Abstract
Background
Clinical examination and management of patients with meningiomas is primarily dependent upon appropriate diagnosis of tumor type and surgical intervention. Physical therapists should be able to identify patients presenting with signs and symptoms suggestive of potential central nervous system (CNS) disorders and refer the patient appropriately.
Patient characteristics
In this case report, a 52-year-old female was referred to physical therapy after 18 months of unresolved dizziness.
Examination
Oculomotor examination revealed evidence of peripheral vestibular and potential CNS disorders. The physical therapist referred the patient to a physician who ordered magnetic resonance imaging (MRI).
Intervention
The patient received five physical therapy sessions while waiting for the MRI which revealed a meningioma. The meningioma was surgically removed and the patient was subsequently relieved of all symptoms.
Outcomes
Despite the presence of the meningioma, the patient reported improved stability during work-related activities and decreased dizziness as a result of physical therapy intervention pre-operatively.
Discussion
This case report emphasizes the importance of a physical therapists ability to perform and interpret an oculomotor examination in a patient presenting with signs consistent with peripheral vestibular and CNS disorders. It also demonstrates the role of physical therapy in collaboration with physicians in order to provide appropriate patient care management.
Keywords: Differential diagnosis, Meningioma, Physical therapy, Vestibular rehabilitation
Dizziness is a common problem in the elderly as well as in young and middle-aged adults.1,2 Yardley et al.1 indicated that 23.3% of people aged 18–64 years reported having symptoms of dizziness within the past month that interfered with their activities of daily living (ADLs). Estimates of the prevalence of dizziness in community-dwelling adults have ranged from 1 to 35%.3 When the specific symptom of vertigo, or the illusion of motion, is used, the prevalence has been estimated at 6.7%.3 It has also been suggested that 5.5% of people in the USA develop dizziness each year.3 According to Aggarwal and colleagues,2 40.6% of people who experienced dizziness did not consult with a physician and only a third of those who consulted with a physician received treatment.
People who experience dizziness report a significant reduction in quality of life with limitations in their ability to perform ADLs and activities associated with life roles such as work.3–7 In cases where dizziness is due to vestibular system pathology, people often demonstrate gait and balance deficits leading to self-imposed activity restriction and decreased independence;8 and it has been hypothesized that a fear of falling may contribute to these limitations.1 People with dizziness, instability, and fear of falling are commonly referred to a physical therapist who is trained to screen for and identify a variety of potential problems and act accordingly.9 Dizziness is a vague term used to describe symptoms of some underlying pathology and can be difficult to diagnose. The National Institute of Health reported that patients with vestibular pathologies see an average of 4.5 physicians before receiving a correct diagnosis.10 Potential causes of dizziness include orthostatic hypotension, vertebrobasilar insufficiency, anxiety, medication, peripheral vestibular disorders, benign paroxysmal positional vertigo, central nervous system (CNS) disorders, and cervical dysfunction.1,2,11–13
CNS disorders that produce symptoms of dizziness include multiple sclerosis, cerebrovascular accidents, and tumors.14 In general, masses in the posterior fossa occur with some frequency and include vestibular and facial nerve schwannomas, primary cholesteatomas, and meningiomas.15 However, the prevalence of meningiomas within the cerebellopontine angle is relatively rare representing 3.1–15% of all intracranial meningiomas.15–18 Meningiomas represent about 20% of all intracranial tumors and have a 2∶1 female/male ratio. A meningioma within the cerebellopontine angle can cause symptoms including ataxia, imbalance, hearing loss, facial pain, facial numbness, weakness of facial muscles, and dizziness.15–18
CNS disorders are suspected when corrective saccades are observed during non-vestibular tests of extraocular movements such as smooth pursuit, vestibulo-ocular reflex (VOR) cancellation, and when target overshooting or undershooting is consistently observed during saccades testing.19,20 CNS disorders are also suspected when nystagmus is observed during the fixation blocked condition, especially if the nystagmus is direction changing, purely vertical, and/or inconsistent with a peripheral vestibular hypofunction, and when other CNS signs are present.19–21 Lastly, dynamic visual acuity may be impaired with CNS disorders due to the cerebellum’s contribution to the adjustment and maintenance of VOR gain and duration.19,22–24 Table 1 provides a description of room light and fixation blocked tests useful in examining a person who presents with dizziness.
Table 1. Physical therapy oculomotor examination summary45.
| Smooth pursuit | Description: observe the eyes as they track a moving object at 20°/s with the head stationary |
| Result: corrective saccades observed with pursuit to the left suggesting potential CNS dysfunction (*Red Flag) | |
| Saccades | Description: observe the eyes as they quickly look back and forth between two horizontally or vertically placed objects |
| Result: target reached with one corrective saccade and was normal | |
| VOR cancellation | Description: observe the eyes as they fixate on the clinician’s nose while the clinician moves the patient’s head horizontally at a speed of 1 Hz |
| Result: corrective saccades observed with head rotations to left suggesting potential CNS dysfunction (*Red Flag) | |
| Head thrust test | Description: an unpredictable, small amplitude head thrust to each side is applied with high acceleration. The patient’s head is flexed 30° and they are instructed to fixate on the examiner’s nose |
| Result: corrective saccade observed with head thrusts to the left suggesting VOR dysfunction due to central or peripheral dysfunction | |
| Dynamic visual acuity test | Description: the patient reads eye chart with head stationary then again while the head is oscillated in the horizontal plane at a speed of 2 Hz |
| Result: visual acuity decreased by eight lines suggesting VOR deficit due to CNS or peripheral dysfunction | |
| Spontaneous nystagmus test | Description: observe the eyes for nystagmus while patient is sitting stationary |
| Result: spontaneous left beating nystagmus observed suggesting imbalance of the resting firing rate of vestibular neurons due to peripheral or central dysfunction | |
| Gaze holding nystagmus | Description: observe the eyes while the patient holds their gaze about 30° to the right, left, up, and down |
| Result: left beating nystagmus observed with left gaze, right beating nystagmus observed with right gaze (direction changing) suggesting potential CNS dysfunction (*Red Flag) | |
| Head-shake test | Description: the patient’s head is flexed 30° and oscillated horizontally 20 times at 2 Hz, the eyes are observed upon cessation of head movement |
| Result: nystagmus not observed and was normal | |
| Hallpike–Dix test | Description: the patient’s head is rotated 45° to the right/left while in sitting, the patient is then assisted into supine with the head extended about 30° |
| Result: purely torsional nystagmus (the absence of vertical component) towards the left was elicited suggesting potential CNS dysfunction (*Red Flag) |
Tests of posture and gait are also used in the examination of a person with dizziness.3,19 Computerized dynamic posturography, the dynamic gait index, and the functional gait assessment are tests that can be used to identify instability, fall risk, and disability in this patient population.19,25,26 Many authors report that computerized dynamic posturography can assist in understanding problems with balance that might be caused by biomechanical and/or neurologic disorders.27–31 Computerized dynamic posturography has been compared to electronystagmography in people who had peripheral, central, or both peripheral and central vestibular disorders. Among those included in the study, 42% had abnormal vestibular findings on electronystagmography testing and 83% had an abnormal computerized dynamic posturography score.27,28 A higher prevalence of abnormal computerized dynamic posturography results in those with central vestibular disorders was also identified.27,28 The dynamic gait index is a commonly used outcome measure of gait performance in people with vestibular dysfunction.25 Gait parameters measured by the dynamic gait index are different in people with balance or vestibular disorders compared to controls without such disorders.25 The difference in performance is attributed to difficulty in maintaining trunk and gaze stability during head movement while ambulating.25,32
The purpose of this case report is to discuss how the performance of a complete vestibular and balance examination, including the oculomotor system, can assist a physical therapist in identifying a potential CNS dysfunction in patients with dizziness. Additionally, the importance of collaborating with physicians to optimize the effectiveness and efficiency of patient care is discussed. Physical therapists use room light and fixation blocked oculomotor examinations to assist in their differential diagnostic procedure in patients with dizziness. Room light examinations include assessment of smooth pursuit, saccades, VOR cancellation, head thrust testing, and static and dynamic visual acuity.3,19,22,23 Fixation blocked examinations include observing for the presence of spontaneous, gaze holding, post-head shaking, and positional-provoked nystagmus.3,19,22,23
Patient Characteristics
The patient was a 52-year-old female with a history of approximately 18 months of dizziness. She reported that her symptoms began with a sudden onset of feeling ‘coldness’ in her left ear. Within a few hours, she began experiencing vertigo, which lasted for 1 week, including 2 days of nausea and vomiting. During this time, the patient reported hearing loss in the left ear and because the vertigo was constant, she was forced to miss a week of work. The patient consulted with her primary care physician during that week and was diagnosed with vertigo, and was prescribed meclizine and rest. She returned to work the following week but the dizziness persisted over the next 6 months, prompting her to return to her primary medical doctor who referred her to an otolaryngologist (ENT). The ENT requested a magnetic resonance image (MRI) to rule out a central problem but the request was denied by her insurance company. Instead, a computed tomography (CT) scan was authorized and performed. The ENT also sent the patient for an audiogram which confirmed incomplete hearing loss in her left ear. Electronystagmography testing was not requested at this time. After the CT scan of her brain came back negative, the ENT suggested to the patient that the dizziness might get better over time and no other recommendations were made at that time. After another 8 months of continued dizziness, a friend of the patient suggested that she request a referral to physical therapy. The patient returned to her primary physician who prescribed physical therapy with the diagnoses of ataxia and vertigo.
At the time of the physical therapy evaluation, the patient’s chief complaints were symptoms of instability and blurry vision despite daily use of meclizine. She denied having fallen and reported that her symptoms had remained stable since the cessation of the initial 1-week episode of vertigo and nausea. The instability was aggravated by grocery shopping, walking on compliant surfaces, head or body turns, looking up or down, and negotiating stairs. The patient also reported difficulty reading due to a slowing in her ability to find or focus on the next line of text. Finally, she reported that she had to watch television with her head tilted to her left. The patient was a pre-school teacher and expressed that the feelings of persistent disequilibrium were of concern to her especially while at work.
Examination
Positive findings from the room light oculomotor examination included corrective saccades to the left with smooth pursuit, corrective saccades during VOR cancellation with her head rotating to her left, positive left head thrust test, and impaired dynamic visual acuity (drop of eight lines on the dynamic visual acuity test performed using a modified ETDRS Visual Acuity Eye Charts chart with Sloan letters). Positive findings in the fixation blocked oculomotor examination included spontaneous left beating nystagmus with complaints of a rocking feeling, left beating nystagmus with left gaze, right beating nystagmus with right gaze, the elimination of spontaneous nystagmus post-head shake test, and purely torsional nystagmus towards the left during the Hallpike–Dix testing bilaterally.
Positive findings that suggested a CNS problem included corrective saccades during smooth pursuit and VOR cancellation, a drop of eight lines on the dynamic visual acuity test, spontaneous nystagmus, direction changing nystagmus with gaze holding, and purely torsional nystagmus in the Hallpike–Dix tests. Additionally, a positive head thrust test in this case suggested the presence of a problem at the root entry zone of the vestibular nerve where it meets the vestibular nuclei at the pontomedullary junction.33 Table 1 provides a summary of the oculomotor examination.
The patient’s balance examination included computerized dynamic posturography, the dynamic gait index, and the functional gait assessment. The computerized dynamic posturography test battery included the sensory organization test, motor control test, and adaptation test.34 These tests were performed using the NeuroCom® SMART EquiTest® Balance Master which calculates the body’s anterior–posterior sway in response to different challenges to the support surface and with alterations to a person’s ability to utilize sensory systems in the maintenance of postural control.34 The motor control test measures the timing, strength, and symmetry of automatic responses generated by sudden movements of the support surface.34 The adaptation test measures the response to movements of the support surface that vary from trial to trial.34 The sensory organization test measures abnormalities in the use of somatosensory, visual, and vestibular systems.34 The sensory organization test revealed decreased use of visual and vestibular feedback, and the motor control test and adaptation test were within normal limits.
The patient’s score on the functional gait assessment was 22/30 which was below average for her age group.35 The patient scored 18/24 on the dynamic gait index which indicated that she was at risk for falls. The patient also reported a 4/10 with aggravating movements on the visual analogue scale with 0 representing no dizziness and 10 representing the worst imaginable dizziness.
Clinical Impression
Because the physical therapy examination findings were consistent with both peripheral vestibular and CNS dysfunction, the physical therapist made the clinical decision to refer the patient to a physiatrist specializing in the treatment of patients with brain injuries and vestibular disorders. Based upon the recommendations from the physical therapist, the physiatrist ordered electronystagmography testing as well as MRI with contrast to rule out CNS disorders such as multiple sclerosis or a tumor. Physical therapy was to be continued in the meantime.
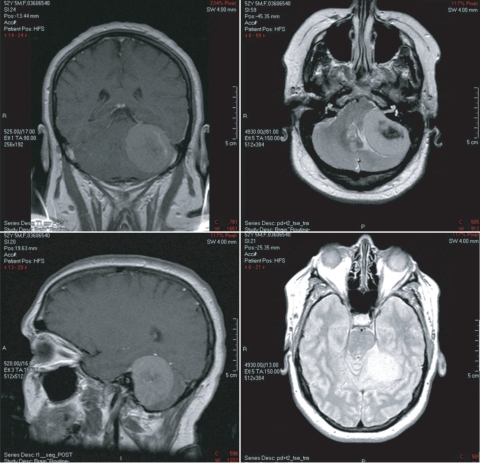
The MRI, which was performed between the patient’s third and fourth physical therapy sessions, revealed a large extra-axial mass within the left cerebellopontine angle which was suspected to be a meningioma (Fig. 1). The electronystagmography testing was cancelled and the patient was referred to a neurosurgeon. Approximately 4 months after her initial physical therapy examination and 3.5 months after seeing the physiatrist, the patient underwent surgical removal of the cerebellopontine angle mass.
Figure 1.
MRI results of patient’s left cerebellopontine angle meningioma.
Intervention
Before the cerebellopontine angle mass being diagnosed, the patient received physical therapy interventions twice per week while awaiting the MRI and electronystagmography testing. Interventions were aimed at improving the balance and dynamic visual acuity deficits that were identified during the examination. Interventions included vestibular adaptation exercises, optokinetic stimulation, dynamic standing balance activities on compliant surfaces with her eyes open and closed, and dynamic locomotor training.19,22 The patient was also prescribed a home exercise program consisting of specific vestibular adaptation exercises (X1 viewing to improve the VOR reflex) and anterior–posterior weight shifting.19,22 The patient was to perform X1 viewing with both horizontal and vertical head turns for 90 seconds each while focusing on a stationary target.19,22 This was performed in standing with a progression to walking five times per day as tolerated. The weight shifting exercise was to be carried out with her eyes closed while standing on a folded towel for 20 repetitions once daily. She was instructed to stand with her back approximately 6 inches from a wall with a chair in front of her. To ensure safety, the patient was observed performing each home exercise in the clinic before its prescription or progression. The patient was notified of her MRI results after her fifth physical therapy visit. Afterwards, the patient returned to physical therapy for one more visit to discuss her diagnosis and to review her home exercise program. She was discharged from physical therapy after six visits and subsequently underwent surgical removal of the cerebellopontine angle mass. Table 2 provides a descriptive treatment summary.
Table 2. Physical therapy intervention summary.
| Visit 1Initial consultation | Subjective exam, oculomotor exam (room light and fixation blocked), patient education, home exercise program (HEP) initiated (X1 viewing standing feet hip width for 60 seconds horizontally and vertically, 5×/day as tolerated) |
| Visit 2Initial consultation continued | Computerized dynamic posturography testing, limits of stability test, functional gait assessment, dynamic gait index, patient education for test findings and treatment plan, HEP progressed (weight-shifting on towel with eyes closed 20×/day as tolerated) |
| Visit 31st post-consultation treatment session | X1* viewing during gait with large target, locomotor training on treadmill with saccades, standing balance with eyes closed on tempur foam, HEP modified to include use of larger target for X1 viewing as tolerated |
| Visit 42nd post-consultation treatment session | X1 viewing during gait, level surface, treadmill with saccades, optokinetics, standing balance with eyes closed on tempur, HEP reviewed (no changes made) |
| Visit 53rd post-consultation treatment session | X1 viewing on tempur, optokinetics, standing balance with eyes closed on tempur and high density foam, HEP progressed to include X1 viewing during forward and backward walking as tolerated |
| Visit 64th post-consultation treatment session | X1 viewing on rocker board, optokinetics, standing balance on high density foam w/eyes open and closed, standing balance on rocker with eyes open and closed, sidestepping on 2×4, HEP reviewed (no changes made) |
| Visit 75th post-consultation treatment session | X1 viewing on high density foam, X1 viewing during gait, sidestepping on 2×4 with resistance, weighted ball toss on 2×4, standing balance with eyes closed on high density foam, HEP progressed to include head turns while standing on a folded blanket with eyes closed as tolerated |
| Visit 86th post-consultation treatment session | Standing balance on high density foam with eyes closed, gait over compliant surface with head turns, patient education and discussion regarding diagnosis, HEP reviewed (no changes made) |
Note: *X1 viewing is a specific type of VOR adaptation exercise aimed at improving gaze stability during head movements. Initially, the patient focuses on a target approximately 3 feet away and moves their head from side-to-side horizontally at a speed that allows them to keep the target in focus. As VOR adaptation occurs over time, the distance and speed of the head movement can be increased and the environment in which the exercises are performed can be made more challenging.
Outcomes
Upon discharge from physical therapy, the intensity of her dizziness had decreased by two points from 4/10 to 2/10. The sensory organization test and dynamic gait index were not performed on her last session in order to allow the patient time to discuss concerns regarding her diagnosis and prognosis and to review her home exercises. After the surgery, re-testing of the sensory organization test and dynamic gait index was performed. All conditions of the sensory organization test were found to be within normal limits and her dynamic gait index score was 22/24 indicating that she was no longer at risk for falls.
Upon follow-up with the patient 3 months post-surgery, she reported complete resolution of her symptoms. The sensory organization test was within normal limits and the dynamic gait index remained at 22/24.
Discussion
The patient in this case report presented with signs consistent with peripheral vestibular and CNS dysfunction. The physical therapy examination findings suggesting a CNS dysfunction were supported through the identification of a meningioma at the cerebellopontine angle via MRI. Meningiomas have a peak incidence at 45–55 years of age, female/male ratio of 9∶5, account for 17% of all brain tumors and up to 10–15% of cerebellopontine angle masses.18 Symptomology is dependent upon the tumor location. In this case report, the patient’s subjective complaints of partial hearing loss, dizziness, and gait instability suggested the presence of a vestibular disorder. The onset, description, and duration of symptoms through the acute stage also suggested a peripheral vestibular disorder such as labyrinthitis. However, the persistence of her symptoms over the course of 18 months despite her continued performance of typical work and daily living tasks indicated that she was not compensating well for the disorder. The lack of compensation could be explained by the meningioma’s interference with central mechanisms needed for adaptation and/or the prolonged use of meclizine.19,36,37
There were several findings in the oculomotor examination that indicated the presence of a potential CNS dysfunction including the corrective saccades observed during smooth pursuit, saccade testing, and VOR cancellation testing. The deficit in dynamic visual acuity indicated the presence of a vestibular disorder of either central or peripheral origin. The positive head thrust test confirmed the presence of a peripheral vestibular disorder, most likely due to compression of the vestibular nerve at the root entry zone. Spontaneous nystagmus in the fixation blocked condition suggested the presence of an uncompensated vestibular disorder or CNS disorder. Direction changing nystagmus in the fixation blocked condition and the presence of purely torsional nystagmus during the Hallpike–Dix testing also suggested a potential CNS disorder.19,38 The results of the sensory organization test, dynamic gait index, and functional gait assessment tests objectified the presence of postural instability during standing and walking. These findings should prompt the physical therapist to refer the patient to a physician for further diagnostic testing.
Literature to support the use of vestibular rehabilitation in the treatment of central vestibular disorders is limited. Furthermore, studies that evaluate the effectiveness of vestibular rehabilitation in this patient population often lack internal validity.14 Therefore, less is known regarding the effect of vestibular rehabilitation with central vestibular disorders such as brain tumors, traumatic brain injury, or multiple sclerosis. Although many aspects of vestibular rehabilitation are similar for patients with peripheral vestibular disorders and central vestibular disorders, studies indicate that people with central vestibular disorders have poorer outcomes and do not progress as quickly when compared to those with pure peripheral vestibular disorders.14
Other studies have indicated that the use of customized vestibular rehabilitation interventions have been effective in improving sensory organization test, dynamic gait index, and dynamic visual acuity scores.19,37,39,40–44 Additionally, it has been shown that people with central vestibular disorders respond to rehabilitation with improvements in subjective measures of the effect of dizziness and instability on daily life.14,39 In this case report, the patient reported improvements in the severity of her symptoms including dizziness and instability during walking and work-related activities over the course of six physical therapy sessions. Owing to the medical implications of her diagnosis and an unexpected discharge from physical therapy services, these statements were not objectified until after her surgery.
This case report brings to light the importance of the inclusion and interpretation of a room light and fixation blocked oculomotor examination in the differential diagnosis of a person presenting to physical therapy with complaints of dizziness and imbalance. These tests take little time to administer and provide valuable information regarding the nature of the person’s complaints. By identifying evidence of potential CNS disorders, the physical therapist is better equipped to manage patients in the most appropriate way. This includes the ability to establish an appropriate prognosis and intervention for the patient, which incorporates communicating with and making referrals to a physician to ensure that the patient is diagnosed and treated efficiently. This translates into fewer unnecessary services and greater cost effectiveness in the delivery of patient care. In conclusion, this case report emphasizes the importance of a physical therapist's ability to perform and interpret an oculomotor examination in patients presenting with complaints of dizziness. It also demonstrates the importance of collaborating with physicians in the management of patients with suspected CNS involvement. Finally, the patient in this case report made progress from physical therapy interventions despite the presence of an undiagnosed meningioma.
Acknowledgments
The authors express their gratitude to Dr. Susan Whitney for providing constructive feedback and suggestions concerning this case report.
References
- 1.Yardley L, Owen N, Nazareth I, Luxon L. Prevalence and presentation of dizziness in a general practice community sample of working age people. Br J Gen Pract 1998;48:1131–5 [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal N, Bennett D, Bienias J, Mendes de Leon CF, Morris MC, Evans DA. The prevalence of dizziness and its association with functional disability in a biracial community population. J Gerontol 2000;55:288–92 [DOI] [PubMed] [Google Scholar]
- 3.Shubert M, Minor B. Vestibulo-ocular physiology underlying vestibular hypofunction. Phys Ther 2004;84:373–82 [PubMed] [Google Scholar]
- 4.Grimby A, Rosenhall U. Heath-related quality of life and dizziness in old age. Gerontology 1985;41:286–98 [DOI] [PubMed] [Google Scholar]
- 5.A report of the Task Force in the National Strategic Research Plan, National Institute on Deafness and Other Communication Disorders Bethesda, MD: National Institutes of Health, National Institute on Deafness and Other Communication Disorders; 1989. p. 74 [Google Scholar]
- 6.Clark MR, Sullivan MD, Katon WJ, Russo JE, Fischl M, Dobie RA, et al. Psychiatric and medical factors associated with disability in patients with dizziness. Psychosomatics 1993;34:409–15 [DOI] [PubMed] [Google Scholar]
- 7.Wrisley D, Whitney S, Furman J. Measurement of health status in patients with dizziness and a history of migraine. J Neurol Phys Ther 2004;28:84–90 [Google Scholar]
- 8.Hall C, Herdman S. Reliability of clinical measures used to assess patients with peripheral vestibular disorders. J Neurol Phys Ther 2006;30:74–81 [DOI] [PubMed] [Google Scholar]
- 9.Herdman SJ. Vestibular rehabilitation. 3rd ed. Philadelphia, PA: F. A. Davis; 2007. p. 228 [Google Scholar]
- 10.Luxon L. Hearing and balance disorders: a new approach. Clin Med 2007;7:318–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroenke K, Lucas LA, Rosenberg ML, Scherokman B, Herbers JE, Wehrle PA, et al. Causes of persistent dizziness: a prospective study of 100 patients in ambulatory care. Ann Intern Med 1992;22:323–34 [DOI] [PubMed] [Google Scholar]
- 12.Sloane PD, Baloh RW. Persistent dizziness in geriatric patients. J Am Geriatr Soc 1989;37:1031–8 [DOI] [PubMed] [Google Scholar]
- 13.Sixt E, Landahl S. Postural disturbances in a 75-year-old population I: prevalence and functional consequences. Age Ageing 1987;16:393–8 [DOI] [PubMed] [Google Scholar]
- 14.Furman J, Whitney S. Central causes of dizziness. Phys Ther 2000;80:179–87 [PubMed] [Google Scholar]
- 15.Desai K, Nadkarni T, Bhayani R, Goel A. Cerebellopontine angle epidermoid tumor presenting with ‘tic convulsif’ and tinnitus — case report. Neurol Med Chir (Tokyo) 2002;42:162–5 [DOI] [PubMed] [Google Scholar]
- 16.Brackman DE, Bartels LJ. Rare tumors of the cerebellopontine angle. Otolaryngol Head Neck Surg 1980;88:555. [DOI] [PubMed] [Google Scholar]
- 17.Mallucci C, Ward V, Carney A, O’Donoghue G, Robertson I. Clinical features and outcomes in patients with non-acoustic cerebellopontine angle tumors. J Neurol Neurosurg Psychiatry 1999;66:768–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haught K, Hogg J, Killeffer J, Voelker JL, Schochet SS, Fujii K, et al. Entirely intracanalicular meningioma: contrast-enhanced MR findings in a rare entity. AJNR Am J Neuroradiol 1998;19:1831–3 [PMC free article] [PubMed] [Google Scholar]
- 19.Whitney SL, Herdman SJ. In: Herdman SJ, editor Vestibular rehabilitation. 3rd ed. Philadelphia, PA: F.A. Davis; 2007. Physical Therapy Assessment of Vestibular Hypofunction; p. 272–308 [Google Scholar]
- 20.Nedzelski JM. Cerebellopontine angle tumors: bilateral flocculus compression as cause of associated oculomotor abnormalities. Laryngoscope 1983;93:1251–60 [DOI] [PubMed] [Google Scholar]
- 21.Wagner J, Glaser M, Brandt T, Strupp M. Downbeat nystagmus: aetiology and comorbidity in 117 patients. J Neurol Neurosurg Psychiatr 2008;79:672–7 [DOI] [PubMed] [Google Scholar]
- 22.Schubert MC. Vestibular disorders. In: O’Sullivan SB, Schmitz TJ, editors. Physical rehabilitation: assessment and treatment. 5th ed. Philadelphia, PA: F.A. Davis; 2007. Vestibular Rehabilitation; p. 999–1029 [Google Scholar]
- 23.Allison LK, Fuller K. In: Umphred DA, editors. Neurological rehabilitation. 5th ed. St Louis, MO: Mosby; 2007. Chapter 25: Balance and Vestibular Disorders; p. 732 [Google Scholar]
- 24.Brandt T, Strupp M. General vestibular testing. Clin Neurophysiol 2009;116:406–26 [DOI] [PubMed] [Google Scholar]
- 25.Marchetti G, Whitney S, Blatt P, Morris LO, Vance JM. Temporal and spatial characteristics of gait during performance of the dynamic gait index in people with and people without balance or vestibular disorders. Phys Ther 2008;88:640–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill-Body K, Beninato M, Krebs D. Relationship among balance impairments, functional performance, and disability in people with peripheral vestibular hypofunction. Phys Ther 2000;80:748–58 [PubMed] [Google Scholar]
- 27.Whitney S, Marchetti G, Schade A. The relationship between falls history and computerized dynamic posturography in persons with balance and vestibular disorders. Arch Phys Med Rehabil 2006;87:402–7 [DOI] [PubMed] [Google Scholar]
- 28.Keim R. Clinical comparisons of posturography and electronystagmography. Laryngoscope 1993;103:713–6 [DOI] [PubMed] [Google Scholar]
- 29.Goebel J, Paige G. Dynamic posturography and caloric test results in patients with and without vertigo. Otolaryngol Head Neck Surg 1989;100:553–8 [DOI] [PubMed] [Google Scholar]
- 30.Voorhees R. The role of dynamic posturography in neurotologic diagnosis. Laryngoscope 1989;99:995–1001 [DOI] [PubMed] [Google Scholar]
- 31.Monsell E, Furman J, Herdman S, Konrad H, Shepard N. Computerized dynamic platform posturography. Otolaryngol Head Neck Surg 1997;117:394–8 [DOI] [PubMed] [Google Scholar]
- 32.Patten C, Horak F, Krebs D. Head and body center of gravity control strategies: adaptations following vestibular rehabilitation. Acta Otolaryngol 2003;123:32–40 [DOI] [PubMed] [Google Scholar]
- 33.Newman-Toker D, Kattah J, Alvernia J, Wang DZ. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology 2008;70:2378–85 [DOI] [PubMed] [Google Scholar]
- 34.NeuroCom International. Available from: http://resourcesonbalance.com [cited Jun 23 2009] [Google Scholar]
- 35.Walker M, Austin A, Banke G, Foxx R, Gaetano L, Gardner LA, et al. Reference group data for the functional gait assessment. Phys Ther 2007;87:1468–77 [DOI] [PubMed] [Google Scholar]
- 36.Morrow MJ, Sharpe JA. Torsional nystagmus in the lateral medullary syndrome. Ann Neurol 1988;24:390–8 [DOI] [PubMed] [Google Scholar]
- 37.Walker M. Treatment of vestibular neuritis. Curr Treat Options Neurol 2009;11:41–5 [DOI] [PubMed] [Google Scholar]
- 38.Lopez L, Bronstein AM, Gresty MA, Rudge P, du Boulay EP. Torsional nystagmus: a neuro-otological and MRI study of thirty-five cases. Brain 1992;115:1107–24 [DOI] [PubMed] [Google Scholar]
- 39.Badke MB, Shea TA, Miedaner JA, Grove CR. Outcomes after rehabilitation for adults with balance dysfunction. Arch Phys Med Rehabil 2004;85:227–33 [DOI] [PubMed] [Google Scholar]
- 40.Herdman SJ, Clendaniel R, Mattox D, Holliday M, Niparko J. Vestibular adaptation exercises and recovery: acute stage after acoustic neuroma resection. Otolaryngol Head Neck Surg 1995;113:77. [DOI] [PubMed] [Google Scholar]
- 41.Herdman SJ, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg 2003;129:819. [DOI] [PubMed] [Google Scholar]
- 42.Horak FB, Jones-Rycewicz C, Black FO, Shumway-Cook A. Effects of vestibular rehabilitation on dizziness and imbalance. Otolaryngol Head Neck Surg 1992;106:175. [PubMed] [Google Scholar]
- 43.Cohen H. Vestibular rehabilitation reduces functional disability. Otolaryngol Head Neck Surg 1992;107:638. [DOI] [PubMed] [Google Scholar]
- 44.Telian SA, Shepard NT, Smith-Wheelock M, Kemink JL. Habituation therapy for chronic vestibular dysfunction: preliminary results. Otolaryngol Head Neck Surg 1990;103:89. [DOI] [PubMed] [Google Scholar]
- 45.Tusa RJ. In: Herdman SJ, editor. Vestibular rehabilitation. 3rd ed. Philadelphia, PA: F.A. Davis; 2007. Chapter 25: Vestibular Rehabiliation; p. 113–22 [Google Scholar]