Abstract
The uptake, biosynthesis and catabolism of polyamines in the microsporidian parasite Encephalitozoon cuniculi are detailed with reference to the effects of oligoamine and arylamine analogues of polyamines. Enc. cuniculi, an intracellular parasite of mammalian cells, has both biosynthetic and catabolic enzymes of polyamine metabolism, as demonstrated in cell-free extracts of mature spores. The uptake of polyamines was measured in immature, pre-emergent spores isolated from host cells by Percoll gradient. Spermine was rapidly taken up and metabolized to spermidine and an unknown, possibly acetamidopropanal, by spermidine/spermine N1-acetyltransferase (SSAT) and polyamine oxidase (PAO). Most of the spermidine and the unknown product were found in the cell incubation medium, indicating they were released from the cell. bis(Ethyl) oligoamine analogues of polyamines, such as SL-11144 and SL-11158, as well as arylamine analogues [BW-1, a bis(phenylbenzyl) 3-7-3 analogue] blocked uptake and interconversion of spermine at micromolar levels and, in the case of BW-1, acted as substrate for PAO. The Enc. cuniculi PAO activity differed from that found in mammalian cells with respect to pH optimum, substrate specificity and sensitivity to known PAO inhibitors. SL-11158 inhibited SSAT activity with a mixed type of inhibition in which the analogue had a 70-fold higher affinity for the enzyme than the natural substrate, spermine. The interest in Enc. cuniculi polyamine metabolism and the biochemical effects of these polyamine analogues is warranted since they cure model infections of Enc. cuniculi in mice and are potential candidates for human clinical trials.
INTRODUCTION
The microsporidia are a group of obligate intracellular parasites of the phylum Microspora. With over 144 genera and 1200 species, these organisms parasitize a wide range of hosts from insects to fish, non-primate mammals and man (Wittner & Weiss, 1999). Microsporidia are true eukaryotes containing a nucleus, nuclear membrane, intracytoplasmic membrane system, a Golgi apparatus, and chromosome separation on mitotic spindles. No mitochondria or centrioles are present. Microsporidia form characteristic unicellular spores which extrude a polar tube through which sporoplasm is passed to inoculate a host cell (Wittner & Weiss, 1999). Little is known concerning the metabolism of the microsporidia, except for a few studies identifying glycolytic enzymes and metabolites which are excystment requirements (Weidner et al., 1999). Molecular studies have recently characterized RNA and the full genome sequence of Encephalitozoon (Enc.) cuniculi is now known (Wittner & Weiss, 1999; Katinka et al., 2001).
The microsporidia have become increasingly important as human pathogens in AIDS. There are approximately eight genera with species proven to be pathogenic in humans. Enterocytozoon (Ent.) bienusi, an enteric parasite which causes intractable diarrhoea in AIDS, is the most common species (Wittner & Weiss, 1999; Weber et al., 1994).
Chemotherapy for microsporidia infections has relied on the benzimidazoles and fumagillin, which are generally effective with exceptions: albendazole is not effective versus Ent. bienusi and fumagillin has cytotoxic effects, especially in AIDS patients (Didier, 1997; Costa & Weiss, 2000; Dieterich et al., 1994; Weiss et al., 1994a; Katiyar et al., 1994).
With this as background, our work has focused on polyamine metabolism in the microsporidia using Enc. cuniculi as model organism (Bacchi et al., 2001). Polyamines are low-molecular-mass polycations, found universally in cells, which function as cofactors for macromolecule synthesis, in osmotic control, and as structural facilitators for nucleic acids (Cohen, 1998). The major polyamines are putrescine, spermidine and spermine. Polyamines are absolutely required for cell division and differentiation and their internal concentrations are carefully controlled by synthesis, degradation, excretion and uptake from the environment. In mammals and many protozoa, synthesis occurs from the conversion of ornithine to putrescine through ornithine decarboxylase (ODC) (Bacchi & Yarlett, 2002), although the enteric parasite Cryptosporidium parvum has a plant-like pathway in which arginine is the starting point for putrescine synthesis through arginine decarboxylase (Keithly et al., 1997). One or two aminopropyl groups, originating from decarboxylated S-adenosylmethionine, are added to putrescine to form spermidine and spermine, respectively. The limiting enzymes of polyamine synthesis are ODC and S-adenosylmethionine decarboxylase (AdoMetdc), both of which are highly inducible and have short half-lives in the cell (Marton & Pegg, 1995). Mammalian cells, as well as some protozoa, take up spermine and spermidine, interconverting them to spermidine and putrescine, respectively. The key enzymes in interconversion are spermidine/spermine N1-acetyltransferase (SSAT) and polyamine oxidase (PAO). SSAT is also highly inducible by exogenous polyamines, and the trio of ODC, AdoMetdc and SSAT serves to keep intracellular polyamines at relatively stable levels (Marton & Pegg, 1995).
Chemotherapeutic interest in the polyamine pathway was initially spurred by the development of enzyme-activated inhibitors of ODC [dl-α-difluoromethylornithine hydrochloride (DFMO)] and AdoMetdc (MDL 73811, AbeAdo; Bacchi & Yarlett, 2002). Although these were not effective clinically as anti-tumour agents, DFMO found use clinically against African sleeping sickness (Schechter & Sjoerdsma, 1989) and AbeAdo in experimental trypanosome infections (Bitonti et al., 1990). More recent attention, however, has focused on polyamine analogues such as bis(ethyl)norspermine (BE 3-3-3) and bis(ethyl)homospermine (BE 4-4-4) as anti-tumour agents. These agents are taken into mammalian cells on polyamine transporters and down-regulate ODC and AdoMetdc, while up-regulating SSAT. The net result is that polyamine synthesis is shut down while remaining intracellular polyamines are acetylated and excreted. The resulting decline in polyamine levels causes apoptosis and cell death, since the analogues do not substitute functionally for natural polyamines (Marton & Pegg, 1995; Casero & Woster, 2001; Frydman & Valasinas, 1999; Bernacki et al., 1995).
Our recent work has demonstrated that several classes of polyamine analogues block in vitro growth of Enc. cuniculi and sterilize host monolayers at micromolar levels. These agents also cured well-validated laboratory model infections of Enc. cuniculi (Zou et al., 2001; Bacchi et al., 2002). In the present study, we examine these analogues for effects on polyamine metabolism in Enc. cuniculi.
METHODS
Strain and growth conditions
Enc. cuniculi was obtained from Dr Ann Cali of Rutgers University. It was cultivated in RK-13 (rabbit kidney) or HEK (human embryonic kidney, ATCC CRL-1573) cells. Cells were grown to 80% confluency in Falcon T-75 flasks (Becton-Dickinson) at 37 °C in 5% CO2/air, and infected with 5 × 105 spores. Infected monolayers were also trypsinized and split into additional flasks. After 1 week of culture, greater than 50% infectivity was obtained. The medium used was Minimal Eagle’s Medium, Earle’s salts, l-glutamine, 7% (w/v) fetal bovine or newborn calf serum (Visvesvara et al., 1991). The medium was changed twice (RK-15 cells) or three times (HEK cells) weekly, and the old medium containing mature spores was pooled and aseptically stored at 4 °C.
Enzyme preparations
Intact mature spores were obtained from supernatant culture media and stored aseptically at 0–4 °C (Bacchi et al., 2001). Spores were washed in 150 mM PBS [0·9% (w/v) NaCl] containing 1% (w/v) SDS and resuspended in PBS prior to breaking. Spores were poured into 2 ml microfuge tubes containing 425–600 nm glass beads (Sigma) and disrupted (6–8 min at 4 °C) using a Mini Bead Beater (Bio Spec Products) or Vortex Mixer and adapter (#13000-50-V1; Mo Bio Laboratories) (Weiss et al., 1994b). Homogenate preparations with greater than 90% breakage (by microscopy) were collected and cleared by centrifugation at 2200 g for 10 min. The cleared supernatants were used to determine SSAT and PAO activity.
Gradient purification of pre-emergent spores
The large-scale gradient procedure described previously (Bacchi et al., 2001) was used to obtain purified preparations of pre-emergent spores. Usually 10 heavily infected (50–80 %) monolayers from Falcon T75 flasks were trypsinized, harvested and ground in a glass vessel for 6–8 min (3–4 × 2 min grinding) at 0–4 °C. The breakage medium contained W-T medium (described below under ‘Incubation studies using pre-emergent spores’) plus a protease inhibitor cocktail containing 2·7 mM Nα-p-tosyl-l-lysine chloromethyl ketone hydrochloride (TLCK), 0·15 mM aprotinin, 2·2 mM leupeptin and 0·5 mM DTT in 50 mM KCl/0·15 M Na3PO4, pH 7·4. Cell homogenates were passed through 12 µm and 5 µm filters and the final filtrate was centrifuged for 10 min at 15 000 g on 50% (v/v) stock isotonic Percoll (1 ml/11 ml Beckman 14 × 89 mm tube; Green et al., 1999). Two bands were obtained, a broad one at 1·018–1·035 g ml−1 (light band) and a narrower one at 1·102–1·119 g ml−1 (heavy band). We had demonstrated from the previous study that the heavy band contained pre-emergent spores and immature spores while the light band contained debris, spore cases and some immature stages (Bacchi et al., 2001). Since the heavy fraction exhibited far greater metabolic activity than the light fraction (Bacchi et al., 2001), we used it for the data presented in the present study. Cell counts of suspensions in several experiments prior to and post-incubation indicated that no lysis of these forms had occurred.
Polyamine analysis
Polyamine content was quantified by reversed-phase HPLC using a Perkin-Elmer LC-410 pump and a percosphere C18-5 µ 150×4·6 mm column (Perkin Elmer). Heavy gradient fractions (pre-emergent spores) were extracted with 10% (w/v) trichloroacetic acid (TCA) at 0–4 °C. Denatured protein was removed by centrifugation and extracts were pre-column derivatized with 0·8 g o-phthalaldehyde l−1 (2 : 1) dissolved in 3 ml methanol added to 30·9 g boric acid l−1 containing 24 g KOH l−1 (pH 10·4). The derivatized samples were quantified with a Perkin-Elmer LC 240 fluorescence detector using an excitation wavelength of 320 nm and an absorption wavelength of 455 nm. Signals were integrated using β-ram (IN/US Systems) Version 3.1 software. Concentrations of polyamines were determined relative to standards. The HPLC solvent gradient used and other details have been described previously (Yarlett et al., 1994).
Enzyme assays
All incubations were at 37 °C. SSAT was measured according to Libby et al. (1989) in a reaction containing 0·06 M Bicine buffer (pH 8·0), 0·15–6 mM spermine or spermidine, 0·1 µCi (0·5 nmol) [1-14C]acetyl-CoA and 50 µl spore enzyme preparation. Reactions were incubated for 30 min, stopped by addition of 20 µl 0·5 M hydroxylamine, and placed on ice. Reaction tubes were heated for 3 min in a boiling water bath and centrifuged to remove protein. Clarified reactions were spotted on P-81 phosphocellulose filters. Filters were washed with distilled water, then methanol, dried and counted. Activity is expressed as nmol (mg protein)−1 (30 min)−1.
PAO was measured spectrophotometrically (Beckman DU 640) according to the method of Childs & Bardsley (1975) in 0·1 M glycine buffer (pH 7·0) containing 10 units of horseradish peroxidase [Sigma Type VIA: 1 unit will oxidize 1 µmol 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) min−1 at 25 °C at pH 5·0], 5 mM ABTS and 22–220 µg Enc. cuniculi protein. Final volume was 1 ml. Assays (in duplicate) were started by addition of 1 mMN1-acetylspermine. Assays were linked to reduction of ABTS by peroxidase and measurement of oxidized product at 420 nm, using a six-compartment cell holder at 37 °C. The extinction coefficient of ABTS at 420 nm is E=3·6 × 104 M−1 cm−1 (Childs & Bardsley, 1975). Studies examining polyamine analogues as substrates of PAO had N1-acetylspermine left out of the reaction. Blanks containing protein but no N1-acetylspermine were run with each reaction, and subtracted from substrate runs. Initial velocity plots were obtained using Beckman kinetics Software (Beckman Instruments).
Incubation studies using pre-emergent spores
The effect of polyamine analogues on polyamine synthesis and interconversion was examined on intact pre-emergent spores. Heavy gradient preparations were incubated in W-T medium based on those employed by Weidner & Trager (1973) and Weidner et al. (1999) in their studies of Nosema excystation and containing 3 mM ATP, 1 mM GTP, 0·5 mM NAD+, 2 mM glucose 6-phosphate, 0·5 mM acetyl-CoA, 1 mM sodium pyruvate, 2 mM glucose in 8 mM NaCl, 138 mM Na3PO4, pH 6·8. We had previously used 150 mM Na3PO4/0·8% NaCl (Bacchi et al., 2001), but obtained better uptake and synthesis activity with W-T incubation medium.
Pre-emergent spores from heavy gradient fractions were incubated in the presence of 5 µCi (125 pmol) l-[2,3-3H]ornithine/4 nmol l-methionine for polyamine synthesis and 0·25 µCi (2·1 nmol) [1-14C]spermine for polyamine interconversion in suspensions containing a 250 µl aliquot of cell suspension (100–500 µg protein) in W-T medium. Polyamine analogues and radiolabelled substrates were added at the start of the 1 or 2 h incubation period. After incubation, preparations were centrifuged at 12 000 g for 10 min, and pellets were washed in incubation medium and lysed with 250 µl of 10% (w/v) TCA. Lysates were kept at 0–4 °C overnight and frozen until analysis. Supernatants from incubations were aspirated from pellets and frozen until analysis. All incubations were done in duplicate.
HPLC analysis
Lysates and supernatant incubation media from incubations of intact pre-emergent spores with l-[2,3-3H]ornithine or [1-14C]spermine were analysed by HPLC using procedures outlined previously (Bacchi et al., 2001). An IN/US flow-through radio-detector monitored radioactivity and the IN/US β-2 ram software package (IN/US Systems) was used for data peak integration. Using this system, we obtained the following retention times, based on radiolabelled standards: ornithine, 12 min; putrescine, 24 min; spermidine, 28 min; spermine, 31 min. Acetamidopropionaldehyde had a retention time of 3·5 min, based on UV absorption analysis.
Uptake studies
Pre-emergent spore preparations were incubated for 15 min in duplicate with varying concentrations of [1–4 terminal methylenes-14C]spermine, [terminal methylenes-3H(N)]spermidine [1,4-14C]putrescine or l-[2,3-3H]ornithine. The W-T incubation medium was used, with 0·2 ml reaction mixtures centrifuged through 0·2 ml mineral oil at 12 000 g for 0·5 min. Aqueous and oil layers were aspirated, and the tips of microfuge tubes were cut off and analysed by liquid scintillation counting in the presence of Beckman Ready Protein scintillation fluor (Beckman Instruments) as in Goldberg et al. (1998).
Protein determination
Protein content was estimated by the Bradford method, using BSA as standard.
Chemicals
Radiolabelled chemicals were purchased as follows: [1–4 terminal methylenes-14C]spermine (113 mCi mmol−1; 4·18 GBq mmol−1), Amersham Pharmacia Biotech; l-[2,3-3H]ornithine (40 mCi mmol−1; 1·48 GBq mmol−1), Moravek Biochemicals; [1-14C]acetyl-CoA (59 mCi mmol−1; 2·2 GBq mmol−1); [terminal methylenes-3H(N)]spermidine (25 mCi mol−1; 0·93 GBq mmol−1); [1,4-14C]putrescine (110 mCi mmol−1; 4·07 GBq mmol−1), NEN Life Science Products.
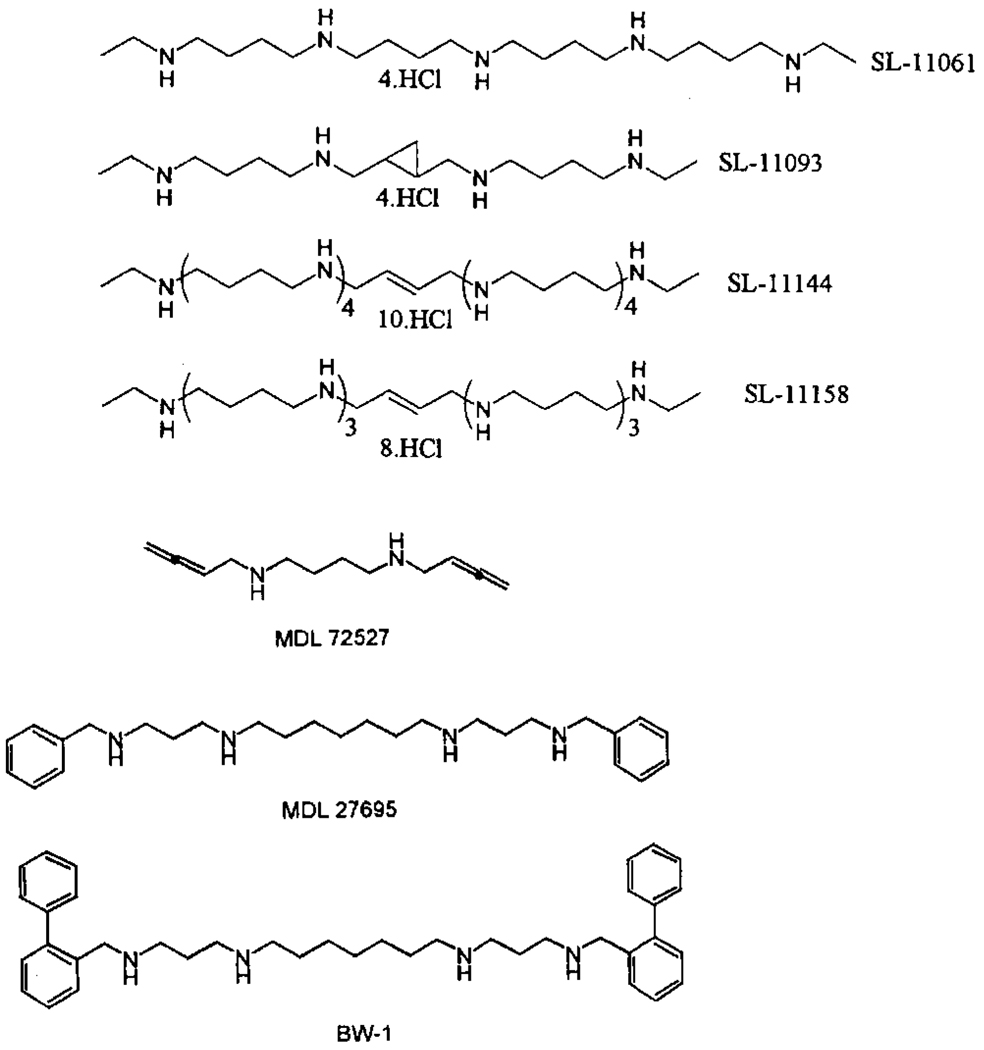
Polyamine analogues were obtained as follows: SL-11061 (BE 4×4), SL-11093, SL-11144, SL-11158, Dr Benjamin Frydman, SLIL Biomedical (synthesis: Valasinas et al., 2001; Bacchi et al., 2002); BW-1, Dr Patrick Woster, Wayne State University (synthesis: Zou et al., 2001). MDL 72521, MDL 72527, MDL 27695, DFMO and difluoromethylarginine were gifts from Marion Merrell Dow. Other chemicals were obtained from Sigma.
RESULTS
Polyamine content of pre-emergent spores
Heavy gradient fractions were analysed by HPLC and fluorescence detection for polyamine content after isolation and washing. Analysis of seven separate preparations yielded a mean of 38·15±27·8 nmol (mg protein)−1 for spermine and 23±15·8 nmol (mg protein)−1 for spermidine. Neither putrescine nor cadaverine were found using techniques sensitive to 50 pmol per sample (Yarlett et al., 1994). We had earlier reported polyamine content of mature, released spores to include low levels of putrescine as compared to spermidine and spermine (Coyle et al., 1996).
Enzymes of polyamine metabolism
Mature spores were broken using the beadbeater technique (Weiss et al., 1994b), clarified by centrifugation and, used as cell-free extracts, assayed for SSAT and PAO, enzymes of polyamine degradation. Results in Table 1 are compared to previously reported data for the synthetic enzymes ODC, SSAT and AdoMetdc (Bacchi et al., 2001). PAO is four times more active than AdoMetdc and >17-fold more active than ODC. Spermine was the preferred substrate for SSAT, while N1-acetylspermine was the most active substrate for PAO. Other activity/inhibitor data for SSAT and for PAO are given under ‘Effects of polyamine analogues on polyamine metabolism of pre-emergent spore preparations’. Arginine decarboxylase activity was less than 0·0036 nmol (mg protein)−1 (2 h)−1 – below the lower limit of detection for the assay. This activity was not inhibitable by difluoromethylarginine (DFMA), a specific inhibitor of the enzyme (Bitonti et al., 1982). Likewise, DFMA was not an inhibitor of ODC, while DFMO at 1 mM was 55% inhibitory (Bacchi et al., 2001).
Table 1. Activities of enzymes of polyamine metabolism in freshly isolated mature spores of Enc. cuniculi.
Spores were obtained aseptically from supernatants of infected HEK or RK-13 cells. Enzyme preparations were made using glass beads as described in Methods. Data are the mean of (n) 4–13 preparations, assayed in duplicate. Ranges are in parentheses. Data for ODC, arginine decarboxylase (ADC) and AdoMetdc were reported previously (Bacchi et al., 2001) and are included for comparative purposes.
| Enzyme | Substrate | n | Activity [nmol (mg protein)−1 (2 h)−1] |
|---|---|---|---|
| ODC | 1-[14C]Ornithine | 4 | 25·35 (18·6–32·1) |
| ADC | [U-14C]Arginine | 4 | <0·0036 |
| AdoMetdc | S[carboxyl-14C]AdoMet | 4 | 123·6 (28·6–242·3) |
| SSAT | [1-14C]Acetyl-CoA | 13 | 24·12 (5·6–52·8) |
| PAO | N1-Acetylspermine | 5 | 438·15 (81·02–818·3) |
Polyamine synthesis and uptake by heavy gradient fractions
We had initially examined polyamine metabolism in mature spores freshly isolated from supernatant culture media, but found this activity to be very low (Bacchi et al., 2001). Early studies on polyamine metabolism in pre-emergent spores used both heavy and light gradient fractions incubated in 150 mM Na3PO4-buffered 0·8% (w/v) NaCl, yielding wide-ranging results (Bacchi et al., 2001). Nevertheless, these data indicated that uptake and conversion of l-[2,3-3H]ornithine + 500 µM l-methionine to putrescine, spermidine and spermine occurred at about 12% of the rate of interconversion of [1-14C]spermine to spermidine and putrescine [55·4 nmol (mg protein)−1 (2 h)−1, 12 preparations, versus 444·8 nmol (mg protein)−1 (2 h)−1, 9 preparations, respectively; Bacchi et al., 2001].
We also examined uptake of polyamines in pre-emergent spores to compare rates of uptake. Table 2 lists Km and Vmax values of an experiment in which pre-emergent spores were examined in a rapid uptake study. Incubation mixtures (0·2 ml) containing increasing concentrations of radiolabelled ornithine, putrescine, spermidine and spermine were centrifuged through 0·2 ml mineral oil at 12 000 g. Incubations of 15 min were used in a procedure we have used for uptake studies with African trypanosomes and obtained negligible carry-over of exogenous label (Goldberg et al., 1998). Table 2 shows that, of the four molecules studied, spermine had the lowest Km value (12·5 nmol) and the highest Vmax [10 000 nmol (15 min)−1]. One gauge of the efficacy of transport is the Vmax/Km ratio. For spermine, this ratio was 800, which was >100-fold higher than ratios obtained with data from other substrates. Other preparations yielded similar results.
Table 2. Polyamine and amino acid uptake by Enc. cuniculi pre-emergent pores.
Organisms were harvested from HEK cells as described. The spore suspension (240 µg protein) was incubated in W-T medium for 15 min with 25–500 µM of [1-14C]spermine, [terminal methylenes-3H(N)]spermidine, [1,4-14C]putrescine or l-[2,3-3H]ornithine. Cells were centrifuged through 200 µl mineral oil; the supernatant medium was aspirated. Tips of tubes were cut off and counted in Beckman Ready Protein. Michaelis–Menten kinetics were used to analyse data.
| Substrate | Km (nmol) | Vmax [nmol (15 min)−1] | Vmax/Km |
|---|---|---|---|
| [1-14C]Spermine | 12·5 | 10 000 | 800 |
| [terminal methylenes-3H(N)]Spermidine | 90 | 660 | 7·3 |
| [1,4-14C]Putrescine | 285 | 2 220 | 7·7 |
| l-[2,3-3H]Ornithine | 250 | 1 810 | 7·2 |
In a series of experiments, we also examined the effects of various ionophores and ion channel reagents on spermine uptake. Used at 10–100 µM versus [1-14C]spermine uptake, the following agents had little (<10 %) or no effect over 15 min uptake (two preparations, duplicate determinations): gramicidin S, amiloride, nifedipine, nitrendipine, monensin. At 100 µM, valinomycin was 57% inhibitory (two determinations: range 10–100 µM) while KCN was 31% inhibitory and sodium azide was not inhibitory.
Effects of polyamine analogues on polyamine metabolism of pre-emergent spore preparations
In recent work, we had demonstrated that polyamine analogues having a backbone of repeating N-butyl subunits, such as pentamines, oligoamines or bis(aryl)-substituted 3-7-3 analogues (Fig. 1), sterilized Enc. cuniculi-infected monolayer cells and cured two murine model infections (Bacchi et al., 2002). We began mechanistic studies with some of the compounds that proved to be active in vivo, incubating pre-emergent spore preparations with 8 µM (0·25 µCi) [1-14C]spermine for 1 or 2 h, and examining the resulting 10% TCA extracts by HPLC and radiodetection for reduction in uptake and interference with spermine interconversion. In an initial series of three experiments, we found that 10 µM SL-11144 and SL-11158 inhibited spermine uptake by 66% (59–77 %) and 63% (32–89 %), respectively. With 0·25 µCi [terminal methylenes-3H(N)]-spermidine as substrate, SL-11144 also inhibited uptake 88·5% (single preparation) and SL-11158 58·8% (48·6–68·9%; two preparations) (data not shown).
Fig. 1.
Structures of polyamine analogues used in this study.
In the course of these studies, we found a peak appearing at 3–5 min, which did not correspond to known polyamine standards or N1-acetylspermine. The peak did, however, correspond to acetamidopropionaldehyde, a byproduct of polyamine interconversion which is excreted from cells (Marton & Pegg, 1995). The confirmation of the identity of this peak, however, awaits MS analysis.
In several experiments, we examined the effects of polyamine analogues on overall spermine uptake, and production of spermidine and acetamidopropionaldehyde. Table 3 gives results of a typical experiment (duplicate incubations) using the analogues SL-11061, SL-11158 and BW-1. In this experiment, we monitored internal (pellet) as well as external (supernatant medium) concentrations of spermine and products. All three analogues caused significant (70–80%) reduction in total spermine uptake, and higher inhibition of spermidine (82–95 %) and acetamidopropionaldehyde (76–92 %) production. SL-11061 had somewhat lower inhibition of total uptake (69·3%) as well as lower inhibition of spermidine (82·6%) and acetamidopropionaldehyde (76·3%) production. BW-1, although having a median effect on total uptake (73·4 %), inhibited production of both metabolites ≥90 %. Although little spermidine was found as excretion product, acetamidopropionaldehyde was present in control incubations, and was reduced in SL-11158 (20 %) and BW-1 (27 %) supernatants. If incubations continued for 2 h, supernatant media of analogue-treated cells had 3·2- to 11·5-fold more spermine remaining in the medium than controls incubated without analogues (not shown), indicating that less uptake was occurring in these cells.
Table 3. Effects of polyamine analogues on uptake and interconversion of [1-14C]spermine by Enc. cuniculi pre-emergent spores.
Preparations were incubated for 1 h in W-T medium with [1-14C]spermine. At 1 h, incubation mixtures were microfuged (12 000 g, 1 min) and the supernatant premium was aspirated and frozen. Pellets were extracted with 10% TCA overnight and frozen. Pellet extracts and supernatant medium were assayed by HPLC as described in Methods. Results are from duplicate incubations. Retention time values: unknown (possibly acetamidopropionaldehyde), 3–5 min; spermidine, 26 min; spermine, 30 min. All analogues were added at 10 µM.
| Preparation | Activity [nmol (mg protein)−1 (2 h)−1] |
|||
|---|---|---|---|---|
| Unknown | Spermidine | Spermine | Total | |
| Control pellet | 8·16 | 1·45 | 14·6 | 24·25 |
| +SL-11061 | 1·93 (−76·3%) | 0·25 (−82·6%) | 5·25 (−64 %) | 7·44 (−69·3%) |
| +SL-11158 | 0·58 (−92·8%) | 0·23 (−83·5%) | 3·71 (−74·5%) | 4·54 (−81·3%) |
| +BW-1 | 0·77 (−90·5%) | 0·06 (−95·6%) | 5·59 (−61·7%) | 6·43 (−73·4) |
| Control supernatant | 3·81 | – | 5·86 | 10·35 |
| +SL-11061 | 4·29 (+12·5%) | 0·37 | 5·03 (−14·1%) | 8·25 |
| +SL-11158 | 3·04 (−20·2%) | – | 3·96 (−32 %) | 5·33 |
| +BW-1 | 2·78 (−27 %) | – | 6·24 (+6·4%) | 4·11 |
Effects of SL-11158 on Enc. cuniculi SSAT
The key enzyme involved in polyamine interconversion is SSAT which in Enc. cuniculi has two to three times the activity with spermine rather than spermidine as substrate. In mammalian cells, SSAT has a short (15 min) t1/2 and is highly inducible (to 1000-fold, or 1% of total cell protein; Casero & Pegg, 1993).
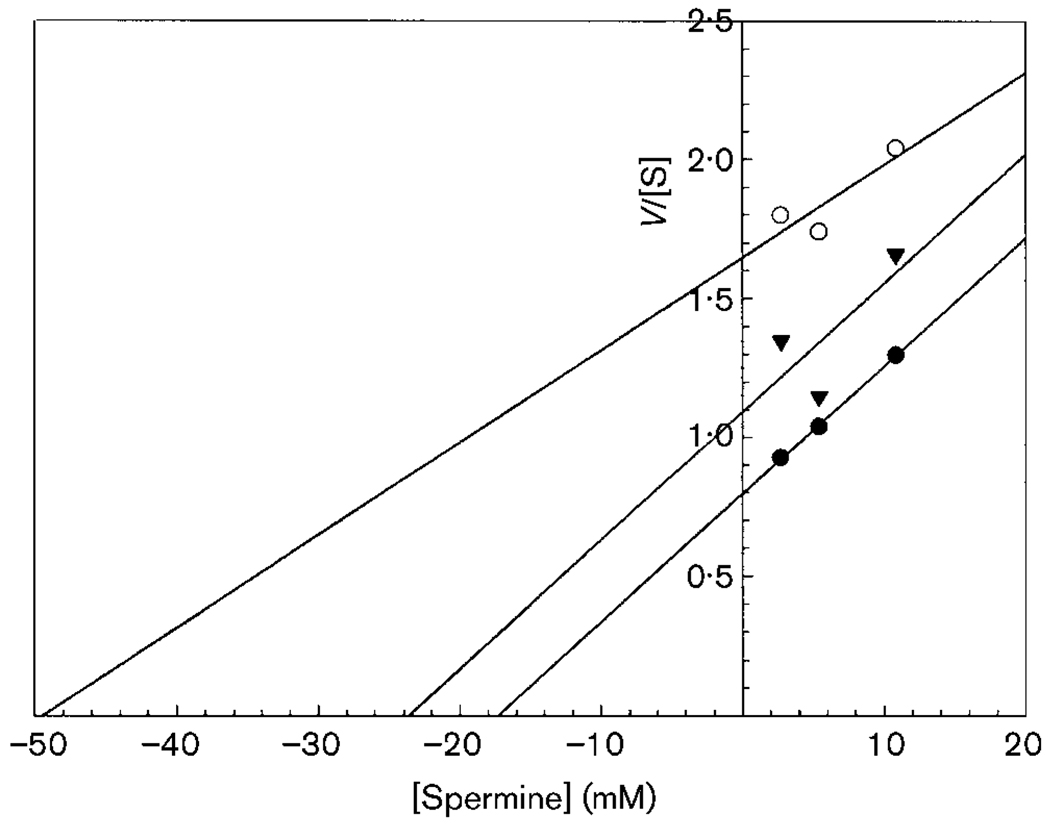
To determine the effect of SL-11158 on enzyme kinetics of Enc. cuniculi SSAT, we measured enzyme activity in the presence of none, 0·54 and 1·08 mM SL-11158 with increasing concentrations of spermine (2·7, 5·4 and 10·8 mM). Incubations contained 100 µM Bicine buffer, pH 8·0, 17 µM (60 µCi mmol−1) [1-14C]acetyl-CoA, inhibitor and spermine and 114 µg Enc. cuniculi spore protein preparation. After 30 min, reactions (in triplicate) were spotted on filter paper discs, washed and counted in Ominfluor. Blank reactions without spermine and protein were also run and subtracted. Results were analysed using a Hanes–Woolf primary plot (V/[S] versus substrate concentration). A Km value of 17·3 mM was obtained for spermine and a Ki value of 0·24 mM was obtained for SL-11158 from a secondary Hanes–Woolf plot of Km versus inhibitor concentration (Fig. 2). This is a mixed type of inhibition in which the inhibitor has an affinity for the enzyme which is much higher than that of the substrate.
Fig. 2.
Hanes–Woolf plot of Enc. cuniculi SSAT activity with spermine as substrate versus SL-11158 as inhibitor. Inhibitor concentrations: ●, none; ▼, 0·54 mM; ○, 1·08 mM. Spermine concentrations were 2·1, 5·4 and 10·8 mM. The non-converging lines show a mixed type of inhibition. The Km value for spermine was 17·3 mM and the Ki value of 0·24 mM was obtained for SL-11158. Reactions were in triplicate.
Inhibitor/substrate studies on Enc. cuniculi PAO
We examined a number of compounds as substrates or inhibitors of Enc. cuniculi PAO, and compared them to findings in the literature for mammalian PAO (Table 4). None of the compounds, used at the concentrations indicated, affected the commercial horseradish peroxidase reaction in control reactions with H2O2 as substrate, without N1-acetylspermine and homogenate.
Table 4. Characteristics of mammalian and Enc. cuniculi PAO activity.
Data for mammalian enzyme compiled from Seiler (1987, 1995) and Bitonti et al. (1990). Enc. cuniculi PAO prepared from freshly harvested mature spores broken using the mini beadbeater technique (Weiss et al., 1994b); assays as described in legend to Table 5. +, Activity; −, no activity; nd, not determined.
| Substrates | Mammalian PAO | Enc. cuniculi PAO |
|---|---|---|
| N1-Acetylspermine | + | + |
| Spermine | + | − |
| Spermidine | + | − |
| N1,N12-Diacetylspermine | + | nd |
| N1-Acetylspermidine | + | nd |
| N8-Acetylspermidine | − | + |
| bis(Benzyl)putrescine | + | − |
| MDL 27695 | + | +(Inhibits at 100–500 µM) |
| Benzylamine | + | +(16% at 100 µM) |
| Aminoguanidine | − | + (1–100 µM) |
| Cofactor | ||
| FAD | + | − |
| pH optimum | 10·0 | 7·0–8·0 |
| Inhibitors | ||
| MDL 72521 | Ki 0·3 µM | Stimulates activity at 10–100 µM |
| MDL 72527 | Ki 0·1 µM | Stimulates activity at 10–100 µM |
| Pargyline | − | 80% at 50 µM |
| Aminoguanidine | − | 30–90% (at 100–500 µM) |
Enc. cuniculi PAO utilized N1-acetylspermine and N8-acetylspermidine as substrate. It did not utilize spermine, spermidine or bis(benzyl)putrescine. Unfortunately, neither N1- or N8-acetylspermidine nor N1,N12-diacetylspermine were available for study with the Enc. cuniculi PAO, but both are substrates for mammalian PAO. In contrast, mammalian PAO utilizes spermine N1- but not N8-acetylspermidine, and uses bis(benzyl)putrescine. MDL 27695 is a bis(benzyl) 3-7-3 polyamine analogue which is debenzylated by mammalian PAO (Bitonti et al., 1990), and which is also used as substrate by the Enc. cuniculi PAO. Aminoguanidine was not a substrate for mammalian enzyme but, at concentrations below 100 µM, was a substrate for Enc. cuniculi enzyme.
The pH optima of the enzymes also differed: pH 10·0 for mammalian PAO and pH 7·0–8·0 for the protozoan enzyme (Table 4). FAD superadded to control reaction mixtures did not stimulate the rate of the Enc. cuniculi enzyme. Two enzyme-activated irreversible inhibitors of mammalian PAO were developed by Merrell Dow Research Laboratories, MDL 72521 and MDL 72527 (Bey et al., 1985). These exhibited time- and dose-dependent kinetics and had Ki values of 0·1–0·3 µM for the mammalian enzyme (Seiler, 1987). These analogues, used at up to 100 µM, stimulated the Enc. cuniculi enzyme by up to 70% (Table 5).
Table 5. Polyamine analogues as substrates or inhibitors of Enc. cuniculi PAO.
Enzyme reactions contained 0·1 M glycine buffer pH 7·0, 10 units horseradish peroxidase (Sigma), 5 mM 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 20–200 µg Enc. cuniculi mature spore cell-free homogenate, in a final volume of 1 ml. Assays of analogues as substrates had no N1-acetyl-spermine and analogues were added to start the reaction; assays of analogues as inhibitors had 1 mM N1-acetylspermine added to start the reaction. Assays were linked to reduction of ABTS by peroxidase and measurement of the oxidized product at 420 nm (Childs & Bardsley, 1975). All activities were determined in duplicate and corrected for blanks run with extract, but without N1-acetylspermine. Data are compiled from eight separate experiments in which the mean specific activity of full activity controls was 21·1 nmol (mg protein)−1 min−1 [range: 5·0–75 nmol (mg protein)−1 min−1]. +, Stimulation of activity; nd, not determined.
| Compound | Concentration | Percentage of control activity (substrate) |
Percentage inhibition of activity |
|---|---|---|---|
| N1-Acetylspermine (control) | 1 mM | 100 | 0 |
| bis(Benzyl)putrescine | 1 mM | 0 | nd |
| SL-11061 | 100 µM | 0 | +6 |
| SL-11093 | 100 µM | 0 | 13 |
| SL-11144 | 100 µM | 0 | 75 |
| SL-11158 | 100 µM | 0 | 57 |
| MDL 27695 | 1 mM | 33·9 | nd |
| Aminoguanidine | 1 µM | 56·1 | 15 |
| Aminoguanidine | 10 µm | 38·8 | 22 |
| Aminoguanidine | 100 µm | 33·7 | 33 |
| Aminoguanidine | 500 µm | 0 | 88 |
| Benzylamine | 100 µM | 16·4 | nd |
| MDL 72527 | 10 µM | nd | +7·5 |
| MDL 72527 | 50 µM | nd | +29 |
| MDL 72527 | 100 µM | 91·3 | +30 |
| MDL 72521 | 100 µM | 101·3 | +55 |
| MDL 72521 | 10 µM | 103 | +49 |
| MGBG* | 100 µM | nd | +360 |
| BW-1 | 1 mM | 54·9 | +260 |
| Pargyline | 50 µM | nd | 80 |
MGBG, Methylglyoxal-bis(guanylhydrazone).
Table 5 lists activities of polyamine analogues used in this study as substrates or as inhibitors of Enc. cuniculi PAO. Substrate studies were done without N1-acetylspermine in the reaction, while inhibitor studies had 1 mM N1-acetylspermine as substrate. Activities were determined in duplicate and corrected with blanks run with extract without N1-acetylspermine. None of the compounds affected rates with H2O2 as substrate for horseradish peroxidase.
None of the SLIL amine analogues studied (SL-11061, SL-11093, SL-11144, SL-11158) served as substrate for the Enc. cuniculi PAO at 100 µM, and only SL-11144 and SL-11158 were significant inhibitors of the reaction at 100 µM(75 and 57 %, respectively). MDL 27695 (1 mM) was a substrate, having 34% of the activity with the natural substrate, while BW-1 (1 mM), a bis(phenylbenzyl) 3-7-3 analogue of MDL 27695, had 55% of control activity, and stimulated the control reaction (with N1-acetylspermine as substrate) by 40 %. Pargyline is an inhibitor of monoamine oxidases, but inhibited the full reaction by 80% and did not affect the mammalian enzyme (Table 5). These analogues did not affect the horseradish peroxidase reaction with H2O2 as substrate.
DISCUSSION
Although the microsporidia have been studied as unusual eukaryotic intracellular parasites for many years, little is known regarding their metabolism. Weidner et al. (1999) summarized the metabolic capabilities of Ameson michaelis, a parasite of the blue crab, indicating glycolysis proceeded in isolated sporoplasms when exposed to exogenous glucose and ATP. Related studies (Dolgikh et al., 1997) indicated at least eight glycolytic enzymes (but not hexokinase) were present in spores of Nosema grylli, and concluded that it is likely that glycolytically produced NADH is recycled through a glycerol-3-phosphate dehydrogenase, since other glycolytic dehydrogenases were not detectable. Since hexokinase was also not detectable in several studies, it was postulated that the microsporidia may utilize hexose phosphates directly from the host (summarized in Weidner et al., 1999).
Similarly, the ability of Enc. cuniculi to take up spermine and convert it to spermidine and putrescine, coupled with the high efficacy of spermine uptake as opposed to other polyamines or precursors, makes it likely that this microsporidian utilizes polyamine salvaged from the host cell as a major mechanism of acquiring polyamines. The kinetics of transport indicate it is used by Enc. cuniculi to scavenge any available polyamines from the host cytoplasm. Other protozoan parasites such as Trypanosoma cruzi and Trichomonas vaginalis are also believed to obtain most of their polyamines via exogenous sources (Le Quesne & Fairlamb, 1996; Ariyanayagam & Fairlamb, 1997; Yarlett et al., 1994). Since spermine uptake was not blocked by monensin, gramicidin S, ouabain or amiloride, but was inhibited by valinomycin, it appears that Na+/K+ ATPase was not involved in uptake, but a universal cation transporter may be responsible. Relatively low inhibition (31 %) of transport obtained with KCN reinforces the finding that the microsporidia have neither mitochondria nor a functional cytochrome system (Undeen, 1990).
The high activity of exogenous spermine uptake in pre-emergent spores, and the increasing focus on polyamine analogues as anti-tumour agents (Casero & Woster, 2001; Frydman & Valasinas, 1999), made it imperative that we examine analogues as anti-microsporidial agents (Bacchi et al., 2002). In mammalian cells, these are taken up through polyamine transporters and down-regulate polyamine synthesis while inducing SSAT and promoting excretion of acetylated polyamines. As polyamine levels fall in treated cells, the concentrations of analogues increase. Since they do not function in cell metabolism as do polyamines, division stops and apoptosis begins (Casero & Woster, 2001; Frydman & Valasinas, 1999; Ha et al., 1998).
In the present study, we worked with pre-emergent spore fractions, which, although relatively homogeneous, were not actively dividing cells. Thus, effects of analogues seen on dividing mammalian cells such as SSAT induction, reduction in ODC and AdoMetdc activities, could not be readily observed. However, these analogues had several definitive effects on polyamine metabolism of Enc. cuniculi, including reduction of spermine uptake and interconversion (SL-11144, SL-11158) and mixed inhibition of SSAT (SL-11158). BW-1 was a substrate for PAO and in vivo is thus a likely competitive inhibitor of the enzyme with N1-acetylspermine as substrate. The related analogue MDL 27695 [a bis(benzyl) 3-7-3 analogue; Fig. 1] is debenzylated by mammalian PAO (Bitonti et al., 1990). MDL 27695 at 1 mM had modest (33 %) substrate activity in the Enc. cuniculi PAO assay. BW-1, a bis(phenylbenzyl) 3-7-3 analogue, had higher substrate activity (54·9%) in this system, and, used in the presence of the natural substrate, stimulated enzyme activity, by 2·6-fold (Table 5).
These studies indicate that Enc. cuniculi has significant polyamine scavenging potential with spermine the preferred substrate and it is likely that interconversion of polyamines readily occurs in this intracellular parasite. SL-11144, SL-11158 and BW-1 interfere with polyamine uptake and interconversion in the intact pre-emergent spores, while SL-11158 gives a mixed type of inhibition of parasite SSAT. BW-1 is a substrate for parasite PAO and NMR studies in progress indicate two products are formed from [13C]BW-1, a carboxylic acid and an aldehyde, which is consistent with an oxidation pathway (P. Woster, personal communication). All these effects occur at similar analogue concentrations, indicating that the mechanism of action of these agents in vivo targets this pathway.
Since existing chemotherapeutic agents for the microsporidia are not universally curative in human infections (Wittner & Weiss, 1999), more effective agents are needed. Continuing studies are aimed at developing more active agents by examining structure–activity relationships and clarifying mechanism(s) of action.
ACKNOWLEDGEMENTS
This work has been supported by grants from the National Institutes of Health (#2RO1-041398-06 to M. W. and A44-AI43094-03 to B. F.), and a Pace University Scholarly Research Award (C. J. B.). The authors thank Jenny Gallardo, Amy Kirner and Maryann Soliman for technical help.
Abbreviations
- AdoMetdc
S-adenosylmethionine decarboxylase
- DFMO
dl-α-difluoromethylornithine hydrochloride
- ODC
ornithine decarboxylase
- PAO
polyamine oxidase
- SSAT
spermidine/spermine N1-acetyl-transferase
REFERENCES
- Ariyanayagam MR, Fairlamb AH. Diamine auxotrophy may be a universal feature of Trypanosoma cruzi epimastigotes. Mol Biochem Parasitol. 1997;84:111–121. doi: 10.1016/s0166-6851(96)02788-0. [DOI] [PubMed] [Google Scholar]
- Bacchi CJ, Yarlett N. Polyamine metabolism as chemotherapeutic target in protozoan parasites. Mini Rev Med Chem. 2002;2:553–563. doi: 10.2174/1389557023405549. [DOI] [PubMed] [Google Scholar]
- Bacchi CJ, Lane S, Weiss LM, Yarlett N, Takvorian P, Cali A, Wittner M. Polyamine synthesis and interconversion by the microsporidian Encephalitozoon cuniculi. J Eukaryot Microbiol. 2001;48:374–381. doi: 10.1111/j.1550-7408.2001.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Bacchi CJ, Weiss LM, Lane S, et al. Novel synthetic polyamines are effective in the treatment of experimental microsporidiosis, an opportunistic AIDS-associated infection. Antimicrob Agents Chemother. 2002;46:55–61. doi: 10.1128/AAC.46.1.55-61.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacki RJ, Oberman EJ, Seweryniak KE, Atwood A, Bergeron RJ, Porter CW. Preclinical antitumor efficacy of the polyamine analogue N1,N11-diethylnorspermine administered by multiple injection or continuous infusion. Clin Cancer Res. 1995;1:847–857. [PubMed] [Google Scholar]
- Bey P, Bolkenius FN, Seiler N, Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985;28:1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- Bitonti AJ, McCann PP, Sjoerdsma A. Restriction of bacterial growth by inhibition of polyamine biosynthesis by using monofluoromethylornithine, difluoromethylarginine and dicyclohexylammonium sulphate. Biochem J. 1982;208:435–441. doi: 10.1042/bj2080435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti AJ, Dumont JA, Bush TL, Stemerick DM, Edwards ML, McCann PP. Bis(benzyl) polyamine analogs as novel substrates for polyamine oxidase. J Biol Chem. 1990;265:382–388. [PubMed] [Google Scholar]
- Casero RA, Jr, Pegg AE. Spermidine/spermine N1-acetyltransferase – the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- Casero RA, Jr, Woster PM. Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem. 2001;44:1–26. doi: 10.1021/jm000084m. [DOI] [PubMed] [Google Scholar]
- Childs RE, Bardsley WG. The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J. 1975;145:93–103. doi: 10.1042/bj1450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SS. A Guide to the Polyamines. New York: Oxford University Press; 1998. pp. 69–93. [Google Scholar]
- Costa SF, Weiss LM. Drug treatment of micro-sporidiosis. Drug Resist Updat. 2000;3:384–399. doi: 10.1054/drup.2000.0174. [DOI] [PubMed] [Google Scholar]
- Coyle C, Bacchi CJ, Yarlett N, Tanowitz HB, Wittner M, Weiss LM. Polyamine metabolism as a therapeutic target for Microsporidia. J Eukaryot Microbiol. 1996;43:96S. doi: 10.1111/j.1550-7408.1996.tb05020.x. [DOI] [PubMed] [Google Scholar]
- Didier ES. Effects of albendazole, fumagillin, and TNP-470 on microsporidial replication in vitro. Antimicrob Agents Chemother. 1997;41:1541–1546. doi: 10.1128/aac.41.7.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DT, Lew EA, Kotler DP, Poles MA, Orenstein JM. Treatment with albendazole for intestinal disease due to Enterocytozoon bieneusi in patients with AIDS. J Infect Dis. 1994;169:178–183. doi: 10.1093/infdis/169.1.178. [DOI] [PubMed] [Google Scholar]
- Dolgikh VV, Sokolova JJ, Issi IV. Activities of enzymes of carbohydrate and energy metabolism of the spores of the microsporidian, Nosema grylli. J Eukaryot Microbiol. 1997;44:246–249. doi: 10.1111/j.1550-7408.1997.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Frydman B, Valasinas A. Polyamine-based chemotherapy of cancer. Exp Opin Ther Patents. 1999;9:1055–1068. [Google Scholar]
- Goldberg B, Rattendi D, Lloyd D, Sufrin JR, Bacchi CJ. Effects of intermediates of methionine metabolism and nucleoside analogs on S-adenosylmethionine transport by Trypanosoma brucei brucei and a drug-resistant Trypanosoma brucei rhodesiense. Biochem Pharmacol. 1998;56:95–103. doi: 10.1016/s0006-2952(98)00118-x. [DOI] [PubMed] [Google Scholar]
- Green LC, Didier PJ, Didier ES. Fractionation of sporogonial stages of the microsporidian Encephalitozoon cuniculi by Percoll gradients. J Eukaryot Microbiol. 1999;46:434–438. doi: 10.1111/j.1550-7408.1999.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Ha HC, Woster PM, Casero RA., Jr Unsymmetrically substituted polyamine analogue induces caspase-independent programmed cell death in Bcl-2-overexpressing cells. Cancer Res. 1998;58:2711–2714. [PubMed] [Google Scholar]
- Katinka MD, Duprat S, Cornillot E, et al. Genome sequence and genome compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature. 2001;414:450–453. doi: 10.1038/35106579. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD. Antiprotozoal activities of benzimidazoles and correlations with β-tubulin sequence. Antimicrob Agents Chemother. 1994;38:2086–2090. doi: 10.1128/aac.38.9.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithly JS, Zhu G, Upton SJ, Woods KM, Martinez MP, Yarlett N. Polyamine biosynthesis in Cryptosporidium parvum and its implications for chemotherapy. Mol Biochem Parasitol. 1997;88:35–42. doi: 10.1016/s0166-6851(97)00063-7. [DOI] [PubMed] [Google Scholar]
- Le Quesne SA, Fairlamb AH. Regulation of a high-affinity diamine transport system in Trypanosoma cruzi epimastigotes. Biochem J. 1996;316:481–486. doi: 10.1042/bj3160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby PR, Henderson M, Bergeron RJ, Porter CW. Major increases in spermidine/spermine-N1-acetyltransferase activity by spermine analogs and their relationship to polyamine depletion and growth inhibition in L1210 cells. Cancer Res. 1989;49:6226–6231. [PubMed] [Google Scholar]
- Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- Schechter PJ, Sjoerdsma A. Therapeutic utility of selected enzyme-activated irreversible inhibitor. In: Palfreyman MG, McCann PP, Lovenberg W, Temple JG, Sjoerdsma A, editors. Enzymes as Targets for Drug Design. San Diego, CA: Academic Press; 1989. pp. 201–210. [Google Scholar]
- Seiler N. Inhibition of enzymes oxidizing polyamines. In: McCann PP, Pegg AE, Sjoerdsma A, editors. Inhibition of Polyamine Metabolism. Orlando, FL: Academic Press; 1987. pp. 49–77. [Google Scholar]
- Undeen AH. A proposed mechanism for the germination of microsporidian (Protozoa: Microspora) spores. J Theor Biol. 1990;142:223–235. [Google Scholar]
- Valasinas A, Sarkar A, Reddy VK, Marton LJ, Basu HS, Frydman B. Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J Med Chem. 2001;44:390–403. doi: 10.1021/jm000309t. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS, Leitch GJ, Moura H, Wallace S, Weber R, Bryan RT. Culture, electron microscopy, and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J Protozool. 1991;38:105S–111S. [PubMed] [Google Scholar]
- Weber R, Bryan RT, Schwartz DA, Owens RL. Human microsporidian infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E, Trager W. Adenosine triphosphate in the extracellular survival of an intracellular parasite (Nosema michaelis, Microsporidia) J Cell Biol. 1973;57:586–591. doi: 10.1083/jcb.57.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E, Findley AM, Dolgikh V, Sokolova J. Microsporidian biochemistry and physiology. In: Wittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. Washington, DC: American Society for Microbiology; 1999. pp. 172–195. [Google Scholar]
- Weiss LM, Michalakakis E, Coyle CM, Tanowitz HB, Wittner M. The in vitro activity of albendazole against Encephalitozoon cuniculi. J Eukaryot Microbiol. 1994a;41:65S. [PubMed] [Google Scholar]
- Weiss LM, Zhu X, Cali A, Tanowitz H, Wittner M. Utility of microsporidian rRNA in diagnosis and phylogeny: a review. Folia Parasitol (Praha) 1994b;41:81–90. [PubMed] [Google Scholar]
- Wittner M, Weiss LM. The Microsporidia and Microsporidiosis. Washington, DC: American Society for Microbiology; 1999. [Google Scholar]
- Yarlett N, Lindmark DG, Goldberg B, Moharrami MA, Bacchi CJ. Subcellular localization of the enzymes of the arginine dihydrolase pathway in Trichomonas vaginalis and Tritrichomonas foetus. J Eukaryot Microbiol. 1994;41:554–559. doi: 10.1111/j.1550-7408.1994.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Zou Y, Wu Z, Sirisoma N, Woster PM, Casero RA, Jr, Weiss LM, Rattendi D, Lane S, Bacchi CJ. Novel alkylpolyamine analogues that possess both antitrypanosomal and antimicrosporidial activity. Bioorg Med Chem Lett. 2001;11:1613–1617. doi: 10.1016/s0960-894x(01)00315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]