Abstract
To record sleep, actigraph devices are worn on the wrist and record movements that can be used to estimate sleep parameters with specialized algorithms in computer software programs. With the recent establishment of a Current Procedural Terminology code for wrist actigraphy, this technology is being used increasingly in clinical settings as actigraphy has the advantage of providing objective information on sleep habits in the patient’s natural sleep environment. Actigraphy has been well validated for the estimation of nighttime sleep parameters across age groups, but the validity of the estimation of sleep-onset latency and daytime sleeping is limited. Clinical guidelines and research suggest that wrist actigraphy is particularly useful in the documentation of sleep patterns prior to a multiple sleep latency test, in the evaluation of circadian rhythm sleep disorders, to evaluate treatment outcomes, and as an adjunct to home monitoring of sleep-disordered breathing. Actigraphy has also been well studied in the evaluation of sleep in the context of depression and dementia. Although actigraphy should not be viewed as a substitute for clinical interviews, sleep diaries, or overnight polysomnography when indicated, it can provide useful information about sleep in the natural sleep environment and/or when extended monitoring is clinically indicated.
Clinical Use of Wrist Actigraphy
The first major medical use of actigraphy was for attempting to evaluate psychologic disorders in the pediatric population using purely mechanical sensors first conceived in the 1950s.1 Over subsequent decades, the development of piezoelectric sensors, lithium batteries, and digital data storage has enhanced accuracy, reliability, and storage capacity, and devices can now record objective, long-term data regarding a patient’s daily activity level. This is rapidly developing into a significant asset for sleep medicine clinicians. The field of actigraphy owes much of its increasing usefulness to the advancement of devices (actigraphs) used for measuring body movement with increasing frequency and precision, with current devices able to record and store information for weeks or months, and to the development of automatic scoring algorithms in available software packages for the identification of sleep vs wakefulness. Current devices also have the advantage of small size and light weight, making the devices unobtrusive and convenient for patients. Through the collection of data representing body movement over time, the actigraph paints a picture of daily sleep-wake cycles, which can be useful in the diagnosis and evaluation of several clinical sleep disorders and treatment outcomes.
In 1995, the American Academy of Sleep Medicine (AASM) concluded that actigraphy was useful as a research tool for the study of sleep, but that its clinical usefulness remained uncertain.2 This statement was expanded in 2002 to indicate the potential use of actigraphy to measure sleep in clinical settings; however, the strength of the evidence remained relatively weak.3 In an updated report in 2007, growing research literature supported the use of actigraphy for clinical application, particularly in the evaluation of circadian rhythm disorders, insomnia, hypersomnia, and obstructive sleep apnea (OSA).4 This report contributed to the establishment of a category 3 Current Procedural Terminology code (ie, emerging technology) for actigraphy, which was advanced to a category 1 Current Procedural Terminology code in 2009.
There are multiple methods for evaluating patients’ sleep complaints, including clinical interviews, sleep diaries, polysomnography (PSG) (laboratory or home based), and actigraphy. The usefulness of actigraphy depends on the specific presenting complaint, and each of the aforementioned assessment methods has both advantages and disadvantages. This article reviews recent studies on the validity of wrist actigraphy in the assessment of sleep patterns, discusses the use of actigraphy relative to other tools available to the sleep medicine clinician, and outlines key considerations in the use of actigraphy in specific sleep disorders patients.
Validation of Wrist Actigraphy vs PSG
In recent years, multiple studies have been published on the validity of wrist actigraphy. Tables 15-15 and 216-19 summarize 15 studies published since 2004 that compared wrist actigraphy to other established methods of assessing sleep. In total, 10 studies compared actigraphy to PSG in adults of various ages, and two compared actigraphy to videosomnography in infants. Three studies compared wrist actigraphy to daily sleep diaries. Taken together, these studies suggest that wrist actigraphy is useful in the estimation of total sleep time (TST), sleep percentage, and wake after sleep onset (WASO). Findings are less consistent, however, in terms of estimating sleep-onset latency (SOL), particularly among patients with sleep disorders. Studies of adults are shown in Table 1; studies of infants and children are shown in Table 2. Three additional studies with publication dates prior to 2004 are listed in the AASM practice parameters as providing level 1 evidence for the use of actigraphy.4 We have focused our discussion on the most recent studies, given the changes in devices and software that may decrease the relevance of older studies.
Table 1.
—Summary of Actigraphy Validation Studies in Adults (Published 2004–2010)
| Study/Year | Total Sample Size, No. (Mean Age, y) | Study Subjects | Comparison Methodology | Device (Manufacturer) | Software/Protocol | Main Findings/Conclusions |
| Wang et al5/2010 | 39 (61.5) | Non-small cell lung cancer patients | Actigraphy vs sleep diary | MicroMini (Ambulatory Monitoring, Inc) | Action 3.8 (Ambulatory Monitoring, Inc) | 87% congruency between actigraphy and sleep diaries. Actigraphy showed significantly more nighttime awakenings. |
| Sánchez-Ortuño et al6/2010 | 62 (28.4) | Insomnia sufferers (n = 31); control subjects (n = 31) | Actigraphy vs PSG vs sleep diary | Mini-Mitter (Mini-Mitter Co) | Not specified | No significant difference in WASO, TST, or SE for control subjects or insomnia patients. Stronger correlation between actigraphy and sleep diaries than PSG and sleep diaries for SOL. |
| Natale et al7/2009 | 408 (40.4) | Insomnia sufferers (n =126); control subjects (n = 282) | Actigraphy in insomniacs vs control subjects | Basic Mini-Motionlogger (Ambulatory Monitoring, Inc) | ACT Millennium (Ambulatory Monitoring, Inc) | Actigraphy showed significant differences in SOL, TST, WASO, SE, and NA > 5 between insomnia sufferers and control subjects. TIB showed no significant difference. |
| Chae et al8/2009 | 33 (54) | 20 subjects with OSA, 13 with ≥ 5 periodic limb movements/h | Actigraphy (with different sensitivity thresholds and protocols for defining SO) vs PSG | Actiwatch-L (Mini-Mitter-Respironics, Inc) | Actiware 5.0 (Mini-Mitter-Respironics, Inc) | 5-min immobility protocol most accurate for identifying SO using actigraphy. SL was underestimated when PSG latencies were short and overestimated when PSG latencies were long. |
| Blackwell et al9/2008 | 68 women (81.9) | Women age ≥ 65 y at risk for osteoporotic fractures (SOF study) | Actigraphy (TAT, ZCM, and PIM modalities) vs PSG | Sleepwatch-O (Ambulatory Monitoring, Inc) | Action W-2 using the Cole-Kripke algorithm | Actigraphy underestimated TST by 68 min for those sleeping ≤ 5 h and overestimated TST by 31 min for subjects with SE < 70%, as compared with PSG. Using the best modality (PIM), sleep parameters TST, WASO, and SE did not differ from PSG by more than 17.9, 6.8 min, and 3.8%, respectively. Lower agreement was found for TAT and ZCM. |
| Paquet et al10/2007 | 15 (Not mentioned- adults) | Healthy subjects (caffeine vs placebo) undergoing nighttime PSG or recovery sleep PSG after SD | Actigraphy (2 sensitivity modes) vs PSG | Actiwatch-L (Mini-Mitter-Respironics, Inc) | Actiware 5 (Cambridge Neurotechnology) | Significant overestimations of TST and SE in the setting of non-nighttime PSG, which had low SE. Specificity for sleep was 50% overall despite > 90% sensitivity. |
| Bradshaw et al11/2007 | 54 (30.7) | EDS patients referred for MSLT with PSG | Actigraphy vs PSG vs sleep diary | Unspecified model actigraphic watch (Precision Control Design) | Action W, version 2.4.20 (Ambulatory Monitoring, Inc) | The mean SL found in nights prior to MSLT using actigraphy was significantly correlated with PSG SL during MSLT. SL from sleep diaries significantly overestimated SL and underestimated TST. |
| García-Díaz et al12/2007 | 62 (Not mentioned- adult) | Suspected OSA | Respiratory polygraphy device with actigraphy vs PSG | Apnoescreen II (Eric Jaeger GmbH & Co) | Not specified | Addition of actigraphy to home-based OSA diagnostic device modestly improved sensitivity for patients with RDI ≥30, but there was no change in patients with RDI of 15–30. Actigraphy overestimated TST and SE. |
| Sivertsen et al13/2006 | 34 (60.5) | Chronic primary insomnia | Actigraphy vs PSG | Actiwatch Plus (Cambridge Neurotechnology) | Actiwatch Sleep Analysis 2001, version 1.19 (Cambridge Neurotechnology) | Actigraphy had a sensitivity for detecting sleep of 95.2% but a specificity of only 36.3% for detecting wakefulness, for an accuracy of 83.1%. Actigraphy underestimated total wake time and SOL and overestimated TST and SE. |
| Lichstein et al14/2006 | 57 (Not mentioned-adult) | Primary and comorbid insomnia | Actigraphy vs PSG vs sleep diary | Actiwatch AW64 (Mini-Mitter Co) | Actiware Sleep 3.3 (Mini-Mitter Co) | WASO, TST, and SE were not significantly different among PSG, actigraphy, and sleep diaries. Actigraphy and PSG were not significantly different in SOL and NA. Sleep diaries correlated with PSG in these measures but did not correlate significantly. |
| Hedner et al15/2004 | 228 (48.8) | Suspected OSA | Actigraphy vs PSG | Watch_PAT100 System (Itamar Medical) with built-in actigraph (Ambulatory Monitoring, Inc) | ASWA in the zzzPAT package (Itamar Medical) | SE, TST, and SL between PSG and actigraphy were not different among patients with mild, moderate, or severe OSA. Actigraphy overestimated SL, especially among those with mild OSA. |
EDS = excessive daytime somnolence; MSLT = multiple sleep latency test; NA = number of awakenings; OSA = obstructive sleep apnea; PIM = proportional integration mode; PSG = polysomnography; RDI = respiratory disturbance index; SD = sleep deprivation; SE = sleep efficiency; SL = sleep latency; SO = sleep onset; SOF = study of osteoporotic fractures; SOL = sleep-onset latency; TAT = time above threshold; TIB = time in bed; TST = total sleep time; WASO = wake after sleep onset; ZCM = zero crossing mode.
Table 2.
—Summary of Actigraphy Validation Studies in Infants and Children (Published 2004–2010)
| Study/Year | Total Sample Size, No. (Mean Age) | Study Subjects | Comparison Methodology | Device (Manufacturer) | Software/Protocol | Main Findings/Conclusions |
| Sung et al16/2009 | 10 with 38 overnight studies total (31.2 wk) | Baseline studies across gestational ages | Actigraphy (with different sensitivity thresholds) vs video somnography | Actiwatch AW64 (Mini-Mitter Co) | Actiware Sleep 3.3 (Mini-Mitter Co) | The predictive value of sleep using actigraphy ranged from 91.3% to 96.5% across threshold settings with a sensitivity of 88.2% to 96.8% vs video analysis. Device was not reliable for predicting wakefulness. |
| Werner et al17/2008 | 50 (5.9 y) | Baseline study | Actigraphy vs sleep diary | Actiwatch Plus AW4 (Cambridge Neurotechnology) | Actiware 5 (Cambridge Neurotechnology) | Satisfactory agreement between actigraphy and sleep diary for sleep start, end, and assumed sleep. Insufficient agreement between actual sleep time and nocturnal awake time. |
| Sitnick et al18/2008 | 58 (47 mo) | 22 subjects with autism, 11 subjects with nonspecific developmental delays, and 25 control subjects | Actigraphy vs video somnography | Actiwatch AW64 (Mini-Mitter Co) | Unspecified Mini-Mitter software | In an epoch-by-epoch analysis, there was 94% agreement, 97% sensitivity, and 24% specificity for sleep compared with video somnography. Sleep-onset time, SOL, sleep end time, TST, number of awakenings, and total sleep duration and number of nocturnal awakenings correlated significantly. |
| Hyde et al19/2007 | 45 (5.8 y) | Healthy children (age 1–12 y) | Actigraphy vs PSG | Actiwatch AW64 (Mini-Mitter Co) | Actiware Sleep 3.3 (Mini-Mitter Co) | With epoch-by-epoch comparison, agreement rates were high (85.1%–88.6%). Predictive value for sleep (91.6%–94.9%) and sensitivity for sleep (90.1%–97.7%) were high. Predictive value for wake (46.7%–65.6%) and specificity (39.4%–68.9%) were low. No effect of age, AHI, or PSG arousal index. |
AHI = apnea-hypopnea index. See Table 1 legend for expansion of other abbreviations.
Actigraphy vs Other Methods of Sleep Assessment
When a patient presents with a sleep complaint, clinicians typically begin with a detailed clinical interview. At times, this interview is sufficient to diagnose a sleep disorder, whereas at other times additional assessments are needed. When making the decision regarding which assessments are most appropriate, one must consider the process of differential diagnosis, patient burden, cost, and the importance of understanding sleep in the natural sleep environment vs documenting the characteristics of sleep architecture. Table 3 outlines several considerations one might reflect on in determining the appropriate assessment method(s) to employ.
Table 3.
—Main Advantages and Disadvantages of Actigraphy vs Other Sleep Assessment Methods
| Sleep Assessment Method | Main Advantages | Main Disadvantages |
| Sleep questionnaires | Brief, easily administered in conjunction with clinical interview Low patient burden |
Subject to recall biases Limited usefulness in patients who are unable to self-report reliably (eg, young children, dementia patients) |
| Limited validity compared with PSG | ||
| Sleep diary | Provides documentation of daily variability | Patient burden higher than questionnaires; requires patient to complete diary each day for maximum validity |
| Documents habits in the home sleep environment | Influenced by patient’s expectations about sleep | |
| Less recall bias than questionnaires because information is recorded daily | ||
| Actigraphy | Provides objective information about daily variability and sleep quality | Limited usefulness in assessment of SOL |
| Records information in the home sleep environment | Higher cost than sleep diaries | |
| Not influenced by patient expectations, recall bias, or memory impairments | Patients should complete sleep diaries concurrently to enhance quality of information | |
| Lower cost than PSG | ||
| Laboratory PSG | “Gold standard” objective assessment of sleep | High participant burden |
| High cost | ||
| Does not provide information on sleep habits at home | ||
| Can lead to a “first night effect” phenomenon |
See Table 1 legend for expansion of abbreviations.
Sleep diaries represent an important clinical tool and are often used in the behavioral treatment of sleep disorders such as insomnia.20 Despite their wide-spread use, sleep diaries have some limitations. Self-documentation of sleep frequency and duration can be prone to systematic biases that have clinical implications. For example, when documenting adherence to a sleep schedule using a sleep diary, Carney et al21 reported that bedtime was a full hour earlier than the bedtime reflected in the actigraphy data. Other research has shown significant differences in self-reported documentation of nap frequency, nap duration, nighttime awakenings, and sleep latency (SL) compared with actigraphy.22 Parents of pediatric patients have reported TSTs that are significantly greater (by an average of 1-2 h) than those reflected in actigraphy recordings of their child.17,23 Self-reported sleep duration in sleep diaries also seems to overestimate time asleep in both young adults24 and elderly subjects who lack sleep complaints,25 compared with actigraphy. Although these studies do not imply that sleep diaries are not clinically useful, they do suggest that actigraphy may be useful when patient documentation of sleep habits does not align with other aspects of the clinical presentation.
Strategies to Enhance Wrist Actigraphy Data
As with any home-based monitoring, the patient must be provided with detailed instructions about the device and information on technical support should problems arise. Typically, the duration of recording should be 1 week; however, depending on the specific diagnostic question, recordings of longer or shorter duration are sometimes indicated. The patient should be informed that the device records movement (and light, if relevant) and should be provided with instructions for the removal of the device if it is not fully waterproof. The patient must then return the device so the data recorded can be uploaded onto a computer for scoring and analysis.
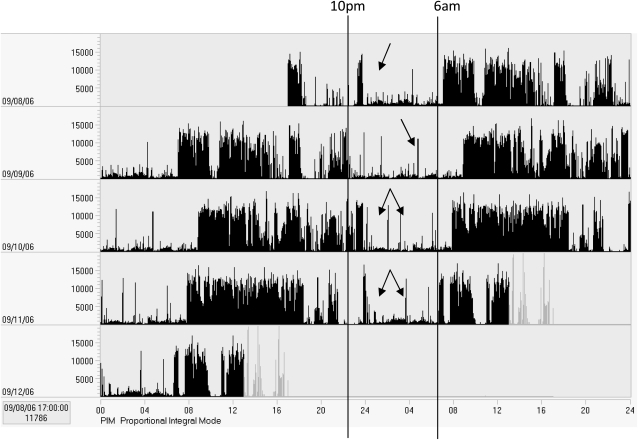
The scoring of actigraphy should follow an established protocol. Although there is no consensus in the field on the precise protocol, in general, specific rules should be in place for the review of recordings for artifacts, for determining the major sleep period (typically from “bedtime” to “rise time”), and for the information to be gleaned from the report. As described previously, clinicians can have confidence in measures of TST, sleep percentage, and amount of time awake at night. Other variables, such as daytime napping and SOL, should be interpreted more cautiously. Often, review of the recording in its entirety can be informative; however, to view night-to-night patterns, a “double plot” can be useful (as in Fig 1). In these plots, each day is repeated adjacent to and below the previous day. This “lines up” the nights of data and can be particularly useful in depicting circadian rhythm sleep disorders.
Figure 1.
Example of a double-plot of actigraphy data. This plot shows nights of data aligned one above the next. This 5-day recording is from a 92-year-old female resident of an assisted-living facility and shows a pattern of abnormally increased activity during the nighttime hours (indicated by arrows).
The use of a concurrent sleep diary during actigraphy recording provides multiple additional benefits: The diary can contribute backup sleep/wake data in the event of actigraph malfunction or noncompliance with its use. The diary can help differentiate awake but relatively motionless periods from true sleep when interpreting the actigraph data and, thus, can potentially improve the accuracy of sleep-onset identification. The diary provides a place where a patient can document his/her reasons for napping, arousals, uncharacteristic behavior, and so forth, which would assist the clinician in finding an underlying cause of the disorder.
The exact information gathered depends, in part, on the device used and the differential diagnostic considerations. For example, some devices may have an “event marker” that patients can press when getting in and out of bed. A diary may, therefore, be needed only to document unusual activities during the day that might impact the recording (eg, device removal for sports, travel across time zones). At a minimum, the patient is typically asked to document the times he/she got into bed for the night and the time he/she last arose in the morning. A sample sleep diary is shown in Figure 2.
Figure 2.
Sample instructions and daily patient log for use with wrist actigraphy.
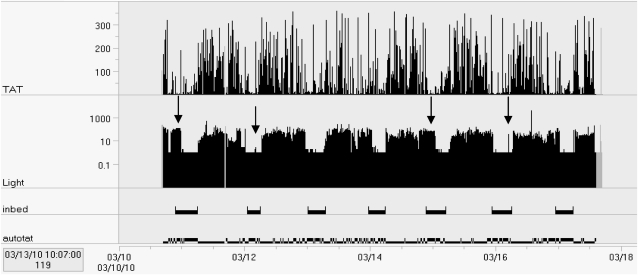
Another strategy to enhance the quality of information from actigraphy is the inclusion of a light sensor on the actigraphy device. This can be particularly useful when there are concerns about the reliability of the diary (eg, patient is cognitively impaired or may be malingering), or simply to verify times noted in the sleep diary. Commonly, scorers use a sharp decrease in light levels near bedtime to help define the major sleep period. Because timed exposure to light is sometimes used in the treatment of circadian rhythm sleep disorders, the light sensor on the actigraphy can be used to monitor adherence to light therapy as well. Figure 3 shows an example of an actigraphy recording completed in an inpatient hospital that shows the increase in light levels during nighttime awakenings.
Figure 3.
Seven-day recording of a 66-year-old woman during hospitalization following ankle injury. Arrows indicate periods when lights were left on after the patient indicated “trying to fall asleep” and occasions when lights were turned on at night. During the day, light levels reached no higher than 450 lux during the 1-week recording period, confirming that the patient did not go outdoors on any occasion.
Use of Actigraphy Prior to a Multiple SL Test
Patients with disorders of excessive daytime sleepiness may sometimes be investigated using a multiple SL test (MSLT) to help characterize the severity of daytime sleepiness or to identify the early onset of rapid eye movement sleep during the day (which is characteristic of narcolepsy).26,27 The MSLT is typically performed in a sleep laboratory where the patient is put to bed multiple times at set intervals throughout the day with the opportunity to sleep. EEG is used to measure the moment of sleep and rapid eye movement onset during each of the sleep opportunities. It is critical, however, that the clinician distinguish between daytime sleepiness resulting from insufficient nighttime sleep before the MSLT and daytime sleepiness of an organic origin (eg, narcolepsy). For this reason, it is necessary to document the patient’s sleep habits for a period of time prior to the MSLT. Wrist actigraphy represents a useful tool for objectively documenting sleep habits prior to an MSLT.27
Several studies suggest that actigraphy may be superior to other methods for documenting sleep habits prior to an MSLT in clinical settings. In a study of 54 subjects comparing self-reported sleep schedules,11 sleep log recordings, and actigraphy 2 weeks prior to a MSLT, sleep logs showed an average of approximately 1.5 h more sleep a night than actigraphy. Based on self-report alone, 32% of participants were sleeping <6 h a night. In this study, actigraphy was the only modality to show a significant relationship between average nightly sleep duration and mean SL on the MSLT. The authors suggest that patients were inclined to minimize their nighttime sleep disruption on sleep logs, because the MSLT results often had job status implications. The benefits of actigraphy to establish a patient’s sleep schedule prior to an MSLT have been recognized by the AASM4 and in The International Classification of Sleep Disorders, 2nd edition27 for use in conjunction with, or as a replacement for, sleep diaries. The 2007 practice parameters published by the AASM suggest an optional patient-care strategy to use actigraphy for assessing sleep time in hypersomnia when sleep log data collection is not ideal, specifically suggesting actigraphy for patients with “impaired cognition, literacy or motivation.”4
Of note, studies typically have not included a comparison of wrist actigraphy with PSG for the assessment of daytime sleep among patients with daytime sleepiness. Although one advantage of actigraphy is the ability to collect information across the 24-h day, the validity of algorithms used to score sleep at night may not be directly translatable into scoring of sleep during the day. Additional research is needed in this area.
Insomnia
Actigraphy can be a useful tool for evaluating insomnia, particularly because insomnia sufferers have a greater propensity for misperceiving their sleep time than individuals without insomnia and overall tend to significantly underestimate sleep time.28 Studying insomnia using a single-night PSG can conceivably help quantify TST; however, the “first night effect” (ie, the impact of testing in the unfamiliar and restrictive environment of the sleep laboratory) frequently leads to artificially reduced sleep efficiency and time, and there is evidence that this “first night effect” is more pronounced in insomniacs29 and can take as much as four polysomnographic studies to resolve.30 Furthermore, insomniacs have considerable night-to-night variability in sleep parameters, which makes actigraphy better suited for an extended data-collection period that would be much too costly and resource intensive for PSG.31,32
Actigraphy has been shown to accurately reflect several important sleep parameters for the characterization of insomnia. When comparing multinight PSG with actigraphy, it has been shown that the measurements of WASO, TST, and sleep efficiency do not differ significantly.6,14 In an article by Sánchez-Ortuño et al,6 the ability of actigraphy to assess sleep parameters in insomnia was similar to the accuracy in 31 normal sleeping control subjects. In a retrospective study comparing insomnia patients and normal sleepers, Natale et al7 showed the clinical importance of the sleep parameters of SOL, TST, WASO, sleep efficiency, number of awakenings > 5, and mean motor activity by demonstrating that they are significantly worse in insomniacs than in control subjects when studied by actigraphy. The study found that the combination of TST, SOL, and number of awakenings >05 into a single formula was the most effective for distinguishing insomnia patients from normal sleepers, with a positive predictive value of 81% and a negative predictive value of 83%.
As mentioned previously, the reliability of the measurement of SOL by actigraphy as compared with PSG has been controversial, with studies showing it to be underestimated13,33 or inconsistent.14 Nonetheless, some studies have shown that SOL measurements correlate with PSG significantly9,34 and correlated better than PSG with subjective perceptions of sleep.6
Interpreting sleep onset from lack of movement sensed by the actigraph can be difficult in sufferers of insomnia, especially among those patients with difficult sleep initiation, where the patient may lay quietly for extended periods of time while awake in bed.10,35 The reduced sensitivity of actigraphy in detecting the onset of sleep in disturbed sleepers (approximately 0.55)7 is a major reason why actigraphy is considered a tool to characterize sleep disruptions or follow treatment outcomes in known insomniacs, rather than as a tool to diagnose insomnia.4
Finally, the identification of a large discrepancy between actigraphy and self-reported data could suggest the presence of paradoxical insomnia (ie, the patient’s self-report is not consistent with objective sleep quality)27 and that the patient would potentially benefit from psychologic treatment. The actigraphy tracing can be used clinically in these cases to discuss potential differences between the patient’s perceived sleep and objectively recorded sleep.
Circadian Rhythm Sleep Disorders
Actigraphy has been recognized as a tool capable of diagnosing circadian rhythm sleep disorders.4 In one study, the actigraph device was able to detect and characterize significant phase advancement (ie, abnormally early sleep timing) in elderly, compared with younger, subjects.36 Cases of phase advancement, as well as phase delay (ie, abnormally late sleep timing), were identified in an adult population of 350 subjects showing excellent concordance with established questionnaires about circadian preferences.37 In this study, actigraphy-calculated bed times and wake times differed by approximately 10 and 20 min, respectively. Actigraphy has demonstrated usefulness in identifying shift-work sleep disorder by documenting shortened sleep times during rest periods classified subjectively as being of poor quality.38 Blind subjects with free-running clocks were identified and had TST characterization on par with PSG.39 Jet lag is another condition in which actigraphs have been used to successfully identify a sleep rhythm disorder.40,41
The use of actigraphy in circadian rhythm disorders is a practical way of logging sleep/wake data for extended periods of time, which is sometimes necessary to identify the patterns of circadian rhythm disorders. The International Classification of Sleep Disorders, 2nd edition27 requires that at least 7 days of actigraphy be performed with a sleep diary to demonstrate consistency in the pathology. The optimum duration for an actigraph study to diagnose circadian rhythm disorders has not been established, but Berger et al42 suggest that as many as 3 weeks are necessary to obtain valid patterns of activity and sleep to describe weekly or social rhythms.
Actigraphy can also be used to assess treatment effects in circadian rhythm disorders. A number of studies have demonstrated the successful use of actigraphy to follow the treatment of phase advancement,43 delay,44 jet lag,45,46 and shift work sleep disorder.47,48
Sleep-Disordered Breathing
Approximately 9% of women and 24% of men under the age of 60 are thought to have OSA.49 Given the high prevalence, full PSG is not a convenient or cost-effective diagnostic modality to keep up with the need for screening large numbers of people, many of whom live far from sleep disorders centers capable of laboratory PSG. The advent of portable devices that record overnight pulse oximetry with the addition of oronasal airflow, respiratory inductance plethysmography, snoring, body position and/or heart rate monitors in the early 1990s50-53 started the process toward successful home diagnosis of OSA.
To provide an optimal assessment of the severity of breathing disturbances during sleep, such devices should accurately assess TST so that a similar hourly index of respiratory events can be compared with those measured by laboratory PSG. A key concern of respiratory monitoring is that patients with sleep apnea may spend significant time awake in bed; therefore, the respiratory disturbance indices may be lower when the total recording time is used in the denominator of the index calculation. In one study, the use of total time in bed yielded a sensitivity of only 50% for OSA as compared with PSG. The sensitivity improved to 88% when actigraphy TST was used instead of total time in bed, suggesting that some technique to assess TST should be used.54
One method of circumventing this limitation of home-based recording is to use an actigraph to estimate TST during the recording; ideally, the actigraph should be integrated into the apnea-monitoring system. TST calculated from actigraph data had only a mean difference from PSG of 2.5 min in a study of 24 OSA patients,55 although the agreement between the two methods was found to decrease as OSA severity worsened.15 Hedner et al15 found good correlation between actigraphy and PSG for TST, sleep efficiency, and SL in OSA subjects, and these findings contributed to the AASM’s recommendation for the use of actigraphy to aid in the diagnosis of OSA by providing an estimate of TST during recording.4 A later study by García-Díaz et al12 confirmed that sensitivity in the detection of OSA is enhanced by the addition of actigraphy in home ambulatory sleep studies. The improvement in sensitivity, though, was seen only in patients with severe OSA. The authors concluded that home studies were useful in OSA detection and were felt to be an acceptable substitute for laboratory PSG among patients with a high pretest probability of sleep-disordered breathing. Although one could make the case that actigraphy is a more critical addition to home-based OSA screening when the degree of suspicion is lower, the improved sensitivity among patients with severe OSA suggests its routine use is clinically indicated in conjunction with home-based OSA screening.
Actigraphy for the Assessment of Treatment Outcomes
Following the changes in sleep schedule over time allows for actigraphy to help assess and guide the management of circadian rhythm disorders. Actigraphy has also been used to assess sleep in a number of clinical trials of both pharmacologic56 and behavioral treatments for insomnia.57,58 It has also been used widely in studies of institutionalized older adults, such as those in nursing homes, to assess response to nonpharmacologic treatments.59,60 The AASM mentions the use of actigraphy for such treatment management as a guideline.4 Interestingly, simply the awareness that one’s sleep schedule is being monitored objectively by a clinician via actigraphy has been shown to improve compliance with a prescribed sleep routine and thereby adds to its usefulness in management follow-up.21
Sleep Disturbance in Mood Disorders
Sleep abnormalities have been well documented in the setting of depression and may be a complaint in as many as 90% of these patients.61 Actigraphy has demonstrated capabilities in characterizing disturbed sleep in the context of depression. Chung and Tso62 found that actigraphy-derived sleep measures of WASO and TST were independently associated with subjective pain among 91 studied subjects afflicted with major depressive disorder, whereas clinician-rated insomnia and sleep-diary parameters were not significant predictors of pain severity in this study. Variability in sleep duration and fragmentation identified by actigraphy has been significantly correlated with subjects documenting considerable life stressors and especially among those noted to have negative affect.63 Fragmentation of sleep, demonstrated in wrist actigraphy, has also been shown to correlate with postpartum maternal depressive symptoms.64,65 Disrupted circadian patterns (based on wrist actigraphy) were also associated with greater depressive and anxious symptoms among cancer patients,66,67 elderly women,68 children, and adolescents,69 based on multiple studies. Of note, one large study of > 3,000 older men did not find a relationship between actigraphically estimated sleep and depressive symptoms but did find a relationship between self-reported sleep complaints and depression.70
There is some suggestion that actigraphy could be used to follow the progress of depression treatment, given the greater daytime psychomotor retardation demonstrated by subjects with greater depression severity.49,71 Depressed patients whose depression has improved with therapy have shown greater daytime activity levels,72 as well as improvement in SL and sleep percentage estimated by actigraphy.73,74 Therefore, actigraphy may help document the benefits (or lack thereof) of a specific treatment plan for depression in a given patient. More research is needed regarding how sensitive and specific actigraphy is for the characterization of this change in psychologic state.
Sleep Disturbance in Dementia
Assessing sleep among patients with dementia presents a particular challenge to clinicians. Because sleep disruption is a common behavioral symptom of dementia and contributes to significant challenges for caregivers, it can become an important focus of clinical evaluation and intervention.75 Actigraphy has several advantages for patients who are incapable of reliably completing questionnaires or sleep diaries because of cognitive limitations, and in cases in which the disruptions associated with laboratory (or even home-based) PSG may be impossible for the patient to tolerate. Nursing home patients are an example of a group with considerable challenges in the attainment of good sleep history or overnight PSG. In nursing home settings, actigraphy has been validated for the study of nighttime sleep76 and can provide useful information on the patient’s sleep habits in the context of the nursing home environment.77-81
Key Limitations of Wrist Actigraphy
Although actigraphy is an objective measure of sleep vs wakefulness, it has not been validated for measuring sleep stages. Actigraphy is also prone to overestimating sleep in certain patient groups. A discussion of the limitations of this technology is warranted. When comparing actigraphy’s ability to assess sleep parameters to the “gold standard” of PSG, it has shown excellent concordance in the measurement of TST among healthy subjects, with a sensitivity > 90%.9,10,82-85 However, the ability to detect sleep is substantially reduced in patients with disturbed sleep (ie, those who have frequent arousals and reduced TST).9,10,85
With actigraphy, because sleep is inferred from lack of movement, subjects who are awake but lie motionless can be classified incorrectly as being asleep, and thus the technique is biased toward overestimating TST, which may lead to incorrectly minimizing the severity of sleep disturbances. This may present a specific challenge for patients with insomnia, and may partially explain the limited validity of wrist actigraphy for estimating SOL. This may also be a concern among individuals who are hospitalized or bed bound, because these individuals may not have as much activity during wakefulness.
Finally, the process of scoring wrist actigraphy data is substantially simplified when a concurrent sleep diary is maintained by the patient. This enables the clinician to determine the key period for analyzing sleep parameters. Typically, the time window between the patient’s bedtime and morning rise time is considered the “major sleep period” and used for analysis. In the absence of such documentation, actigraphy can still provide a useful estimate of sleep habits over a 24-h period, but parameters such as TST and WASO may be of more limited use. A sample “actigraphy log” is shown in Figure 2.
Summary and Conclusions
Actigraphy represents a useful diagnostic tool for the sleep medicine practitioner, allowing for assessment of sleep over extended periods of time in the natural sleep environment. Actigraphy appears to provide a valid estimate of TST, sleep percentage, and WASO, but the validity of actigraphy for measuring SOL remains suboptimal. Although actigraphy cannot be viewed as a replacement for other assessment tools such as clinical interviews, sleep diaries, or overnight PSG, it can provide useful information in the evaluation of insomnia and circadian rhythm sleep disorders, in the measurement of sleep habits prior to an MSLT, and as a way to estimate TST in the recording of sleep-related breathing disorders. Key limitations remain the absence of validation studies with many of the commercially available devices and the use of actigraphy in the assessment of daytime sleeping.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Martin receives research funding from the National Institutes of Health and the Department of Veterans Affairs and presents educational workshops on the use of wrist actigraphy. Dr Hakim has reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- AASM
American Academy of Sleep Medicine
- MSLT
multiple sleep latency test
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SL
sleep latency
- SOL
sleep-onset latency
- TST
total sleep time
- WASO
wake after sleep onset
Footnotes
Funding/Support: This work was supported by National Institutes of Health/National Institute on Aging K23 AG028452 (Dr Martin); VA RR&D IIR 1RX000135 (Dr Martin), Cedars Sinai Sleep Medicine Fellowship Program (Dr Hakim); and the VA Greater Los Angeles Healthcare System Geriatric Research, Education and Clinical Center (Dr Martin).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Tryon W, Bellak A, Hersen M. Activity Measurement in Psychology and Medicine. New York, NY: Plenum Press; 1991. [Google Scholar]
- 2.Thorpy M, Chesson A, Derderian S, et al. American Sleep Disorders Association Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. Sleep. 1995;18(4):285–287. doi: 10.1093/sleep/18.4.285. [DOI] [PubMed] [Google Scholar]
- 3.Littner M, Kushida CA, Anderson WM, et al. Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26(3):337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 4.Morgenthaler T, Alessi C, Friedman L, et al. Standards of Practice Committee; American Academy of Sleep Medicine Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 5.Wang SY, Chang HJ, Lin CC. Sleep disturbances among patients with non-small cell lung cancer in taiwan: congruence between sleep log and actigraphy. Cancer Nurs. 2010;33(1):E11–E17. doi: 10.1097/NCC.0b013e3181b3278e. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Ortuño MM, Edinger JD, Means MK, Almirall D. Home is where sleep is: an ecological approach to test the validity of actigraphy for the assessment of insomnia. J Clin Sleep Med. 2010;6(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- 7.Natale V, Plazzi G, Martoni M. Actigraphy in the assessment of insomnia: a quantitative approach. Sleep. 2009;32(6):767–771. doi: 10.1093/sleep/32.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chae KY, Kripke DF, Poceta JS, et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10(6):621–625. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell T, Redline S, Ancoli-Israel S, et al. Study of Osteoporotic Fractures Research Group Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31(2):283–291. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30(10):1362–1369. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw DA, Yanagi MA, Pak ES, Peery TS, Ruff GA. Nightly sleep duration in the 2-week period preceding multiple sleep latency testing. J Clin Sleep Med. 2007;3(6):613–619. [PMC free article] [PubMed] [Google Scholar]
- 12.García-Díaz E, Quintana-Gallego E, Ruiz A, et al. Respiratory polygraphy with actigraphy in the diagnosis of sleep apnea-hypopnea syndrome. Chest. 2007;131(3):725–732. doi: 10.1378/chest.06-1604. [DOI] [PubMed] [Google Scholar]
- 13.Sivertsen B, Omvik S, Havik OE, et al. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29(10):1353–1358. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- 14.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 15.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004;27(8):1560–1566. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 16.Sung M, Adamson TM, Horne RS. Validation of actigraphy for determining sleep and wake in preterm infants. Acta Paediatr. 2009;98(1):52–57. doi: 10.1111/j.1651-2227.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- 17.Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children’s sleep patterns. Arch Pediatr Adolesc Med. 2008;162(4):350–358. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- 18.Sitnick SL, Goodlin-Jones BL, Anders TF. The use of actigraphy to study sleep disorders in preschoolers: some concerns about detection of nighttime awakenings. Sleep. 2008;31(3):395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyde M, O’Driscoll DM, Binette S, et al. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. J Sleep Res. 2007;16(2):213–216. doi: 10.1111/j.1365-2869.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 20.Chesson AL, Jr, Anderson WM, Littner M, et al. Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for the nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine report. Sleep. 1999;22(8):1128–1133. doi: 10.1093/sleep/22.8.1128. [DOI] [PubMed] [Google Scholar]
- 21.Carney CE, Lajos LE, Waters WF. Wrist actigraph versus self-report in normal sleepers: sleep schedule adherence and self-report validity. Behav Sleep Med. 2004;2(3):134–143. doi: 10.1207/s15402010bsm0203_2. [DOI] [PubMed] [Google Scholar]
- 22.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8(3):175–183. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 23.So K, Adamson TM, Horne RS. The use of actigraphy for assessment of the development of sleep/wake patterns in infants during the first 12 months of life. J Sleep Res. 2007;16(2):181–187. doi: 10.1111/j.1365-2869.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 24.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17(3):295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 26.Richardson GS, Carskadon MA, Flagg W, Van den Hoed J, Dement WC, Mitler MM. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol. 1978;45(5):621–627. doi: 10.1016/0013-4694(78)90162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Academy of Sleep Medicine . The International Classification of Sleep Disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 28.Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003;4(4):285–296. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 29.Toussaint M, Luthringer R, Schaltenbrand N, et al. First-night effect in normal subjects and psychiatric inpatients. Sleep. 1995;18(6):463–469. doi: 10.1093/sleep/18.6.463. [DOI] [PubMed] [Google Scholar]
- 30.Le Bon O, Staner L, Hoffmann G, et al. The first-night effect may last more than one night. J Psychiatr Res. 2001;35(3):165–172. doi: 10.1016/s0022-3956(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 31.Edinger JD, Marsh GR, McCall WV, Erwin CW, Lininger AW. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991;14(1):13–17. [PubMed] [Google Scholar]
- 32.Vallières A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14(4):447–453. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 33.Vallières A, Morin CM. Actigraphy in the assessment of insomnia. Sleep. 2003;26(7):902–906. doi: 10.1093/sleep/26.7.902. [DOI] [PubMed] [Google Scholar]
- 34.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26(1):81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 35.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12(1):23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Kripke DF, Youngstedt SD, Elliott JA, et al. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22(4):695–709. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- 37.Ando K, Kripke DF, Ancoli-Israel S. Delayed and advanced sleep phase symptoms. Isr J Psychiatry Relat Sci. 2002;39(1):11–18. [PubMed] [Google Scholar]
- 38.Borges FN, Fischer FM. Twelve-hour night shifts of healthcare workers: a risk to the patients? Chronobiol Int. 2003;20(2):351–360. doi: 10.1081/cbi-120019341. [DOI] [PubMed] [Google Scholar]
- 39.Leger D, Guilleminault C, Santos C, Paillard M. Sleep/wake cycles in the dark: sleep recorded by polysomnography in 26 totally blind subjects compared to controls. Clin Neurophysiol. 2002;113(10):1607–1614. doi: 10.1016/s1388-2457(02)00221-3. [DOI] [PubMed] [Google Scholar]
- 40.Grajewski B, Nguyen MM, Whelan EA, Cole RJ, Hein MJ. Measuring and identifying large-study metrics for circadian rhythm disruption in female flight attendants. Scand J Work Environ Health. 2003;29(5):337–346. doi: 10.5271/sjweh.740. [DOI] [PubMed] [Google Scholar]
- 41.Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. Advancing circadian rhythms before eastward flight: a strategy to prevent or reduce jet lag. Sleep. 2005;28(1):33–44. doi: 10.1093/sleep/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008;36(2):191–199. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ondzé B, Espa F, Ming LC, Chakkar B, Besset A, Billiard M. Advanced sleep phase syndrome [in German] Rev Neurol (Paris) 2001;157(11 Pt 2):S130–S134. [PubMed] [Google Scholar]
- 44.Nagtegaal JE, Kerkhof GA, Smits MG, Swart AC, Van Der Meer YG. Delayed sleep phase syndrome: a placebo-controlled cross-over study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J Sleep Res. 1998;7(2):135–143. doi: 10.1046/j.1365-2869.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- 45.Beaumont M, Batéjat D, Piérard C, et al. Caffeine or melatonin effects on sleep and sleepiness after rapid eastward transmeridian travel. J Appl Physiol. 2004;96(1):50–58. doi: 10.1152/japplphysiol.00940.2002. [DOI] [PubMed] [Google Scholar]
- 46.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18(4):318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubo T, Takahashi M, Tachi N, et al. Characterizing recovery of sleep after four successive night shifts. Ind Health. 2009;47(5):527–532. doi: 10.2486/indhealth.47.527. [DOI] [PubMed] [Google Scholar]
- 48.Bjorvatn B, Stangenes K, Oyane N, et al. Randomized placebo-controlled field study of the effects of bright light and melatonin in adaptation to night work. Scand J Work Environ Health. 2007;33(3):204–214. doi: 10.5271/sjweh.1129. [DOI] [PubMed] [Google Scholar]
- 49.Lemke MR, Broderick A, Zeitelberger M, Hartmann W. Motor activity and daily variation of symptom intensity in depressed patients. Neuropsychobiology. 1997;36(2):57–61. doi: 10.1159/000119362. [DOI] [PubMed] [Google Scholar]
- 50.Stoohs R, Guilleminault C. MESAM 4: an ambulatory device for the detection of patients at risk for obstructive sleep apnea syndrome (OSAS) Chest. 1992;101(5):1221–1227. doi: 10.1378/chest.101.5.1221. [DOI] [PubMed] [Google Scholar]
- 51.Penzel T, Peter JH. Ambulatory diagnosis of sleep-related breathing disorders. Sleep. 1992;15(6)(Suppl):S9–S12. doi: 10.1093/sleep/15.suppl_6.s9. [DOI] [PubMed] [Google Scholar]
- 52.Esnaola S, Durán J, Infante-Rivard C, Rubio R, Fernández A. Diagnostic accuracy of a portable recording device (MESAM IV) in suspected obstructive sleep apnoea. Eur Respir J. 1996;9(12):2597–2605. doi: 10.1183/09031936.96.09122597. [DOI] [PubMed] [Google Scholar]
- 53.Zucconi M, Ferini-Strambi L, Castronovo V, Oldani A, Smirne S. An unattended device for sleep-related breathing disorders: validation study in suspected obstructive sleep apnoea syndrome. Eur Respir J. 1996;9(6):1251–1256. doi: 10.1183/09031936.96.09061251. [DOI] [PubMed] [Google Scholar]
- 54.Elbaz M, Roue GM, Lofaso F, Quera Salva MA. Utility of actigraphy in the diagnosis of obstructive sleep apnea. Sleep. 2002;25(5):527–531. [PubMed] [Google Scholar]
- 55.Gagnadoux F, Nguyen XL, Rakotonanahary D, Vidal S, Fleury B. Wrist-actigraphic estimation of sleep time under nCPAP treatment in sleep apnoea patients. Eur Respir J. 2004;23(6):891–895. doi: 10.1183/09031936.04.00089604. [DOI] [PubMed] [Google Scholar]
- 56.Wilson SJ, Rich AS, Rich NC, Potokar J, Nutt DJ. Evaluation of actigraphy and automated telephoned questionnaires to assess hypnotic effects in insomnia. Int Clin Psychopharmacol. 2004;19(2):77–84. doi: 10.1097/00004850-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13(1):17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 58.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 59.Ancoli-Israel S, Gehrman PR, Martin JL, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1(1):22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 60.Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53(5):803–810. doi: 10.1111/j.1532-5415.2005.53251.x. [DOI] [PubMed] [Google Scholar]
- 61.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 62.Chung KF, Tso KC. Relationship between insomnia and pain in major depressive disorder: A sleep diary and actigraphy study. Sleep Med. 2010;11(8):752–758. doi: 10.1016/j.sleep.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Mezick EJ, Matthews KA, Hall M, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34(9):1346–1354. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goyal D, Gay C, Lee K. Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Arch Women Ment Health. 2009;12(4):229–237. doi: 10.1007/s00737-009-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Posmontier B. Sleep quality in women with and without postpartum depression. J Obstet Gynecol Neonatal Nurs. 2008;37(6):722–735. doi: 10.1111/j.1552-6909.2008.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2009;18(1):105–114. doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 67.Du-Quiton J, Wood PA, Burch JB, et al. Actigraphic assessment of daily sleep-activity pattern abnormalities reflects self-assessed depression and anxiety in outpatients with advanced non-small cell lung cancer. Psychooncology. 2010;19(2):180–189. doi: 10.1002/pon.1539. [DOI] [PubMed] [Google Scholar]
- 68.Spira AP, Stone K, Beaudreau SA, Ancoli-Israel S, Yaffe K. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136–143. doi: 10.1097/JGP.0b013e3181871345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armitage R, Hoffmann R, Emslie G, Rintelman J, Moore J, Lewis K. Rest-activity cycles in childhood and adolescent depression. J Am Acad Child Adolesc Psychiatry. 2004;43(6):761–769. doi: 10.1097/01.chi.0000122731.72597.4e. [DOI] [PubMed] [Google Scholar]
- 70.Paudel ML, Taylor BC, Diem SJ, et al. Osteoporotic Fractures in Men Study Group Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56(7):1228–1235. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finazzi ME, Mesquita ME, Lopes JR, Fu LI, Oliveira MG, Del Porto JA. Motor activity and depression severity in adolescent outpatients. Neuropsychobiology. 2010;61(1):33–40. doi: 10.1159/000262178. [DOI] [PubMed] [Google Scholar]
- 72.Todder D, Caliskan S, Baune BT. Longitudinal changes of day-time and night-time gross motor activity in clinical responders and non-responders of major depression. World J Biol Psychiatry. 2009;10(4):276–284. doi: 10.3109/15622970701403081. [DOI] [PubMed] [Google Scholar]
- 73.Brzezinski A, Lynch HJ, Wurtman RJ, Seibel MM. Possible contribution of melatonin to the timing of the luteinizing hormone surge. N Engl J Med. 1987;316(24):1550–1551. [PubMed] [Google Scholar]
- 74.Coffield TG, Tryon WW. Construct validation of actigraphic sleep measures in hospitalized depressed patients. Behav Sleep Med. 2004;2(1):24–40. doi: 10.1207/s15402010bsm0201_3. [DOI] [PubMed] [Google Scholar]
- 75.Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6(Suppl 1A):S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- 76.Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason WJ. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997;20(1):24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fetveit A, Bjorvatn B. Sleep disturbances among nursing home residents. Int J Geriatr Psychiatry. 2002;17(7):604–609. doi: 10.1002/gps.639. [DOI] [PubMed] [Google Scholar]
- 78.Harper DG, Stopa EG, McKee AC, et al. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Arch Gen Psychiatry. 2001;58(4):353–360. doi: 10.1001/archpsyc.58.4.353. [DOI] [PubMed] [Google Scholar]
- 79.Hatfield CF, Herbert J, van Someren EJW, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain. 2004;127(Pt 5):1061–1074. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 80.Paavilainen P, Korhonen I, Lötjönen J, et al. Circadian activity rhythm in demented and non-demented nursing-home residents measured by telemetric actigraphy. J Sleep Res. 2005;14(1):61–68. doi: 10.1111/j.1365-2869.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 81.Greco KE, Deaton C, Kutner M, Schnelle JF, Ouslander JG. Psychoactive medications and actigraphically scored sleep quality in frail nursing home patients. J Am Med Dir Assoc. 2004;5(4):223–227. doi: 10.1097/01.JAM.0000131499.71697.9E. [DOI] [PubMed] [Google Scholar]
- 82.Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105(2):185–191. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 83.Signal TL, Gale J, Gander PH. Sleep measurement in flight crew: comparing actigraphic and subjective estimates to polysomnography. Aviat Space Environ Med. 2005;76(11):1058–1063. [PubMed] [Google Scholar]
- 84.Johnson NL, Kirchner HL, Rosen CL, et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. Sleep. 2007;30(7):899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]