levels of sputum mediators noted. However, despite these limitations, in contrast to previous studies a significant strength of this study is the phenotyping of all subjects as having COPD-E based on chest CT scan at entry into the study and documenting changes in extent of COPD-E following 4 years of tobacco smoke cessation (documented by cotinine levels). This longitudinal biomarker study design differs from previous biomarker studies in COPD-E in which serial CT scans, biomarkers, and cotinine levels to verify smoking status were not obtained. In addition, prior studies have predominantly examined the effect of discontinuing smoking on end points other than emphysema (such as symptoms associated with chronic bronchitis, lung function, and airway hyperresponsiveness).
In summary, in this study we have demonstrated that in subjects with GOLD stage IIb COPD-E, even after at least 4 years of not smoking, airway inflammation persists and that this is associated with continued airspace destruction as revealed by increased emphysema on CT scan. This continued inflammation and airspace destruction in ex-smokers with GOLD stage IIb COPD-E could likely be more extensive if these subjects continued to smoke, and thus it remains important that smokers with COPD should quit smoking. However, this study provides further evidence that once tobacco smoke initiates and causes progression as far as GOLD stage IIb COPD-E, discontinuing smoking may slow but not necessarily halt the persistent inflammation and progression of this severity of COPD-E. These studies underscore the need to identify novel therapies to prevent the progression of moderate to severe COPD-E even in ex-smokers.
Supplementary Material
Acknowledgments
Author contributions: Dr Miller: contributed to processing and analyzing sputum samples, performing statistical analysis of results, and editing the manuscript.
Dr Cho: contributed to processing and analyzing sputum samples, performing statistical analysis of results, and editing the manuscript.
Ms Pham: contributed to processing and analyzing sputum samples, performing statistical analysis of results, and editing the manuscript.
Dr Friedman: contributed to interpreting and scoring of chest CT scans, analyzing the results, and editing the manuscript.
Dr Ramsdell: contributed to clinical characterization of study subjects, analyzing the results, and editing the manuscript.
Dr Broide: contributed to study design, supervising the measurements made, analysis of results, and writing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Friedman interprets chest CT scans for COPDGene, a National Institutes of Health-supported research project, and consults for Broncus Technologies on CT scans in emphysema. Drs Miller, Cho, Ramsdell, and Broide, and Ms Pham have reported that no potential conflict of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendix and e-Table can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/139/6/1380/DC1.
Abbreviations
- COPD-E
COPD-emphysema
- Dlco
diffusing capacity of lung for carbon monoxide
- ELISA
enzyme-linked immunosorbent assay
- GOLD
Global Initiative for Chronic Lung Disease
- HU
Hounsfield unit
- LTB4
leukotriene B4
- MCP-1
monocyte chemoattractant protein-1
- MMP-9
matrix metalloprotease-9
- MPO
myeloperoxidase
Footnotes
Funding/Support: This work was supported by the National Institutes of Health [Grants HL72342, and GCRC MO1RR000827].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;41(6):631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 2.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 3.Willemse BWM, Postma DS, Timens W, ten Hacken NH. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23(3):464–476. doi: 10.1183/09031936.04.00012704. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1(6077):1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med. 1999;106(4):410–416. doi: 10.1016/s0002-9343(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 6.Willemse BWM, ten Hacken NHT, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26(5):835–845. doi: 10.1183/09031936.05.00108904. [DOI] [PubMed] [Google Scholar]
- 7.Willemse BWM, ten Hacken NHT, Rutgers B, Lesman-Leegte IG, Timens W, Postma DS. Smoking cessation improves both direct and indirect airway hyperresponsiveness in COPD. Eur Respir J. 2004;24(3):391–396. doi: 10.1183/09031936.04.00117603. [DOI] [PubMed] [Google Scholar]
- 8.Mullen JB, Wright JL, Wiggs BR, Paré PD, Hogg JC. Structure of central airways in current smokers and ex-smokers with and without mucus hypersecretion: relationship to lung function. Thorax. 1987;42(11):843–848. doi: 10.1136/thx.42.11.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JL, Hobson JE, Wiggs B, Pare PD, Hogg JC. Airway inflammation and peribronchiolar attachments in the lungs of nonsmokers, current and ex-smokers. Lung. 1988;166(5):277–286. doi: 10.1007/BF02714058. [DOI] [PubMed] [Google Scholar]
- 10.de Boer WI, Sont JK, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS. Monocyte chemoattractant protein 1, interleukin 8, and chronic airways inflammation in COPD. J Pathol. 2000;190(5):619–626. doi: 10.1002/(SICI)1096-9896(200004)190:5<619::AID-PATH555>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Turato G, Di Stefano A, Maestrelli P, et al. Effect of smoking cessation on airway inflammation in chronic bronchitis. Am J Respir Crit Care Med. 1995;152(4 pt 1):1262–1267. doi: 10.1164/ajrccm.152.4.7551380. [DOI] [PubMed] [Google Scholar]
- 12.Gamble E, Grootendorst DC, Hattotuwa K, et al. Airway mucosal inflammation in COPD is similar in smokers and ex-smokers: a pooled analysis. Eur Respir J. 2007;30(3):467–471. doi: 10.1183/09031936.00013006. [DOI] [PubMed] [Google Scholar]
- 13.Bhowmik A, Seemungal TAR, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto C, Yoneda T, Yoshikawa M, et al. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest. 1997;112(2):505–510. doi: 10.1378/chest.112.2.505. [DOI] [PubMed] [Google Scholar]
- 15.Hill AT, Bayley DY, Campbell EJ, Hill SL, Stockley RA. Airways inflammation in chronic bronchitis: the effects of smoking and α1-antitrypsin deficiency. Eur Respir J. 2000;15(5):886–890. doi: 10.1034/j.1399-3003.2000.15e12.x. [DOI] [PubMed] [Google Scholar]
- 16.Miller M, Ramsdell J, Friedman PJ, Cho JY, Renvall M, Broide DH. Computed tomographic scan-diagnosed chronic obstructive pulmonary disease-emphysema: eotaxin-1 is associated with bronchodilator response and extent of emphysema. J Allergy Clin Immunol. 2007;120(5):1118–1125. doi: 10.1016/j.jaci.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol. 2009;182(1):684–691. doi: 10.4049/jimmunol.182.1.684. [DOI] [PubMed] [Google Scholar]
- 18.Friedman PJ. Imaging studies in emphysema. Proc Am Thorac Soc. 2008;5(4):494–500. doi: 10.1513/pats.200708-128ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statement of the American Thoracic Society Standardization of spirometry—1987 update. Am Rev Respir Dis. 1987;136(5):1285–1298. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 20.Verebey KG, DePace A, Mulé SJ, Kanzler M, Jaffe JH. A rapid, quantitative GLC method for the simultaneous determination of nicotine and cotinine. J Anal Toxicol. 1982;6(6):294–296. doi: 10.1093/jat/6.6.294. [DOI] [PubMed] [Google Scholar]
- 21.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46(8):798–825. [PubMed] [Google Scholar]
- 22.Capelli A, Di Stefano A, Gnemmi I, et al. Increased MCP-1 and MIP-1β in bronchoalveolar lavage fluid of chronic bronchitics. Eur Respir J. 1999;14(1):160–165. doi: 10.1034/j.1399-3003.1999.14a27.x. [DOI] [PubMed] [Google Scholar]
- 23.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 24.D’Armiento JM, Scharf SM, Roth MD, et al. Eosinophil and T cell markers predict functional decline in COPD patients. Respir Res. 2009;10:113–126. doi: 10.1186/1465-9921-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boschetto P, Quintavalle S, Zeni E, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61(12):1037–1042. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finlay GA, Russell KJ, McMahon KJ, et al. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax. 1997;52(6):502–506. doi: 10.1136/thx.52.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parr DG, White AJ, Bayley DL, Guest PJ, Stockley RA. Inflammation in sputum relates to progression of disease in subjects with COPD: a prospective descriptive study. Respir Res. 2006;7:136–147. doi: 10.1186/1465-9921-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekberg-Jansson A, Andersson B, Bake B, et al. Neutrophil-associated activation markers in healthy smokers relates to a fall in DL(CO) and to emphysematous changes on high resolution CT. Respir Med. 2001;95(5):363–373. doi: 10.1053/rmed.2001.1050. [DOI] [PubMed] [Google Scholar]
- 29.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 30.Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 31.Dirksen A. Monitoring the progress of emphysema by repeat computed tomography scans with focus on noise reduction. Proc Am Thorac Soc. 2008;5(9):925–928. doi: 10.1513/pats.200804-033QC. [DOI] [PubMed] [Google Scholar]
- 32.Roth MD, Connett JE, D’Armiento JM, et al. FORTE Study Investigators Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130(5):1334–1345. doi: 10.1378/chest.130.5.1334. [DOI] [PubMed] [Google Scholar]
- 33.Shaker SB, Dirksen A, Ulrik CS, et al. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD. 2009;6(2):104–111. doi: 10.1080/15412550902772593. [DOI] [PubMed] [Google Scholar]
- 34.Soejima K, Yamaguchi K, Kohda E, et al. Longitudinal follow-up study of smoking-induced lung density changes by high-resolution computed tomography. Am J Respir Crit Care Med. 2000;161(4 pt 1):1264–1273. doi: 10.1164/ajrccm.161.4.9905040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.