Abstract
Networks of blood vessels in normal and tumour tissues have heterogeneous structures, with widely varying blood flow pathway lengths. To achieve efficient blood flow distribution, mechanisms for the structural adaptation of vessel diameters must be able to inhibit the formation of functional shunts (whereby short pathways become enlarged and flow bypasses long pathways). Such adaptation requires information about tissue metabolic status to be communicated upstream to feeding vessels, through conducted responses. We propose that impaired vascular communication in tumour microvascular networks, leading to functional shunting, is a primary cause of dysfunctional microcirculation and local hypoxia in cancer. We suggest that anti-angiogenic treatment of tumours may restore vascular communication and thereby improve or normalize flow distribution in tumour vasculature.
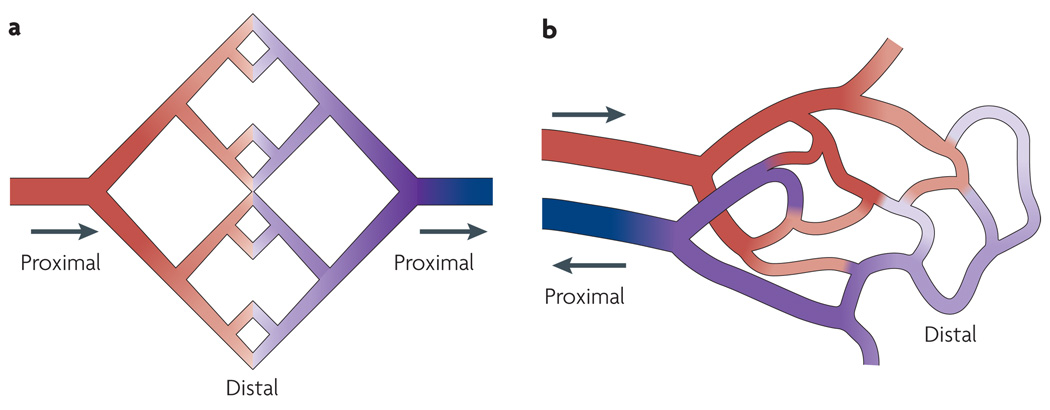
Vascular networks, especially terminal vascular beds of the microcirculation, are often simplistically portrayed as symmetrical arrays of vessel segments in which all flow pathways are equivalent (FIG. 1a). By contrast, real vascular networks have a large degree of topological and structural heterogeneity1–4 and include long and short flow pathways (FIG. 1b). Two main causes for this heterogeneity have been identified4. First, capillaries must supply the entire tissue, whether adjacent to the main feeding vessels or in more remote regions. Second, the generation of vascular networks by angiogenesis is driven by stochastic events, resulting in randomness of the topological arrangement5. Vascular maturation and pruning reduce this irregularity, but substantial heterogeneity remains. Not only does this heterogeneity have important consequences for blood flow distribution2,3,6,7, but it also generates a fundamental biological problem, referred to as the ‘shunt problem’: the potential to form functional shunts — that is, short low-resistance, high-flow pathways that bypass long pathways. If such pathways are present, they can draw blood flow away from more remote regions of a tissue, which consequently reduces the delivery of solutes, including oxygen and drugs, to these regions.
Figure 1. Microvascular networks.
a | A simplified view of a vascular network in which all flow pathways are equivalent, as is often assumed. b | A more realistic configuration, showing that blood flow pathways are heterogeneous, with highly non-uniform lengths. Arrows show blood flow directions in feeding and draining vessels and illustrate the use of the terms ‘proximal’ and ‘distal’ with regard to the circulatory system. Colours indicate the change in blood oxygen levels from high (red) to low (blue) as blood flows through the capillaries.
The control of tumour growth by restricting angiogenesis has attracted tremendous attention in recent years. The initial work using anti-angiogenic drugs alone was promising but did not translate into long-term positive clinical results for cancer patients8. Instead, an alternative strategy has emerged: the use of anti-angiogenic drugs to improve, or ‘normalize’, tumour circulation, thereby improving the delivery of drugs to tumour tissues and increasing the efficacy of chemotherapy9. It is well known that tumour microvasculature shows striking abnormalities, with irregular, leaky vessels, disordered network structure and impaired transport characteristics that lead to highly heterogeneous tissue oxygenation and regions of hypoxia9. However, the anatomical changes involved in vascular normalization and the mechanism or mechanisms by which anti-angiogenic drugs can normalize the circulation have been unclear, and normalization does not occur in all tumour types10.
In this Opinion article, we outline the factors causing the shunt problem and the possible mechanisms by which it is overcome in normal tissues. We argue that impairment of these mechanisms is a primary reason for the aberrant properties of tumour microcirculation. We propose that the restoration of mechanisms that counteract functional shunting underlies the successful normalization of tumour circulation by anti-angiogenic drugs, resulting in improved tumour oxygenation and the more uniform delivery of other chemotherapeutic agents. Failure to prevent functional shunting may result in unsuccessful normalization or resistance to anti-angiogenic therapy11.
The shunt problem
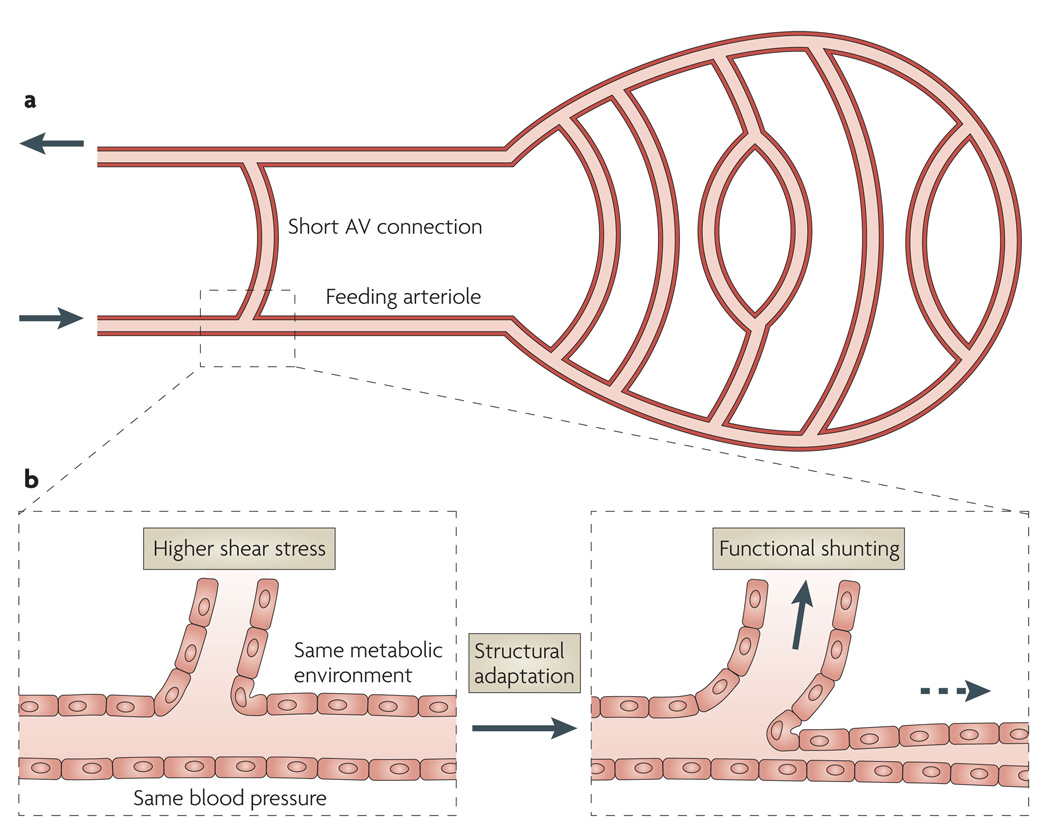
In a heterogeneous vascular network, long flow pathways coexist with short arteriovenous (AV) connections (FIG. 2a). Owing to vascular plasticity12, vessel diameters are subject to remodelling or structural adaptation13– 15 in response to stimuli derived from the metabolic and haemodynamic environment16. Consider first the consequences of such an adaptation for the two vessel segments labelled ‘feeding arteriole’ and ‘short AV connection’ in the schematic network shown in FIG. 2a. Both vessels are embedded in the same parenchymal tissue and carry the same blood; therefore, they receive similar local metabolic signals (FIG. 2b). Blood pressure is also similar in both vessels. The total pressure drop along the long flow pathways containing the feeding arteriole is the same as that across the short AV connection. The wall shear stress varies inversely with vessel length, and so it is higher in the short AV connection than in the feeding arteriole, if they have similar diameters. Increased shear stress triggers an increase in luminal vessel diameter17–20, which tends to decrease blood flow resistance in the short AV connection compared with that in the feeding arteriole and consequently establishes the AV connection as a functional shunt that diverts most of the blood flow away from nutritional vessels. Using model simulations, it can be shown that the same problem can occur in more complex structures with heterogeneous pathway lengths21, and would lead to inadequate or inefficient blood supply to the tissue. In particular, little oxygen can be extracted during the short transit time of blood passing through a shunt with a limited surface area and a long diffusion distance to most tissue cells. Therefore, maldistribution of flow with a high degree of shunting would result in low oxygen extraction22.
Figure 2. The shunt problem.
a | A microvascular network configuration with a short arterio-venous (AV) connection that could potentially form a functional shunt, causing most of the blood flow to bypass the other, longer flow pathways. b | The enlarged section shows factors that influence structural adaptation of the short AV connection and the feeding arteriole. Both vessels experience similar conditions, with the exception that the short AV connection is exposed to a higher wall shear stress. This causes remodelling, with an increase in vessel diameter and, consequently, an increased flow in the short AV connection, leading to functional shunting. Conversely, the feeding arteriole experiences lower wall shear stress and a structural reduction in diameter.
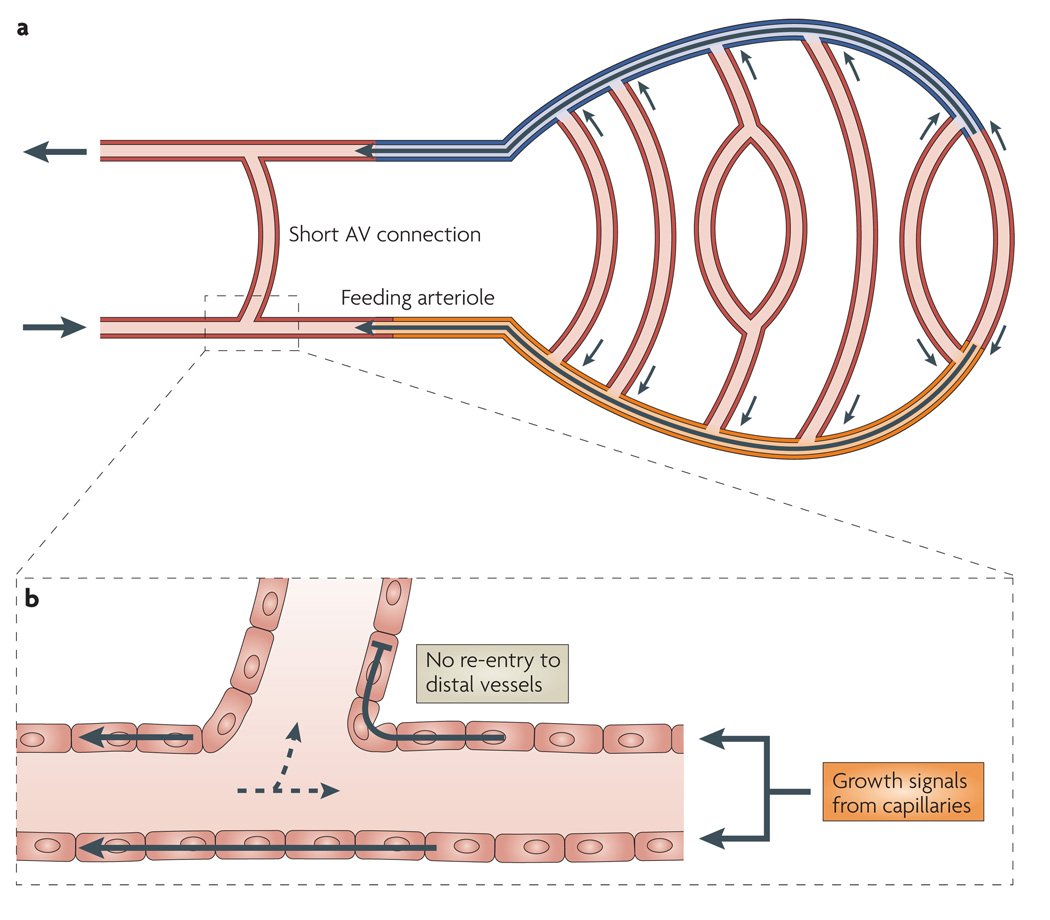
The absence of flow maldistribution and functional shunting in normal vascular networks, as evidenced by the substantial oxygen extractions achievable by normal tissues, implies that powerful biological counter-measures to the shunt problem exist. The arguments given above imply that the mechanisms that prevent functional shunting cannot be based purely on haemodynamic and metabolic signals that are locally generated in each segment but must also respond to the need of the feeding arteriole to supply multiple capillaries and, similarly, the need of the corresponding venule to drain multiple capillaries. This necessitates the transfer of information about the existence of capillaries and/or their metabolic status to their feeding and draining vessels23 on both the arterial and the venous sides (FIG. 3a). The direction of propagation for this information must be exclusively from distal to proximal vessels — that is, against the direction of blood flow in arterial vessels and with the direction of blood flow in venous vessels. A reversal of signal transmission at branch points, resulting in the ‘re-entry’ of the signal into distal segments, would stimulate growth in short AV pathways and so induce functional shunting (FIG. 3b).
Figure 3. Mechanisms for avoidance of the shunt problem.
a | Pathways for information transfer from distal to proximal vessels. The orange shading, the black arrow within it and adjacent small arrows represent information transfer by conducted responses in the upstream direction along the wall of the feeding arteriole. The blue shading, the black arrow within it and the adjacent small arrows represent information transfer by the convection of metabolites in the blood along draining venules. Arrows on the left indicate the direction of blood flow. b | Enlargement to show the paths of the conducted responses. Conducted response signals travelling upstream along feeding arterioles (solid arrows) must be prevented from re-entering smaller downstream side branches to avoid growth of these short arterio-venous (AV) pathways, which could lead to functional shunting. Dashed arrows represent the direction of blood flow.
Mechanisms of information transfer
A probable mechanism for information transfer in the downstream (that is, venous) direction is the generation of metabolites in response to low oxygen availability in the capillary bed; these metabolites can then travel with the blood to larger draining vessels. Many studies have shown the importance of various substances, including ATP and nitric oxide (NO) released from erythrocytes, and adenosine in the control of tone (that is, the degree of vascular contraction)24–28. Prolonged changes in tone evoke corresponding changes in vessel structure12,29,30, providing a potential mechanism for the adaptation of vascular structure to avoid the formation of functional shunts. Information transfer by this convective mechanism is inherent in the downstream direction only.
The situation is more complex for the upstream (that is, arterial) vessels, as information transfer must occur against the direction of blood flow (FIG. 3a), and it is crucial that this direction is maintained throughout the arteriolar vessel tree (FIG. 3b). Conducted responses along vessel walls provide a mechanism for information transfer in the upstream direction. Such conducted responses are known to be involved in the regulation and coordination of vascular tone31–33. They are propagated through gap junctions, which interconnect the endothelial and smooth muscle cells in the vessel wall and provide a pathway for the diffusion of ions and other small solutes34,35. The connexins that form vascular gap junctions are Cx37 (also known as GJα4), Cx40 (also known as GJα5), Cx43 (also known as GJα1) and Cx45 (also known as GJγ1)36–40. Several types of conducted response have been described, with propagation occurring through either endothelial cells or smooth muscle cells, involving different types of gap junctions and ion channels, and decaying at different rates with distance travelled32,41.
Conducted responses along vascular walls can therefore provide the necessary transfer of information from distal to proximal vessels along arterial trees. However, it is not obvious how the re-entry of such signals into downstream branches is prevented (FIG. 3b). One possibility is the participation of heteromeric gap junctions (in which one hemichannel is composed of different connexins) and heterotypic gap junctions (in which hemichannels from two cells forming a gap junction are each composed of a different type of connexin)42–47. Both heteromeric and heterotypic gap junctions may exhibit asymmetrical gating and conduction properties, especially if Cx45 is involved47, and may thus function as rectifiers that allow information transfer in one direction only. The expression of connexins in the vasculature is non-uniform and dependent on flow conditions. For example, blood flow differentially regulates the expression of Cx37, Cx40 and Cx43 (REF. 48); endothelial expression of Cx43 increases with decreasing vessel size49 but is upregulated at branch points, where blood flow is disturbed50. Such connexin expression patterns may lead to gap junction configurations that limit the re-entry of signals into short AV connections.
Effects of faulty anti-shunt mechanisms
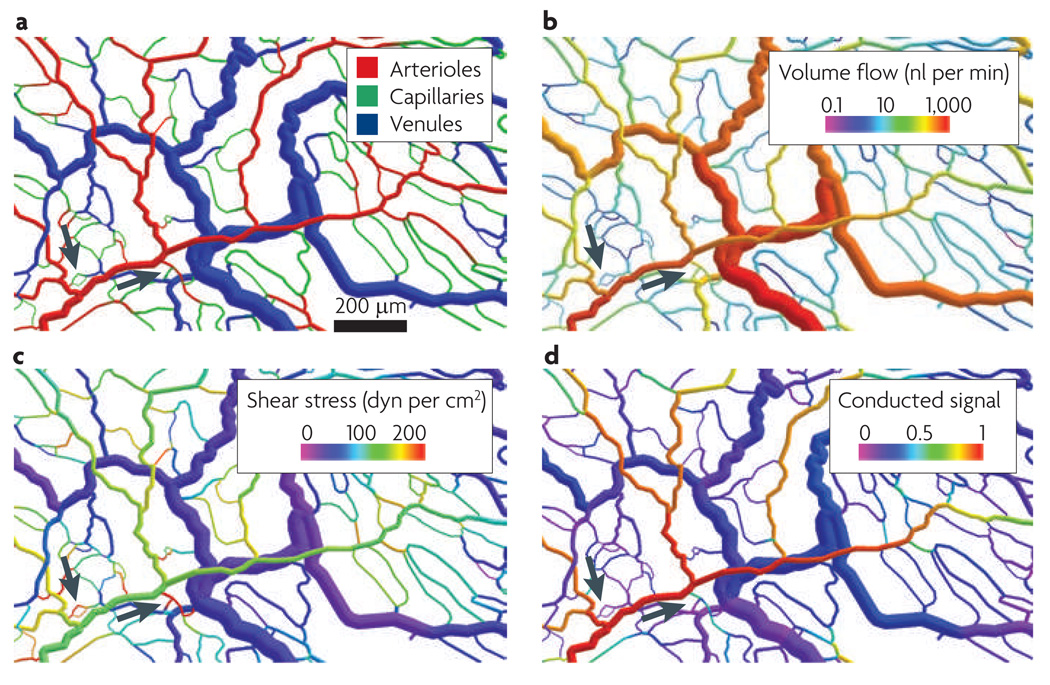
Experimental analysis of the structural effects of reduced information transfer in vivo is difficult owing to problems with suppressing the relevant mechanisms for a prolonged time while monitoring the changes in vascular network structure and function. Mathematical simulations offer a useful alternative, as the effects of parameters and mechanisms implemented in the model can be analysed individually. The mathematical model considered here16,21,23,51 assumes that there is structural vascular adaptation in response to local pressure, wall shear stress and the oxygen partial pressure (Po2), as well as in response to signals transferred upstream by conduction and downstream by the convection of metabolites with the blood (BOX 1). The model was validated by comparing measured and predicted distributions of vessel diameters and flow velocities. FIGURE 4 shows the structure of a microvascular network (specifically, a rat mesentery network) and the simulated distributions of blood flow rate, wall shear stress and upstream conducted signal in the network under normal conditions.
Box 1 | Methods of network modelling.
Network modelling is based on experimental in vivo recordings of microvascular networks with 300–1,000 vessel segments, in which each segment connects two vessel branch points. For these segments, morphological parameters (such as diameter and length) and, in some cases, functional parameters (such as flow velocity and haematocrit) are measured. A computer simulation of network haemodynamics based on these morphological data is used to predict the distribution of blood flow and oxygen, and the results are compared with experimental measurements. The next step is the simulation of vascular-diameter adaptation in response to the following haemodynamic and metabolic stimuli.
Shear stress: to coordinate vessel diameters along flow pathways and avoid excessive viscous energy dissipation in the network
Pressure: to ensure smaller vessel diameters and higher shear stress on the arterial side of the network relative to the venous side, and to avoid high blood pressure in capillaries
Metabolic stimuli (such as oxygen partial pressure): to ensure that blood flow is responsive to local oxygen demand, and to avoid the collapse of parallel flow pathways to a single pathway
Information transfer through downstream convection of metabolites and upstream conducted responses along vessel walls: to avoid enlargement of short arterio-venous connections, which would lead to functional shunting
In the simulation, the diameter of each vessel segment is allowed to vary with time according to the summed effect of these stimuli, until equilibrium is reached. The resulting distributions of structural and haemodynamic variables are compared with observed properties. Distributions corresponding to normal vascular beds cannot be obtained if any of the listed responses is omitted from the model.
Figure 4. Structure and functional characteristics of a normal microvascular network.
An area of the thin mesenteric membrane in a rat was scanned by intravital microscopy, and vessel diameters, segment lengths, topology and vessel location were determined from video recordings. From these data, a digital reconstruction of the network with 546 vessel segments was made and used for mathematical modelling of structural adaptation under normal conditions. Simulated results are shown as colour-coded visualizations. A distal capillary region with a central arteriole and peripheral collecting venules are shown and arrows indicate short arterio-venous (AV) connections that provide a homogeneous capillary density throughout. a | The vessel types. b | Flow rates expressed as blood volume per time. c | Wall shear stress, which is high in short AV connections (arrows). d | Upstream conducted signal levels are high in the feeding arterioles and low in the adjacent short AV connections (arrows). The AV connections remain narrow and do not enlarge to form functional shunts.
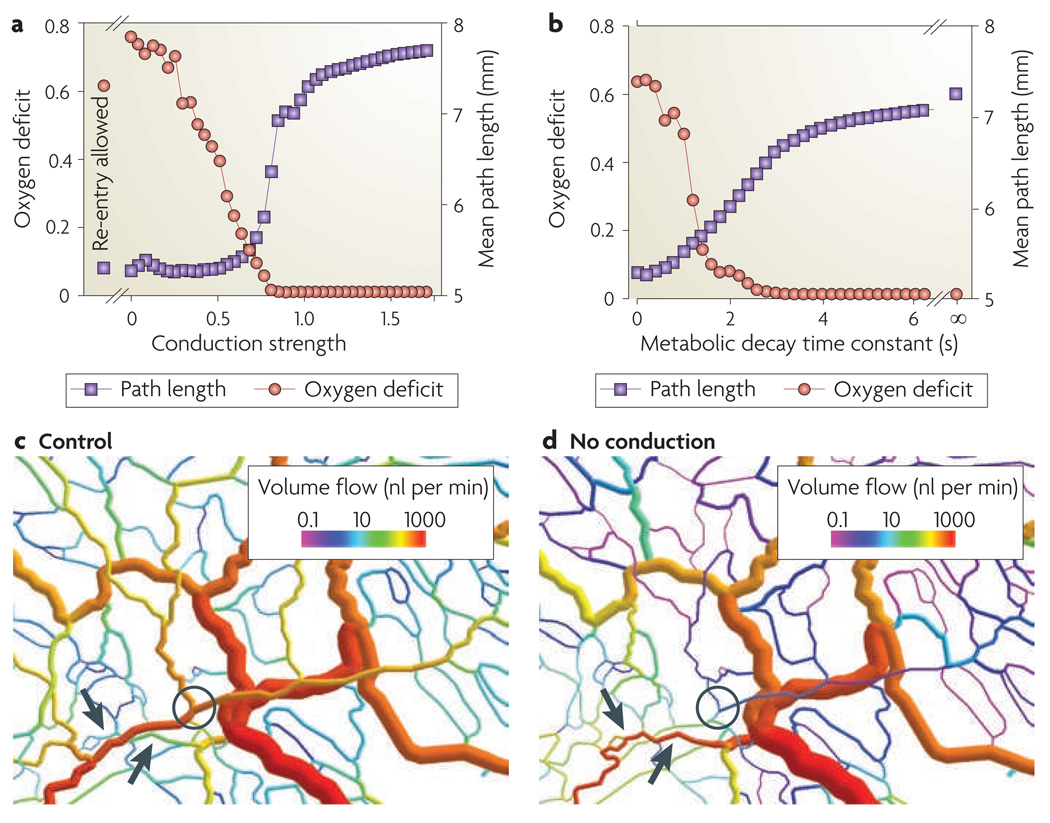
The consequences of changes in information transfer characteristics were simulated using this model. FIGURE 5 shows the effects of changes in the conduction strength constant, with an arbitrary reference value of 1, and the decay time of the metabolic signalling substance, which determines the strength of downstream information transfer. Reducing the conduction strength below a critical value (~0.8) leads to functional shunting, as shown by the reduction in the flow-weighted mean path length for blood travelling through the microvascular network. As a consequence, more distal tissue regions are under-perfused and a substantial oxygen deficit develops. A similar level of oxygen deficit is observed if re-entry of the conducted signal into distal vessels is allowed. The effects of reduced signalling to draining venules by convection (achieved by shortening the decay time of the metabolic signalling substance) are similar but milder, because the resistance to blood flow is lower on the venous side and has less of an impact on flow distribution.
Figure 5. Effects of altered information transfer on predicted network function.
a | The effect of altered upstream information transfer. Conduction strength is given as the relative sensitivity of diameter adaptation to upstream conducted response signals. The oxygen deficit is the length of the vessels that are depleted of oxygen as a fraction of the total vessel length. The mean path length is the flow-weighted mean of all blood flow pathway lengths in the network. Low mean path length values indicate the presence of functional shunting. As conduction strength decreases, so mean path length decreases and the oxygen deficit increases, showing that effective information transfer in the upstream direction, without re-entry of upstream signals, is necessary to avoid functional shunting and an oxygen deficit. Points labelled ‘Re-entry allowed’ are the results obtained when conduction strength equals 1 and re-entry of upstream signal into distal vessels is allowed. b | The effect of altered downstream information transfer. The metabolic decay time constant is the time constant in seconds (s) for the decay or uptake of those metabolites that are responsible for downstream information transfer. As the decay time constant decreases, so mean path length decreases and the oxygen deficit increases, showing that information transfer in the downstream direction is required to avoid functional shunting. c | The distribution of volume flows in a network with normal upstream conducted responses. Arrows show short arterio-venous connections with low flow rates and that do not form functional shunts. The circle indicates that the main arteriole supplying the distal region to the right has a high flow rate. d | The distribution of volume flows in the network in the absence of upstream conducted responses, showing shrinkage and low flow in the main arteriole (circle) supplying the distal region to the right and the enlargement of previously very small capillary segments into a high-flow functional shunt (arrows).
In summary, these simulations show that the absence of anti-shunt mechanisms, especially the upstream conduction of signals along arterial vessels, can lead to the development of functional shunting, such that a large proportion of the blood flow passes through short flow pathways while other regions of the tissue are inadequately supplied. Under these conditions, a high average rate of perfusion may coexist with substantial hypoxia.
In vivo evidence of functional shunting
On the basis of the results from the simulations described above, it is justified to consider experimental or clinical evidence for functional shunting. In situations of shock or ischaemia–reperfusion, functional shunting and maldistribution of the microcirculation are increasingly identified as relevant pathophysiological components and predictive parameters, on the basis of both the measurement of global parameters (such as the oxygen extraction ratio) and intravital microscopic observation of the microcirculation22,52–54. These findings point to the importance of local flow regulation in preventing functional shunting. However, the mechanisms underlying microvascular shunting in these acute events have not been fully elucidated, and the connection to conducted signals remains to be established.
Indications with respect to the possible involvement of gap junctions in the structural adaptation of blood vessels are sparse and derive from studies focused on embryonic vascular development in mice lacking one or more of the vascular connexins Cx37, Cx40, Cx43 and Cx45 (REFS 55–57). Cx45-deficient mice exhibit a vascular phenotype in which initial vessels generated by vasculogenesis fail to develop into appropriately sized mature vascular networks55. According to the concept presented in FIG. 3b, the effect of Cx45 on the maturation of vascular networks during early development may involve its asymmetrical gating characteristics in heterotypic gap junctions47.
Although mice lacking Cx37 or Cx40 do not exhibit notable vascular phenotypes during development, animals with a complete deficiency of both connexins die perinatally, exhibiting severe vascular dysmorphogenesis, haemorrhages and congestion56,57. The described vascular morphology is reminiscent of those seen clinically in vascular anomalies or malformations. For these conditions, several genetic alterations and molecular pathways have been discussed, and some of the mechanisms involved, including those involving Sox genes and Notch–delta-like protein 4 (DLL4) signalling, were shown to be associated with the development of arterial identity58–60. In the vitel-line membrane of the chicken embryo, the expression of Cx40 was recently shown to be one of the earliest and most consistent markers of arterial vessels61. These observations provide further evidence that communication through gap junctions is a crucial component in the development and maintenance of normal vascular structures.
Relevance to tumours
Recently, computational modelling was used to investigate the possible causes for the aberrant structure of tumour microvascular networks, which have high levels of structural and functional heterogeneity51. A near-complete loss of vascular diameter control by conducted signals was identified as the main alteration in structural vascular adaptation. Together with an increased randomness of vascular reaction patterns and a generally higher tendency to grow, this could explain the observed phenotype of tumour microvasculature. In simulations of network adaptation with modified vascular responses, as described above, it was predicted that multiple capillary-type vessels would convert into functional shunts, resulting in flow maldistribution and peripheral under-perfusion (FIG. 6).
Figure 6. Simulated tumour-like structural adaptation.
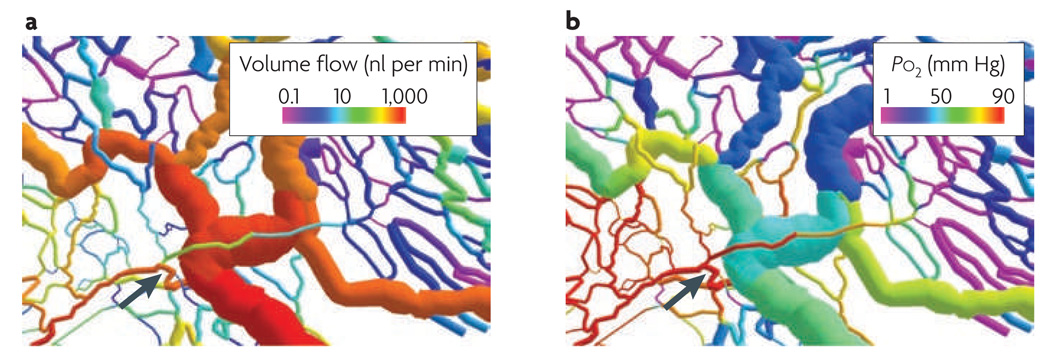
A computer-generated visualization of a microvascular network in rat mesentery, showing a simulated structural adaptation of vessel diameters according to parameters that are typical of tumour networks, including a strongly reduced strength of upstream conducted signals and an increased overall vessel growth tendency. Data are shown for volume flow rate (part a) and intravascular oxygen partial pressure (Po2; part b). The formation of a functional arterio-venous shunt (arrow) leads to decreased flow in the more distal vessels (to the right of the arrow) and, consequently, to low oxygen levels in many vessels.
Few, but partially contradicting, data are available on the differential expression and function of vascular connexins in tumours, perhaps because the corresponding genes are not generally considered to be relevant for angiogenesis and are not included in many studies on this topic62–64. A central part may be played by the tumour microenvironment, which has higher levels of reactive oxygen species (ROS; including H2O2) than normal tissues65,66. Tumours are notoriously hypoxic and, at the same time, subject to increased levels of oxidative and nitrosative stress, and these factors may interfere with the functioning of gap junctions. Hypoxia causes dephosphorylation of Cx43 at Ser365, resulting in the loss of Cx43 function in gap junctions of the myocardium67. Conversely, H2O2 has been reported to cause hyperphosphorylation of Cx43, leading to loss of gap junction function in liver cells68. Vascular endothelial growth factor A (VEGFA) levels are elevated in most solid tumours9, and exposure of endothelial cells to VEGFA in vitro was shown to cause a transient loss of endothelial cell–cell communication69. This effect correlated with changes in the phosphorylation of Cx43. The overexpression of VEGFA in tumours is therefore another potential cause for impaired communication through gap junctions between endothelial cells in vivo.
In this context, it should be noted that the functionality of an anti-shunt mechanism involving conductive information transfer does not necessarily correspond directly to the level of connexin expression or phosphorylation. The ability to suppress functional shunting could be equally compromised by merely disabling the mechanism that prevents re-entry of the signal into downstream vessels at divergent arterial branch points (FIG. 5). Not only the level of connexin expression but also the configuration of gap junctions in vessel segments and at branch points may therefore have major effects on the functional behaviour of the resulting vascular networks.
These considerations support the hypothesis that the deviant properties of tumour microcirculation largely result from disruption of the conducted responses that transmit signals along vessel walls. Anti-angiogenesis therapy that inhibits tumour VEGFA–VEGF receptor (VEGFR) signalling8,9,11,70–72 may restore conducted responses and, therefore, the anti-shunt mechanisms that are present in normal vascular networks, thereby leading to a reduction in functional shunting. This mechanism may underlie the reported normalization of tumour circulation that is caused by the use of anti-angiogenic drugs, leading to improved tumour oxygenation and chemotherapy drug delivery9. Further studies of the expression and function of vascular connexins in tumours are needed to provide in vivo evidence for this hypothesis.
Conclusions
The following conclusions can be drawn from the reasoning and results presented here. Robust anti-shunt mechanisms are needed for the development and maintenance of efficient and functional microvascular networks. Anti-shunt mechanisms require the transfer of information from distal to proximal vessels of both arterial and venous trees. Upstream information transfer is based on the conduction of signals through gap junction channels in the vascular wall. The failure of this mechanism leads to maldistribution of the blood flow and to vascular abnormalities. In tumours, the anti-shunt mechanisms are ineffective, resulting in vascular aberration, heterogeneous distribution of blood flow and local hypoxia, as well as resistance to radiation therapy and some types of chemotherapy. Therefore, improving the function of gap junction channels in tumour-associated endothelial cells and the tumour microvasculature is a potential approach for improving or normalizing the tumour circulation to improve the effectiveness of other chemotherapy drugs.
Many points remain to be investigated. For example, the biological mechanisms that prevent the re-entry of conducted signals into distal vessels are not known. The relationship between tumour development and the impairment of anti-shunt mechanisms is not understood. The effect of genetic variations on the gap junctions that are involved in structural adaptation and their potential effect on the growth of the tumour vasculature should be assessed. Finally, the development of treatment modalities that interfere (positively or negatively) with the anti-shunt mechanisms is still an open challenge.
Acknowledgements
This work was supported by the US National Institutes of Health (NIH) grants CA040355 and HL034555. The authors thank B. Reglin for stimulating discussions and for help with figures 4–6.
Glossary
- Conducted response
A signal that is propagated along the wall of a blood vessel through the gap junctions that connect adjacent endothelial and/or smooth muscle cells. In a conducted vasomotor response, a stimulus applied at one point on a vessel elicits a contraction or dilation at another point. Some studies show that conducted responses can propagate for several millimetres without appreciable decay, implying that they regenerate themselves in a manner analogous to that used by nerve impulses.
- Distal
Further from the heart. Capillaries are the most distal vessels.
- Gap junction
A molecular structure that forms a passage connecting the cytoplasm of two adjacent cells and selectively allows solutes to pass between the cells. A gap junction consists of two linked hemichannels, called connexons, each embedded in the membrane of one of the cells. Each hemichannel consists of six connexins, which are proteins with multiple membrane-spanning domains.
- Proximal
Closer to the heart — that is, upstream in arterial vessels and downstream in venous vessels.
- Structural adaptation
Also known as vascular remodelling. Long-term changes in the structure of blood vessels (such as their diameter and wall mass) that occur during growth, in response to changing functional needs and in various disease states.
- Wall shear stress
The mechanical tangential force per unit area that is exerted on the walls of blood vessels by flowing blood as a consequence of the blood viscosity. It is directed parallel to the surface of the wall in the direction of flow and is transmitted to the endothelial cells, which can respond to changes in wall shear stress. In a cylindrical microvessel, wall shear stress is given by the formula τ = (D/4) × (ΔP/L), where ΔP is the change in pressure, L is the length of the vessel, and D is the diameter of the vessel.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Axel R. Pries’s homepage: http://www.charite.de/ccr/site/html/en/ag_pries.html
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Axel R. Pries, Department of Physiology and the Centre for Cardiovascular Research, Charité Berlin, Thielallee 71, D-14195 Berlin, Germany. Deutsches Herzzentrum Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany.
Michael Höpfner, Department of Physiology and the Centre for Cardiovascular Research, Charité Berlin, Thielallee 71, D-14195 Berlin, Germany..
Ferdinand le Noble, Max-Delbrück-Centrum für Molekulare Medizin, D-13125 Berlin-Buch, Germany..
Mark W. Dewhirst, Department of Radiation Oncology, Duke University Medical Center, Durham, North Carolina 27710, USA.
Timothy W. Secomb, Department of Physiology, University of Arizona, Tucson, Arizona 85724, USA.
References
- 1.Duling BR, Damon DH. An examination of the measurement of flow heterogeneity in striated muscle. Circ. Res. 1987;60:1–13. doi: 10.1161/01.res.60.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Decking UK. Spatial heterogeneity in the heart: recent insights and open questions. News Physiol. Sci. 2002;17:246–250. doi: 10.1152/nips.01393.2002. [DOI] [PubMed] [Google Scholar]
- 3.Pries AR, Secomb TW, Gaehtgens P. Structure and hemodynamics of microvascular networks: heterogeneity and correlations. Am. J. Physiol. 1995;269:H1713–H1722. doi: 10.1152/ajpheart.1995.269.5.H1713. [DOI] [PubMed] [Google Scholar]
- 4.Pries AR, Secomb TW. Origins of heterogeneity in tissue perfusion and metabolism. Cardiovasc. Res. 2009;81:328–335. doi: 10.1093/cvr/cvn318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 6.Gewirtz H, Tawakol A, Bacharach SL. Heterogeneity of myocardial blood flow and metabolism: review of physiologic principles and implications for radionuclide imaging of the heart. J. Nucl. Cardiol. 2002;9:534–541. doi: 10.1067/mnc.2002.125916. [DOI] [PubMed] [Google Scholar]
- 7.Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. Circ. Res. 1989;65:578–590. doi: 10.1161/01.res.65.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nature Rev. Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 10.Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520–11539. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- 11.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature Rev. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 13.Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension. 2005;46:726–731. doi: 10.1161/01.HYP.0000184428.16429.be. [DOI] [PubMed] [Google Scholar]
- 14.Zakrzewicz A, Secomb TW, Pries AR. Angioadaptation: keeping the vascular system in shape. News Physiol. Sci. 2002;17:197–201. doi: 10.1152/nips.01395.2001. [DOI] [PubMed] [Google Scholar]
- 15.Dzau VJ, Gibbons GH. Vascular remodeling: mechanisms and implications. J. Cardiovasc. Pharmacol. 1993;21:S1–S5. [PubMed] [Google Scholar]
- 16.Pries AR, Reglin B, Secomb TW. Structural adaptation of microvascular networks: functional roles of adaptive responses. Am. J. Physiol. 2001;281:H1015–H1025. doi: 10.1152/ajpheart.2001.281.3.H1015. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya A, Ando J, Shibata M, Masuda H. Roles of fluid shear stress in physiological regulation of vascular structure and function. Biorheology. 1988;25:271–278. doi: 10.3233/bir-1988-251-236. [DOI] [PubMed] [Google Scholar]
- 18.Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 19.Langille BL. Arterial remodeling: relation to hemodynamics. Can. J. Physiol. Pharmacol. 1996;74:834–841. [PubMed] [Google Scholar]
- 20.Rodbard S. Vascular caliber. Cardiology. 1975;60:4–49. doi: 10.1159/000169701. [DOI] [PubMed] [Google Scholar]
- 21.Pries AR, Secomb TW, Gaehtgens P. Structural adaptation and stability of microvascular networks: theory and simulations. Am. J. Physiol. 1998;275:H349–H360. doi: 10.1152/ajpheart.1998.275.2.H349. [DOI] [PubMed] [Google Scholar]
- 22.Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H156–H164. doi: 10.1152/ajpheart.2002.282.1.H156. [DOI] [PubMed] [Google Scholar]
- 23.Pries AR, Reglin B, Secomb TW. Structural response of microcirculatory networks to changes in demand: information transfer by shear stress. Am. J. Physiol. 2003;284:H2204–H2212. doi: 10.1152/ajpheart.00757.2002. [DOI] [PubMed] [Google Scholar]
- 24.Berne RM, Knabb RM, Ely SW, Rubio R. Adenosine in the local regulation of blood flow: a brief overview. Fed. Proc. 1983;42:3136–3142. [PubMed] [Google Scholar]
- 25.Arciero JC, Carlson BE, Secomb TW. Theoretical model of metabolic blood flow regulation: roles of ATP release by red blood cells and conducted responses. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1562–H1567. doi: 10.1152/ajpheart.00261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med. Sci. Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- 27.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 28.Stamler JS, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 29.Bakker EN, et al. Small artery remodeling depends on tissue-type transglutaminase. Circ. Res. 2005;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- 30.Bakker EN, et al. Inward remodeling follows chronic vasoconstriction in isolated resistance arteries. J. Vasc. Res. 2002;39:12–20. doi: 10.1159/000048989. [DOI] [PubMed] [Google Scholar]
- 31.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- 32.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Wit C, Wolfle SE, Hopfl B. Connexin-dependent communication within the vascular wall: contribution to the control of arteriolar diameter. Adv. Cardiol. 2006;42:268–283. doi: 10.1159/000092575. [DOI] [PubMed] [Google Scholar]
- 34.Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid. Redox Signal. 2009;11:267–282. doi: 10.1089/ars.2008.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am. J. Physiol. 1995;268:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone S, Isakson B, Locke D. Biological and biophysical properties of vascular connexin channels. Int. Rev. Cell Mol. Biol. 2009;278:69–118. doi: 10.1016/S1937-6448(09)78002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfle SE, et al. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ. Res. 2007;101:1292–1299. doi: 10.1161/CIRCRESAHA.107.163279. [DOI] [PubMed] [Google Scholar]
- 38.de Wit C, Roos F, Bolz SS, Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol. Genomics. 2003;13:169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- 39.Figueroa XF, et al. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ. Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- 40.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc. Natl Acad. Sci. USA. 2001;98:9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Wit C. Different pathways with distinct properties conduct dilations in the microcirculation in vivo. Cardiovasc. Res. 2010;85:604–613. doi: 10.1093/cvr/cvp340. [DOI] [PubMed] [Google Scholar]
- 42.Heyman NS, Kurjiaka DT, Ek Vitorin JF, Burt JM. Regulation of gap junctional charge selectivity in cells coexpressing connexin 40 and connexin 43. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H450–H459. doi: 10.1152/ajpheart.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bukauskas FF, Angele AB, Verselis VK, Bennett MV. Coupling asymmetry of heterotypic connexin 45/ connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms. Proc. Natl Acad. Sci. USA. 2002;99:7113–7118. doi: 10.1073/pnas.032062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cottrell GT, Burt JM. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am. J. Physiol. Cell Physiol. 2001;281:C1559–C1567. doi: 10.1152/ajpcell.2001.281.5.C1559. [DOI] [PubMed] [Google Scholar]
- 45.Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/ heterotypic gap junction channel properties. Am. J. Physiol. Cell Physiol. 2002;282:C1469–C1482. doi: 10.1152/ajpcell.00484.2001. [DOI] [PubMed] [Google Scholar]
- 46.Cottrell GT, Burt JM. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim. Biophys. Acta. 2005;1711:126–141. doi: 10.1016/j.bbamem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Rackauskas M, et al. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys. J. 2007;92:1952–1965. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebong EE, Kim S, DePaola N. Flow regulates intercellular communication in HAEC by assembling functional Cx40 and Cx37 gap junctional channels. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2015–H2023. doi: 10.1152/ajpheart.00204.2005. [DOI] [PubMed] [Google Scholar]
- 49.Theis M, et al. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29:1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 50.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ. Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 51.Pries AR, et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Comput. Biol. 2009;5 doi: 10.1371/journal.pcbi.1000394. e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ince C. The microcirculation is the motor of sepsis. Crit. Care. 2005;9 Suppl. 4:S13–S19. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyml K, Li F, Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit. Care Med. 2005;33:1823–1828. doi: 10.1097/01.ccm.0000172548.34622.de. [DOI] [PubMed] [Google Scholar]
- 54.Lauterbach M, et al. Shunting of the microcirculation after mesenteric ischemia and reperfusion is a function of ischemia time and increases mortality. Microcirculation. 2006;13:411–422. doi: 10.1080/10739680600746032. [DOI] [PubMed] [Google Scholar]
- 55.Kruger O, et al. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 56.Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev. Biol. 2002;251:206–220. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- 57.Simon AM, McWhorter AR. Role of connexin37 and connexin40 in vascular development. Cell Commun. Adhes. 2003;10:379–385. doi: 10.1080/cac.10.4-6.379.385. [DOI] [PubMed] [Google Scholar]
- 58.Limaye N, Boon LM, Vikkula M. From germline towards somatic mutations in the pathophysiology of vascular anomalies. Hum. Mol. Genet. 2009;18:R65–R74. doi: 10.1093/hmg/ddp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brouillard P, Vikkula M. Genetic causes of vascular malformations. Hum. Mol. Genet. 2007;16:R140–R149. doi: 10.1093/hmg/ddm211. [DOI] [PubMed] [Google Scholar]
- 60.Tille JC, Pepper MS. Hereditary vascular anomalies: new insights into their pathogenesis. Arterioscler. Thromb. Vasc. Biol. 2004;24:1578–1590. doi: 10.1161/01.ATV.0000137390.56554.df. [DOI] [PubMed] [Google Scholar]
- 61.Buschmann I, et al. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 2010;137:2187–2196. doi: 10.1242/dev.045351. [DOI] [PubMed] [Google Scholar]
- 62.Elzarrad MK, et al. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008;6:20. doi: 10.1186/1741-7015-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Beijnum JR, et al. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–2348. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- 64.Seaman S, et al. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nebert DW, Mason HS. An electron spin resonance study of neoplasms. Cancer Res. 1963;23:833–840. [PubMed] [Google Scholar]
- 66.Chen CN, Hsieh FJ, Cheng YM, Chang KJ, Lee PH. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in angiogenesis and clinical outcome of human gastric cancer. J. Surg. Oncol. 2006;94:226–233. doi: 10.1002/jso.20372. [DOI] [PubMed] [Google Scholar]
- 67.Solan JL, et al. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J. Cell Biol. 2007;179:1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Upham BL, Kang KS, Cho HY, Trosko JE. Hydrogen peroxide inhibits gap junctional intercellular communication in glutathione sufficient but not glutathione deficient cells. Carcinogenesis. 1997;18:37–42. doi: 10.1093/carcin/18.1.37. [DOI] [PubMed] [Google Scholar]
- 69.Suarez S, Ballmer-Hofer K. VEGF transiently disrupts gap junctional communication in endothelial cells. J. Cell Sci. 2001;114:1229–1235. doi: 10.1242/jcs.114.6.1229. [DOI] [PubMed] [Google Scholar]
- 70.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc. Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jain RK, Finn AV, Kolodgie FD, Gold HK, Virmani R. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nature Clin. Pract. Cardiovasc. Med. 2007;4:491–502. doi: 10.1038/ncpcardio0979. [DOI] [PubMed] [Google Scholar]
- 72.Zhong H, Bowen JP. Antiangiogenesis drug design: multiple pathways targeting tumor vasculature. Curr. Med. Chem. 2006;13:849–862. doi: 10.2174/092986706776361085. [DOI] [PubMed] [Google Scholar]