Abstract
Nitinol usage for biomedical implant devices has received significant attention due to its high corrosion resistance and biocompatibility. However, surface treatments are known to affect surface charge, surface chemistry, morphology, wettability, and corrosion resistance. In this investigation, the corrosion resistance of a binary and various ternary Nitinol alloys was determined after being subjected to electropolishing, magnetoelectropolishing, and water boiling and passivation. Cyclic polarization in vitro corrosion tests were conducted in Phosphate Buffer Saline (PBS) in compliance with ASTM F 2129-08 before and after surface treatments. The concentrations of dissolved metal ions in the electrolyte were also determined by ICPMS.
Keywords: biomaterials, corrosion testing, surface engineering
1. Introduction
Shape memory alloys have recently emerged as materials of choice for biomedical implants by virtue of their unique thermomechanical properties, i.e., shape memory and super-elasticity. The main concern about the use of Nitinol alloys derives from the fact that they contain a large amount of Ni (about 50 at.%). Even though small quantity of Ni is essential to the human body (200-300 μg/day) (Ref 1), excessive amount of Ni release may cause allergic, toxic, and carcinogenic reactions. Metallic materials have the tendency to corrode in the physiological environment thereby accelerating the release of Ni from Nitinol alloys. Titanium oxide films present on these alloys act as an effective barrier to Ni leaching and are responsible for their good corrosion resistance (Ref 2-7). In order to gain wider acceptance of NiTi as an implantable material, it is necessary to improve the surface morphology and structure to inhibit nickel release. Although Nitinol has been the subject of research and development for medical applications since the early 1970s, very little is known about the effect of alloying and surface treatment on the corrosion behavior of these alloys under physiological conditions (Ref 8). In this study, the susceptibility to corrosion of Nitinol alloys was evaluated by conducting in vitro cyclic Polarization tests in accordance with ASTM F 2129-08 (Ref 1, 9-11).
2. Materials
2.1 Nitinol alloys
Nitinol alloys, NiTi NiTiCr, NiTiCu, and NiTiTa, have been prepared by arc melting method at the National Institute of Standards and Technology (NIST). The composition of these alloys is shown in Table 1, where X represents the ternary element. Samples were prepared by cutting the cylindrical ingots with a linear precision saw into cylindrical disks of dimension (1 cm × 2 mm).
Table 1.
Composition of Nitinol alloys (at.%)
| Ni | Ti | X |
|---|---|---|
| 51 | 49 | 0 |
| 45.90 | 44.10 | 10 |
2.2 Reagents
Phosphate Buffered Saline (PBS), a reagent grade chemical conforming to the specifications of the Committee on Analytical Reagents of the American Chemical Society was used as the standard test solution. Distilled water was used for water boiling. 20% concentrated HNO3 was used as the passivation solution.
3. Experimental Methods
3.1 Sample Preparation
All the samples were polished with a series of 200, 320, and 600 grit SiC paper. The samples were then degreased ultrasonically with acetone, rinsed in distilled water, and air-dried. Some of the samples were electropolished and magnetoelectropolished by Electrobright® (Macungie, PA, USA). Water boiling was performed by boiling the samples in distilled water at 132 °C for 30 min followed by the passivation, which is the immersion of water boiled samples in 20% conc. HNO3 at 80 °C for 20 min.
3.2 Corrosion Analysis
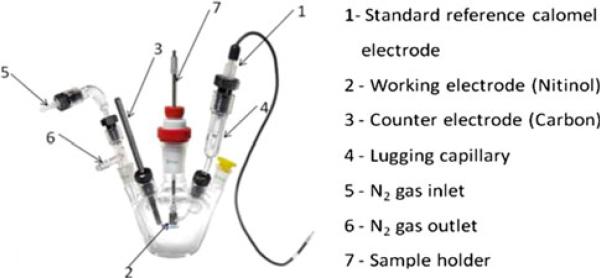
The corrosion cell kit is shown in Fig. 1. The cell was first cleaned with deionized water, rinsed with PBS solution, and filled with approximately 70 mL of PBS. The cell with PBS solution was brought up to 37 °C by placing it in a controlled temperature water bath. The PBS solution was purged with ultra-high-purity nitrogen for 30 min prior to immersion of the sample. A saturated calomel electrode was used as the reference electrode and it was inserted into a Luggin Capillary. The surface area of the sample in contact with PBS was carefully calculated and it was 1 cm2. The cyclic polarization option was then selected on a GAMRY® Instrument Framework Software with a scan rate of 1 mV/s over a potential range between –0.5 and 2.2 V versus a standard calomel electrode (SCE).
Fig. 1.
Corrosion cell kit
4. Results and Discussions
4.1 Localized Corrosion Resistance
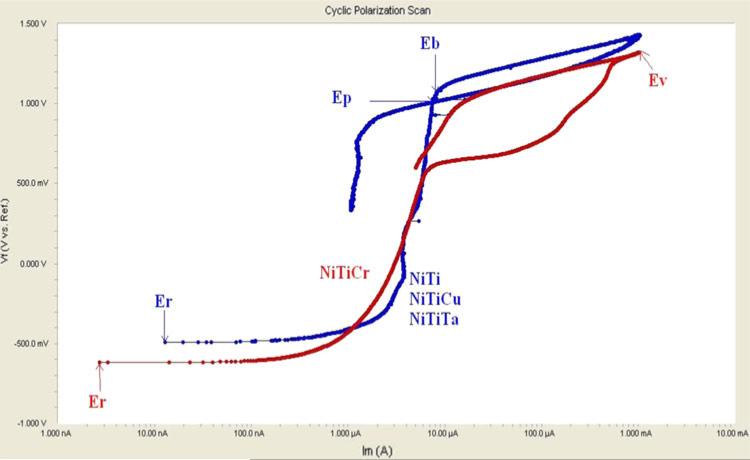
The cyclic potentiodynamic polarization method is very useful for determining the susceptibility of an alloy to pitting and crevice corrosion. Passive metals such as titanium, chromium, and tantalum develop stable oxide layers on Nitinol surfaces, which contribute to their corrosion resistance in physiological conditions. NiTi and NiTiCu forms a TiO2 layer on their surfaces while other ternary Nitinol alloys, NiTiCr and NiTiTa, forms Cr2O3 and Ta2O5 layers, respectively, in addition to TiO2 layer (Ref 12). Typical cyclic potentiodynamic curves for Nitinol alloys are depicted in Fig. 2.
Fig. 2.
Typical cyclic potentiodynamic curves for Nitinol alloys
The corrosion parameters such as break down potential (Eb), protection potential (Ep), vertex potential (Ev), rest potential (Er), and the difference between the break down and the rest potentials (Eb – Er) obtained during cyclic potentiodynamic tests for various untreated and treated binary and ternary Nitinol alloys are given in Table 2. In Table 2, unt stands for untreated alloys while EP, MEP, and WP stand for electropolished, magnetoelectropolished, and water boiled and passivated alloys, respectively.
Table 2.
Corrosion data for Nitinol alloys
| Alloy | Eb | Er | Ep | Ev | Eb – Er |
|---|---|---|---|---|---|
| NiTi-unt | 1.067 | –0.507 | 1.002 | ... | 1.574 |
| NiTi-EP | 1.120 | –0.461 | 1.079 | ... | 1.581 |
| NiTi-MEP | 1.131 | –0.443 | 1.043 | ... | 1.574 |
| NiTi-WP | 1.114 | –0.444 | 1.097 | ... | 1.558 |
| NiTiCu-unt | 1.057 | –0.335 | 1.037 | ... | 1.392 |
| NiTiCu-EP | 1.117 | –0.287 | 1.050 | ... | 1.404 |
| NiTiCu-MEP | 1.143 | –0.300 | 1.083 | ... | 1.443 |
| NiTiCu-WP | 1.189 | –0.278 | 1.126 | ... | 1.467 |
| NiTiTa-unt | 1.110 | –0.296 | 1.090 | ... | 1.407 |
| NiTiTa-EP | 1.145 | –0.466 | 1.127 | ... | 1.611 |
| NiTiTa-MEP | 1.152 | –0.380 | 1.139 | ... | 1.532 |
| NiTiTa-WP | 1.180 | –0.462 | 1.138 | ... | 1.642 |
| NiTiCr-unt | ... | –0.336 | ... | 1.074 | ... |
| NiTiCr-EP | ... | –0.345 | ... | 1.454 | ... |
| NiTiCr-MEP | ... | –0.283 | ... | 1.767 | ... |
| NiTiCr-WP | ... | –0.498 | ... | 1.295 | ... |
Furthermore, the pitting corrosion resistance of the alloys displaying a positive hysteresis (NiTi, NiTiCu, and NiTiTa) was evaluated Eb – Er, whereas, crevice corrosion resistance was evaluated by Ep. The alloys displaying no hysteresis (NiTiCr alloys) were evaluated in terms of Ev.
Among all surface-treated NiTi alloys, NiTi-EP exhibited the highest resistance to pitting corrosion (Eb – Er = 1.581 V). However, NiTi-WP exhibited the highest resistance to crevice corrosion (Ep = 1.097 V) and NiTi-unt had the least resistance to crevice corrosion (Ep = 1.002 V).
Among all the surface-treated NiTiCu alloys, NiTiCu-WP had the highest resistance to pitting (Eb – Er = 1.467 V) and crevice corrosion (Ep = 1.126 V) followed by NiTiCu-MEP, NiTiCu-EP, and NiTiCu-unt. Ni and Cu produced substitutional solid solutions, where the two elements are completely soluble in each other at all proportions. This enhanced the tendency for titanium to diffuse toward the surface to produce a more stable and thicker oxide layer as described in our previous work (Ref 1). The reason for NiTiCu alloy having a thicker oxide layer was attributed to the fact that more titanium atoms relatively close to the surface diffuse toward the surface to produce a thermodynamically stable oxide as compared with remaining in the bulk Ni-Cu solid solution matrix. Similarly, among all the surface-treated NiTiTa alloys, NiTiTa-WP possessed the highest resistance to pitting (Eb – Er = 1.642 V) followed by NiTiTa-EP, NiTiTa-MEP, and NiTiTa-unt. However, NiTiTa-MEP possessed the highest resistance to crevice corrosion (Ep = 1.145 V) followed by NiTiTa-WP, NiTiTa-EP, and NiTiTa-unt. It should be noted that in the case of NiTiCr and NiTiTa, both Cr and Ta compete with Ti to diffuse toward the surface to form the respective oxides (Ref 1, 12).
4.2 Metal Ion Leaching
The ICP-MS (Perkin Elmer Sciex, model ELAN DRC-II) was used to determine the concentration of dissolved metal ions in PBS solution after each corrosion test. Five corrosion tests were conducted with each alloy. After each corrosion test, 5 mL of PBS was collected and sent for ICP-MS analysis. The average concentration of metal ions in three replicates of each sample was determined by ICP-MS as displayed in Table 3.
Table 3.
ICPMS analysis (in ppb)
| Alloy | Ni | Ti | X |
|---|---|---|---|
| NiTi-unt | 69.93 | ND | ... |
| NiTi-EP | ND | ND | ... |
| NiTi-MEP | ND | ND | ... |
| NiTi-WP | ND | ND | ... |
| NiTiCu-unt | ND | 1.63 | 13.77 |
| NiTiCu-EP | ND | ND | 28.47 |
| NiTiCu-MEP | ND | ND | 38.35 |
| NiTiCu-WP | 65.74 | ND | 30.87 |
| NiTiTa-unt | ND | 10.92 | ND |
| NiTiTa-EP | ND | ND | ND |
| NiTiTa-MEP | ND | ND | ND |
| NiTiTa-WP | ND | ND | ND |
| NiTiCr-unt | ND | ND | 300 |
| NiTiCr-EP | ND | ND | 354 |
| NiTiCr-MEP | ND | ND | 1492 |
| NiTiCr-WP | ND | ND | 513 |
ND, not detected (below detection limit after background subtraction)
A large amount of nickel (69.93 μg/L) was observed for NiTi-unt alloy. However, surface-treated samples did not show any metal ion leaching. This is attributed to the fact that electropolishing and passivation are known to be efficient for the elimination of defective surface layers.
A large amount of Ni (65.74 μg/L) was observed for NiTiCu-W&P alloy while no other surface treated alloy showed any Ni release. However, among all the surface-treated NiTiCu alloys, NiTiCu-MEP showed the highest Cu release (38.35 μg/L), followed by NiTiCu-W&P, NiTiCu-EP, and NiTiCu-untreated.
No Ni leaching was observed for NiTiTa alloys. TiO2 and Ta2O5, which were strong passive layers, act as a protective barrier against metal ion leaching. Similarly, no nickel was observed in the PBS solution after corrosion test on NiTiCr alloys, however, relatively large amounts of chromium was observed. This was attributed to surface enrichment of the alloy by highly passivating element, chromium, which leached out during the corrosion tests.
5. Conclusions
Surface engineering have led the development of surface treatments for improved corrosion resistance and reduced metal ion leaching. Surface-treated Nitinol alloys were more resistant to corrosion when compared with untreated Nitinol alloys. Among all the alloys, NiTiTa-WP and NiTiTa-MEP exhibited the highest resistance to pitting and crevice corrosion, respectively.
None of the Nitinol alloys showed any Ni ion release except NiTi-unt and NiTiCu-WP. All NiTiCu alloys showed a small release of Cu ions into the electrolyte. However, all NiTiCr alloys released large amount of Cr.
Acknowledgments
The project described was supported by Award Number SC3GM084816 from the National Institute of General Medical Sciences.
Footnotes
This article is an invited paper selected from presentations at Shape Memory and Superelastic Technologies 2010, held May 16-20, 2010, in Pacific Grove, California, and has been expanded from the original presentation.
Contributor Information
Waseem Haider, Department of Materials Science and Engineering, Penn State University, University Park, PA; Applied Research Center, Florida International University, Miami, FL. Contact whaid001@fiu.edu..
Norman Munroe, Applied Research Center, Florida International University, Miami, FL. Contact.
References
- 1.Haider W. Ph.D. Dissertation. 2010. Enhanced Biocompatibility of NiTi (NITINOL) via Surface Treatment and Alloying. FIU Electronic Theses and Dissertations. Paper 177. [Google Scholar]
- 2.Virtanen S, Milošev I, Gomez-Barrena E, Trebše R, Salo J, Konttinen YT. Special Modes of Corrosion Under Physiological and Simulated Physiological Conditions. Acta Biomater. 2008;4:468–476. doi: 10.1016/j.actbio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Frotscher M, Burow J, Schon P, Neuking K, Bockmann R, Eggeler G. Characterization of the Mechanical Properties Of Ultra-Fine Grained NiTiCr-Wires. Materialwiss. Werkstofftech. 2009;40:1–2. [Google Scholar]
- 4.Haider W, Munroe N, Tek V, Pulletikurthi C, Puneet K, Gill S, Pandya S. Review on Surface Modifications of Nitinol. J. Long Term Effects Med. Implant. 2010;19(2):15–24. doi: 10.1615/jlongtermeffmedimplants.v19.i2.30. [DOI] [PubMed] [Google Scholar]
- 5.Goryczka Tomasz, Van Humbeeck Jan. NiTiCu Shape Memory Alloy Produced by Powder Technology. J. Alloys Compd. 2008;456:194–200. [Google Scholar]
- 6.Neelakantan L. Surface Engineering of Nickel Titanium Shape Memory Alloys, Fakultät für Maschinenbau, Ruhr-Universität-Bochum. 2008. p. 150.
- 7.Neelakantan L, Valtiner M, Eggeler G, Hassel AW. Surface Chemistry and Topographical Changes of an Electropolished NiTi Shape Memory Alloy. Phys. Status Solidi A. 2010;207(4):807–811. [Google Scholar]
- 8.Kanchibbolta S, Munroe N. Amorphization in Ni-Ti-Ta System Through Mechanical Alloying. J. Mater. Sci. 2005;40(18):5003–5006. [Google Scholar]
- 9.Haider W, Munroe N, Pulletikurti C, Gill P, Amruthaluri S. A Comparative Biocompatibility of Ternary Nitinol Alloys. J. Mater. Eng. Perform. 2009;18(5):760–764. doi: 10.1007/s11665-009-9435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munroe ND, Pulletikurthi C, Haider W. Enhanced Biocompatibility of Porous Nitinol. J. Mater. Eng. Perform. 2009;18(5):765–767. doi: 10.1007/s11665-009-9454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider W, Munroe N, Gill P, Pulletikurti C. The Electrochemical Characteristics of Surface Treated Nitinol Alloys. NACE International, Corrosion; San Antonio, TX: Mar 14-18, 2010. pp. 52–54. [Google Scholar]
- 12.Haider W, Munroe N, Tek V, Gill PKS, Tang Y, McGoron AJ. Cytotoxicity of Metal Ions Released from Nitinol Alloys on Endothelial Cells. J. Mater. Eng. Perform. doi: 10.1007/s11665-011-9884-5. doi:10.1007/s11665-011-9884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]