Abstract
Objectives
Studies investigating the efficacy of intra-oral myofascial therapies (IMT) for chronic temporomandibular disorder (TMD) are rare. The objective of this randomized, controlled pilot study was to compare the effects of IMT and the addition of self-care and education over 6 months on four common TMD outcome measures: inter-incisal opening range, jaw pain at rest, jaw pain upon opening, and jaw pain upon clenching.
Participants
Thirty myogenous TMD participants between the ages of 18 and 50 years, experiencing chronic jaw pain of longer than 3-month duration, were recruited for the present study.
Intervention
Included patients were randomized into one of three groups: (1) IMT consisting of two treatment interventions per week for 5 weeks; (2) IMT plus ‘self-care’ involving education and exercises; and (3) wait list control.
Main outcome measures
Range of motion findings were measured in millimetres by vernier callipers and pain scores were quantified using an 11-point self-reported graded chronic pain scale. Measurements were taken at baseline, 6 weeks post-treatment, and 6 months post-treatment.
Results
The results showed statistically significant differences in resting, opening, and clenching pain and opening range scores (P<0.05) in both treatment groups compared to control at 6 months. No significant differences were observed between the two treatment groups during the course of the trial.
Conclusions
This study suggests that IMT alone or with the addition of self-care may be of some benefit in the management of chronic TMD over the short-medium term. A larger scale study over a longer term (1–2 years) may be of further value.
Keywords: Biopsychosocial, Manual therapy, Myofascial, Randomized controlled trial, Temporomandibular disorder
Temporomandibular joint (TMJ) pain and dysfunction is estimated to have an incidence between 10 and 40% of the population ranging across the whole socioeconomic,1 ethnic,2 and age3 spectrum. Temporomandibular disorder (TMD) has been found to be associated with other conditions such as tension headache,4 whiplash,5 fibromyalgia,6 tinnitus,7 vertigo, hearing loss, and otalgia.8 More specific signs and symptoms of TMD include joint sounds (with or without pain), abnormal joint motion (particularly deviation or deflection upon opening), periauricular and masticatory muscle tenderness, restricted inter-incisal opening range, pain at rest or during clenching and mastication, and psychosocial factors such as anxiety, a poor coping profile or tendency towards catastrophizing behaviour.9
The non-dental treatment of masticatory muscles, connective tissue, and local neurological structures to affect symptoms of TMD has been attempted surgically10 and pharmacologically11 for many years and has some support in the literature. Manual therapy has been used for many years by those within the physiotherapy, chiropractic, osteopathic, orthopaedic, and emergency medicine fields; however, much of the literature pertaining to manual therapy of the TMJ has focused on acute articular displacement of the meniscus such as in closed or open ‘lock’.12,13 Several dental studies have investigated the combined use of exercises and mobilizations of the TMJ,14 occlusal adjustment,15 and deprogrammer splint use,16 but such multimodal trials make it difficult to assess the efficacy of individual therapies in a comparative way. Also, some of these studies were poorly designed so their conclusions should be interpreted with caution.17
Intra-oral myofascial therapies (IMT) aimed at influencing craniofacial structures have been described at length18–28 and are familiar to many manual therapists. The techniques usually involve applying digital pressure (known variously as ischaemic compression, pressure release, myotherapy, or acupressure)29 into masticatory muscle trigger points, origins, or insertions, using intra-oral contact points. This pressure may become progressively firmer or may be augmented by other interventions such as proprioceptive neuromuscular facilitation, post-isometric relaxation, or other myofascial releases involving the mandible.28 While reviews of the literature have suggested that ‘manual therapy’ of the jaw may be of benefit in TMD,17,30 there are no studies investigating the effectiveness of IMT techniques. Obviously, the non-invasive nature and minimal risks associated with low-force myofascial techniques may present an attractive adjunct or even alternative to more forceful, invasive, or irreversible interventions.
Several common IMT techniques were combined into a novel treatment protocol that aimed to address chronic contracture, spasms, and adhesions of the TMJ apparatus in chronic myogenous TMD sufferers. It was hypothesized that the intervention protocol would improve range of motion findings and pain scores in the short to medium terms (6 months). Furthermore, it was hypothesized that the addition of education and self-care to such a treatment regime would yield more robust results. A small pilot study was conceived comparing three groups: an IMT group; a combined IMT plus education and self-care group (IMTSCE); and a non-treated wait list control group.
Methods
Design
The pilot study was part of a Masters (Honours) project (undertaken by the primary author) at Macquarie University in the Faculty of Science. Human ethics approval no. HE26AUG2005-M04263 was granted by the Macquarie University Ethics Committee. The trial was registered with the Australian Clinical Trials Register (registration no. ACTRN12610000329066, submitted 12 May 2006).
The study design was a randomized, controlled clinical trial consisting of three participant groups: IMT, IMTESC, and no treatment (wait list) control. The trial team consisted of one receptionist, one study assistant, one assessor, and one practitioner. The receptionist answered telephone queries, checked primary inclusion and exclusion criteria, made appointments, prepared files, and consecutively numbered those of included participants for allocation. She was blinded to the randomization schedule and the assessments. The study assistant’s role was to generate the randomization schedule, off-premises, using a web-based random number generator (http://www.randomizer.org) and using it to allocate each numbered participant file to one of the three groups until the schedule was exhausted. The study assistant was blinded to the assessments.
The assessor was an independent dental nurse previously trained for 6 hours over a 2-week period in administering the research diagnostic criteria (RDC) for TMD assessment by the primary author. Tools used in training the assessor included observation of comprehensive original video footage of the RDC protocol being performed, as well as practice drills of the RDC procedure in order to calibrate for variables such as palpation pressure and location, and instructing of participants. All data collection was taken on the premises by the same assessor, who was blinded as to the group allocation of participants. To ensure consistency within the project, a manual was produced outlining enrollment procedures, assessment and reporting procedures, and definitions. The practitioner (primary author) was blinded to the randomization schedule and assessment outcomes until the conclusion of the study.
Subjects
A 5 km radius was marked on a map around the proposed treatment centre (the primary author’s private practice in Edensor Park, NSW, Australia), and seven dental offices were identified within that zone. Dentists were approached and requested to place an A4 sized advertisement in their waiting room. The advertisement consisted of an outline of the trial, primary inclusion and exclusion criteria, relevant faculty information, and a contact telephone number for further information. Recruitment occurred between March 2006 and March 2007. Interested parties were invited to telephone for further information.
Primary inclusion criteria consisted of an age restriction between 18 and 50 years old, a daily history of periauricular pain with or without joint sounds for at least 3 months, voluntary participation, and a willingness to contribute long-term follow-up data. Primary exclusion criteria were also screened by the receptionist and included a history of previous attendance at the practitioner’s clinic, edentulous (toothless) applicants, a history of malignancy in the last 5 years, other physical contraindications such as active inflammatory arthritides, fractures, dislocations, or known instability of the jaws or neck, metabolic diseases (e.g. gout, osteoporosis, Cushing’s disease, and hyper/hypo-parathyroidism), connective tissue and rheumatologic disorders (e.g. systemic lupus erythematosus and scleroderma), and haematological disorders (e.g. anaemia and leukaemia).
Successfully screened applicants made an appointment to attend the clinic in person, read, and sign their informed consent forms and were then assessed using the RDC,31 which is a comprehensive, valid, and reliable biaxial diagnostic tool32,33 for integrating psychosocial, physiological, and palpatory information on TMDs. It is widely used in TMD research.33 The algorithms employed within the RDC are used to diagnose the type of TMD according to myogenous, discal, or arthritic characteristics through the use of self-reporting of pain and dysfunction, range of motion, and palpation. It also allows for assessment of psychosocial factors, in particular identifying a tendency towards somatization, depression, and anxiety. Given the myofascial nature of the project, the RDC was used to identify myogenous TMD sufferers as a secondary inclusion criterion for the study. Also, minimum baseline graded chronic pain scale (GCPS) scores of 3/10 on each of the three symptom outcome measures were set for enrollment in the study, which is in line with other similar studies.34 Secondary exclusion criteria of severe depression or somatization were based on the RDC psychosocial axis assessment.
Outcome measures
Palpatory pain findings are generally considered less valid than self-reported pain scales or visual analogue scales for masticatory muscles and in TMD studies.35,36 TMJ range of motion measurements, such as lateral deviation, protrusion, and retrusion, has been shown to be unreliable, while measuring opening range has high reliability.36,37 It has further been suggested that dynamic pain measurements (i.e. during movement) are of additional use and perhaps even more reliable than resting pain measurements.38 Other variables such as scoring for quality and intensity of joint sounds (popping/grinding) lack reliability.37 Therefore, three GPCS outcomes were selected by author consensus for this study: 11-point GPCS measures of resting pain, maximum opening pain, and clenching pain. Intra-group minimal detectable change (MDC) in TMD and other pain trials has been reported to be 3/10 on a GCPS.38–41 For the purposes of this pilot study, an intra-group improvement in GPCS from baseline to 6 months greater than the MDC of three points was considered clinically significant.
A secondary scale outcome measure chosen was inter-incisal opening range in millimetres (measured by the assessor with vernier callipers). MDC in inter-incisal opening measures has been reported to be between 5 and 9 mm.40–42 For the purposes of this trial, an intra-group increase in opening range from baseline to 6 months post-treatment of greater than 9 mm was considered clinically significant.
Interventions
Control group participants were informed that following a symptom monitoring period of 6 months, they would be eligible for treatment. As such, they were blinded to their control status during this time.
The IMT group underwent two treatment sessions per week for 5 weeks. Each treatment session lasted approximately 15 minutes and interventions were performed by the primary author. The techniques utilised consisted of the following in sequential order:
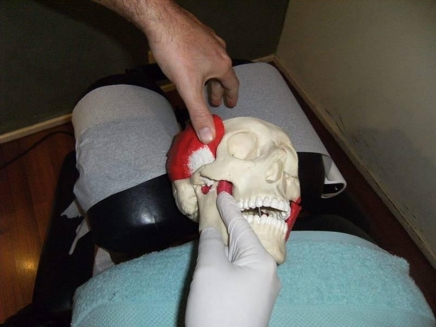
intra-oral temporalis release (Fig. 1). The practitioner was positioned homolateral to the side being treated. A gloved index contact of the caudad hand was placed onto the coronoid process of the mandible, applying light posterior and caudad pressure within pain tolerance of the patient. The cephalad index and middle fingers applied superior pressure longitudinally along the fibres of the temporalis muscle moving gradually anterior to posterior. The patient was asked to incrementally open their mouth to its maximum range. This technique was chosen due to the involvement of the temporalis muscle in various craniofacial pain syndromes;43,44
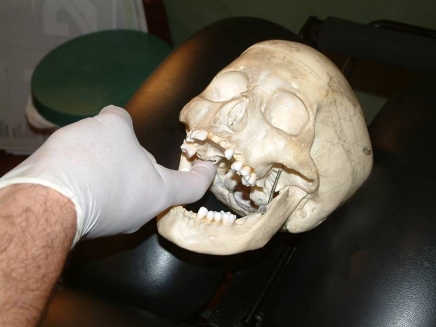
intra-oral medial and lateral pterygoid (origin) technique (Fig. 2). The practitioner was seated either homolateral or contralateral to the side being treated. A gloved index finger was inserted along the lateral wall of the pharynx, posterior to the last molar. Posterior and cephalad pressure was applied into the pharyngeal tissues overlying the pterygoid origins arising from the lateral pterygoid plate of the sphenoid. Care was taken to avoid direct contact of the hamulus. The contact was maintained for 5 seconds. This choice of techniques was based on known pterygoid involvement in chronic degenerative conditions of the TMJ45–47 and the direct influence of the lateral pterygoid in particular on disc position.47–50 Hypertrophy of the pterygoids has also been shown to irritate or compress the auriculotemporal nerve.51 Palpation of the individual medial and lateral pterygoid origins has been described in the literature.28 However, the close proximity of their intra-orally palpable origins on the lateral pterygoid plate, the general sensitivity of pharyngeal tissues, and the tendency towards restricted opening and limited access in TMD patients makes their palpatory differentiation academic rather than practical in many cases; thus each is described together for the purposes of this protocol;
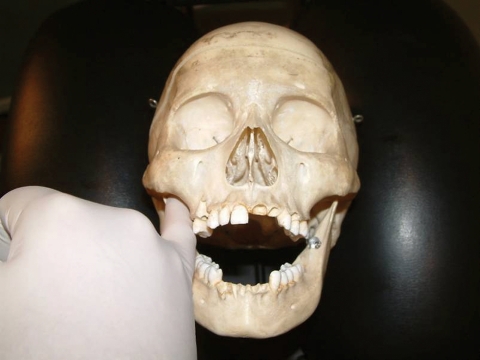
intra-oral sphenopalatine ganglion technique (Fig. 3). The gloved fifth finger of the caudad hand was slowly inserted along the buccal surface of the lightly occluded teeth. The patient was asked to briefly clench their teeth, and upon relaxing, the practitioner gradually worked their finger behind the lingual surface of the masseter and medial pterygoid. This process was repeated until the tip of the finger tip reaches as close to the anterior aspect of the infratemporal fossa/sphenopalatine fossa as is comfortable to the patient. The patient was then asked to lift their head off the table, pushing into the contact. In this way, excessive force by the practitioner was checked by an apprehension response of the patient. After three repetitions, the patient relaxed, resting his/her head back onto the headrest, and gentle buccal pressure was applied into the masseter and medial pterygoid muscles by the practitioner’s finger tip before gently being removed from the mouth. The name of this technique is based on its proposed parasympathetic neurological effects (stimulation of the sphenopalatine ganglion resulting in increased cerebral circulation), with texts suggesting that lacrimation of the ipsilateral eye indicates successful digital application of the technique.19 Evidence in support of this is lacking; nevertheless, the potential utility of myofascial stretch upon the masseter, pterygoid(s), and temporalis muscles commonly encountered in the primary author’s clinical experience was the rationale behind the inclusion of this technique.
Figure 1.
Intra-oral temporalis release.
Figure 2.
Intra-oral pterygoid release.
Figure 3.
Sphenopalatine ganglion technique.
Participants in the IMTESC group (receiving additional education and self-care) were given a scripted short lecture by the practitioner for 2 minutes at the conclusion of each of the first four visits on topics including basic TMJ anatomy; biomechanics, disc displacement, and dysfunction; the role of psycho-emotional factors in TMD particularly relating to para-functional activity; mandibular exercises to be performed at home twice a day (morning and night) as outlined below:
self-administered modification of the chiropractic technique known as ‘mandibular body — condylar cross-pressure chewing technique’ (Fig. 4). The patient applied a thenar or pisiform contact to the condyle of one side of the mandible, while the thenar of the other hand was applied to the ramus of the other side. Both sides exert even pressure upon their contacts, while the patient opens and closes their mouth five times. The contacts were reversed and repeated on the other side;
Post-isometric relaxation stretches — laterotrusion and opening (Figs. 5 and 6). The patient applied a contact to the right side of the chin with the heel of his/her right hand. An isometric resistance was applied to the chin for 10 seconds in a medial direction while the patient laterotruded against their own hands. The chin was then contralaterally laterotruded incrementally, and then the procedure was repeated from that point. Progressing in this way, the chin continues to laterotrude towards its maximum limit. The same procedure was then repeated on the other side and then it is applied to opening of the jaw, with the patient resisting closing of the mandible by cupping his/her chin with his/her hands. Patients were encouraged to perform these two exercises in front of a mirror, to ensure stability and neutrality of their head position in space during the exercises.
Figure 4.
‘Mandibular body — condylar cross-pressure chewing technique’ performed as a self-care exercise.
Figure 5.
Post-isometric relaxation self-care exercise (laterotrusion).
Figure 6.
Post-isometric relaxation self-care exercise (opening).
Visit numbers and frequency determination was without precedent in the literature, so a modification of the 6-week, 12 visit musculoskeletal rehabilitation protocol commonly used within the chiropractic outpatient clinics of Macquarie University (modelled after Mercy Hospital guidelines) was recommended based on the relative sensitivity of orofacial structures, and the personal clinical experience of the primary author in managing TMD patients.
Statistical analysis
All data underwent primary analysis using the Kolmogorov–Smirnov test. Because the data failed to show normality for graded chronic pain scale results, the data were analysed using the non-parametric Kruskal–Wallis test. The alpha-error was set at 0.05. Between group differences were analysed using the Mann–Whitney U test, P values reported as two-tailed. All data were entered into a spreadsheet and analysed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
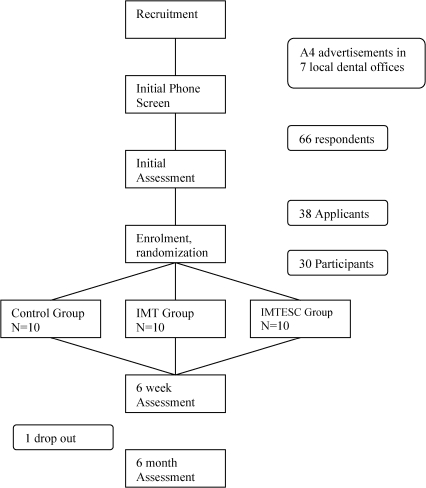
Participant advertisement and recruitment commenced in January 2006 (Fig. 7) and was concluded for this trial in April 2006. There were 66 respondents to the advertisements, of which only 38 met the primary inclusion criteria and qualified for enrolment. Upon baseline assessment, 30 consecutive applicants were enrolled into the study as participants. These were then randomized using a pre-generated, blocked design schedule into three equal groups (n = 10). All participants accepted their allocation. Treatments began in March 2006 and concluded by July 2006. The last of the 6-month assessments were concluded by March 2007. Compliance was good, with no drop outs or scheduling difficulties within the treatment groups during that time. Only one participant from the control group dropped out before the 6-month assessment due to travel commitments. No participants were worse as a result of the study.
Figure 7.
Study flow chart.
The data were analysed on an intention to treat basis with missing values replaced by baseline figures. All outcome data were subjected to Kruskal–Wallis test of comparison between groups. No significant differences were observed for primary outcome measures of pain between the groups at baseline (Table 1). The conclusion of the treatment period at t = 6 weeks (Table 2) showed no significant difference between the groups in primary outcomes of pain at rest and pain on opening; however, pain on clenching and the secondary outcome measure of opening range demonstrated statistically significant changes between groups (P<0.01 and P<0.01, respectively).
Table 1. Background and baseline outcome variables. Data except for gender are represented as mean (standard deviation).
| Detail | Control (n = 10) | IMT (n = 10) | IMTESC (n = 10) | P value* |
| Age in years | 33 (7.38) | 29.7 (8.30) | 37.3 (9.06) | 0.39 |
| Gender (m/f) | 2/8 | 3/7 | 8/2 | |
| Pain† at rest | 4.40 (0.84) | 4.00 (1.33) | 4.10 (0.74) | 0.40 |
| Pain† on opening | 6.10 (2.21) | 4.90 (1.52) | 6.00 (1.94) | 0.30 |
| Pain† on clenching | 6.10 (1.91) | 5.90 (2.08) | 6.20 (1.14) | 0.70 |
| Opening range mm | 35.80 (7.47) | 35.60 (4.85) | 42.10 (7.05) | 0.05 |
Note: *Kruskal–Wallis test for comparison between groups, significant at P<0.05.
†Self-reported graded chronic pain scale 0–10.
Table 2. Means (95% confidence interval) of outcome measures at 6 weeks, i.e. post-treatment.
| Outcome measure | Control (n = 10) | IMT (n = 10) | IMTESC (n = 10) | P value* |
| Pain† at rest | 3.20 (1.95, 4.45) | 1.30 (0.71, 1.89) | 2.70 (0.95, 4.45) | 0.07 |
| Pain† on opening | 4.00 (2.88, 5.12) | 2.40 (1.32, 3.48) | 3.60 (1.84, 5.36) | 0.16 |
| Pain† on clenching | 6.00 (4.74, 7.26) | 3.00 (0.89, 5.11) | 3.50 (1.95, 5.05) | 0.01 |
| Opening range (mm) | 36.40 (31.62, 41.18) | 40.30 (36.41, 44.19) | 46.10 (41.67, 50.53) | 0.01 |
Note: *Kruskal–Wallis test for comparison between groups, significant at P<0.05.
†Self-reported graded chronic pain scale 0–10.
At the 6-month assessment (Table 3), significant differences were observed between groups for all outcome measures, with pain scores (resting, opening, and clenching) and the secondary outcome measure of opening range at significance levels of P⩽0.01. Between-group data analysis using Mann–Whitney U test at 6 months (Table 4) showed statistically significant differences in all pain scores (P<0.01) between the control and IMT groups. Between control and IMTESC groups, there was a statistically significant difference for pain on clenching (P<0.01) and opening range (P<0.01). The IMT group showed statistically significant differences in resting and opening pain compared to the IMTESC group (P = 0.04 and P<0.01, respectively), and also for opening range (P<0.01).
Table 3. Means (95% confidence interval) of outcome measures at 6 months.
| Outcome measure | Control (n = 9) | IMT (n = 10) | IMTESC (n = 10) | P value* |
| Pain† at rest | 3.40 (2.13, 4.67) | 0.60 (0.00, 1.20) | 1.80 (0.74, 2.86) | <0.01 |
| Pain† on opening | 4.40 (2.71, 6.09) | 1.10 (0.01, 2.19) | 2.70 (1.69, 3.71) | <0.01 |
| Pain† on clenching | 5.30 (3.68, 6.92) | 1.50 (0.47, 2.53) | 1.70 (0.87, 2.53) | <0.01 |
| Opening range (mm) | 36.30 (30.11, 42.49) | 41.50 (38.76, 44.24) | 48.30 (44.59, 52.01) | 0.01 |
Note: *Kruskal–Wallis test for comparison between groups.
†Self-reported graded chronic pain scale 0–10.
Table 4. Between group differences at 6 months using Mann–Whitney U test (U).
| Outcome measure | Control versus IMT (U) | P value* | Control versus IMTESC (U) | P value* | IMT versus IMTESC (U) | P value* |
| Pain at rest | 7.00 | <0.01 | 24.00 | 0.05 | 24.00 | 0.04 |
| Pain on opening | 7.50 | <0.01 | 26.50 | 0.06 | 15.00 | <0.01 |
| Pain on clenching | 5.50 | <0.01 | 5.50 | <0.01 | 42.50 | 0.55 |
| Opening range (mm) | 28.00 | 0.09 | 15.00 | <0.01 | 13.50 | <0.01 |
Note: *Two-tailed P values significant at <0.05.
Clinical significance according to an anchored criterion of the MDC being a three-point change in GCPS score at 6 months showed improvement in pain at rest only in the IMT group (−3.4 points shift). Outcomes for opening pain showed improvement scores for both the IMT and IMTESC groups (−3.8 and −4.5 points, respectively). Outcomes for clenching pain showed improvement scores for both the IMT and IMTESC groups (−4.4 and −4.5 points, respectively). For the secondary outcome measure of opening range, none of the three groups met the MDC measure of 9 mm.
Discussion
The interventions were safely employed and the protocols were successfully administered.
The possibility of being placed in a control group can act as a disincentive to enroll, and increases the likelihood of higher drop-out rates particularly if the trial was to run for several years. Our applicants were informed that all participants needed to be monitored and that treatment was guaranteed to each enrolled participant so there may have been a generally more positive response. Larger future studies will need to take into account the need to treat the control group at the conclusion of the study.
Study weaknesses were those common to many pilot studies with a small sample of participants, namely, a tendency towards larger outcome variations, a greater tendency to non-normal distributions, and a greater treatment effect range. Further, in order to keep group numbers consistent, a blocked design was used, which itself has inherent statistical weaknesses. The results noted a trend towards improvement in both treatment groups although between the two groups, we observed no real statistical difference in pain scores at 6 months.
It was noted that the IMTESC group had a much higher proportion of males to females as a result of randomization. There is some epidemiological evidence that females may report greater TMD symptoms than men52 and that they are more susceptible to masticatory myofascial pain.53 However, the relative influence of gender on TMD treatment outcomes, or education and self-care in particular is not well known. The IMT group demonstrated better overall improvement compared to control than the IMTESC group, but the small sample size and gender discrepancy makes interpreting the comparison difficult.
The outcomes of both treatment groups suggest that IMT with or without education and self-care may be of benefit in treating cases of myogenous TMD. It has been suggested that the main mechanism of action of ‘trigger point’ releases and related therapies involves normalization of sarcomere length within contracted muscle units undergoing an energy crisis.28 The improvement observed in both treatment groups is consistent with the notion that IMT may influence chronic masticatory muscle contracture. A larger study is recommended to further define the effects.
It was hypothesized that the addition of self-care exercises and education to the treatment regime would yield stronger results by the 6-month mark, but this was clearly not the case. This may simply reflect the statistical issues inherent in small sample sizes or even the gender considerations discussed above. Compliance with exercise routines and comprehension of educational material was not assessed and might provide variables for consideration in a larger study. It was noted that the strongest outcomes were observed for changes in clenching pain. These findings were both statistically significant and exceeded the MDC. The findings suggest that the muscle fatigue and irritability experienced during sustained loading may have been particularly responsive to the myofascial techniques employed in the treatment. Vasodilation resulting in improved removal of metabolites and reduced inflammatory mediators as well as motor end-plate depolarization due to potassium release from mechanical disruption of muscle cells are two mechanisms that may explain the improvement in end range loading tolerance.28,54,55
Opening pain was only statistically significant for the IMT group, though both treatment groups exceeded the MDC. These results suggest that functional pain outcomes are important variables to consider in cases of myogenous TMD. The results also suggest that the myofascial techniques chosen for the study may be adequate and appropriate for a larger study of myogenous and perhaps even other (e.g. arthritic) TMD patients.
Conclusion
All pilot studies warrant cautious interpretation of results. In a small study such as this, conclusions about effectiveness cannot be made. A sufficiently large pool of participants in a well-designed RCT conducted over at least 1–2 years would be preferable and such a study is currently being undertaken. Nevertheless, this pilot study demonstrated several important considerations necessary in planning a large scale trial. The lack of adverse reactions in any participant during the course of the study should encourage further interest in conservative, non-invasive management strategies for sufferers of myogenous TMD.
References
- 1.Smith JA, Syrop S. TMD incidence and socioeconomic standing. Eradicating the myth of the upper class patient. NY State Dent J 1994;60:36–9 [PubMed] [Google Scholar]
- 2.Goddard G, Karibe H. TMD prevalence in rural and urban Native American populations. Cranio 2002;20:125–8 [DOI] [PubMed] [Google Scholar]
- 3.Morinushi T, Ohno H, Ohno K, Oku T, Ogura T. Two year longitudinal study of the fluctuation of clinical signs of TMJ dysfunction in Japanese adolescents. J Clin Pediatr Dent 1991;15:232–40 [PubMed] [Google Scholar]
- 4.Reik L, Jr, Hale M. The temporomandibular joint pain-dysfunction syndrome: a frequent cause of headache. Headache 1981;21:151–6 [DOI] [PubMed] [Google Scholar]
- 5.Kolbinson DA, Epstein JB, Burgess JA. Temporomandibular disorders, headaches, and neck pain following motor vehicle accidents and the effect of litigation: review of the literature. J Orofac Pain 1996;10:101–25 [PubMed] [Google Scholar]
- 6.Hedenberg-Magnusson B, Ernberg M, Kopp S. Symptoms and signs of temporomandibular disorders in patients with fibromyalgia and local myalgia of the temporomandibular system. A comparative study. Acta Odontol Scand 1997;55:344–9 [DOI] [PubMed] [Google Scholar]
- 7.Ren YF, Isberg A. Tinnitus in patients with temporomandibular joint internal derangement. Cranio 1995;13:75–80 [DOI] [PubMed] [Google Scholar]
- 8.Tuz HH, Onder EM, Kisnisci RS. Prevalence of otologic complaints in patients with temporomandibular disorder. Am J Orthod Dentofacial Orthop 2003;123:620–3 [DOI] [PubMed] [Google Scholar]
- 9.Kalamir A, Pollard H, Vitiello A, Bonello R. TMD and the problem of bruxism: a review. J Bodyw Mov Ther 2007;11:183–93 [Google Scholar]
- 10.Jaeger R. Sphenopalatine neuralgia. Cure of four consecutive cases by removing the retrosal nerves and superior cervical sympathetic ganglion. Med Ann Dist Columbia 1964;33:258–9 [PubMed] [Google Scholar]
- 11.Felisati G, Arnone F, Lozza P, Leone M, Curone M, Bussone G. Sphenopalatine endoscopic ganglion block: a revision of a traditional technique for cluster headache. Laryngoscope 2006;116:1447–50 [DOI] [PubMed] [Google Scholar]
- 12.Segami N, Murakami K, Iizuka T. Arthrographic evaluation of disk position following mandibular manipulation technique for internal derangement with closed lock of the temporomandibular joint. J Craniomand Dis 1990;4:99–108 [PubMed] [Google Scholar]
- 13.van Dyke AR, Goldman SM. Manual reduction of displaced disk. Cranio 1990;8:350–2 [DOI] [PubMed] [Google Scholar]
- 14.Mongini F. A modified extraoral technique of mandibular manipulation in disk displacement without reduction. Cranio 1995;13:22–5 [DOI] [PubMed] [Google Scholar]
- 15.Simmons HC., 3rd Orthodontic finishing after TMJ disk manipulation and recapture. Int J Orthod 2002;13:7–12 [PubMed] [Google Scholar]
- 16.Chiba M, Echigo S. Longitudinal MRI follow-up of temporomandibular joint internal derangement with closed lock after successful disk reduction with mandibular manipulation. Dentomaxillofac Radiol 2005;34:106–11 [DOI] [PubMed] [Google Scholar]
- 17.McNeely ML, Armijo Olivo S, Magee DJ. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys Ther 2006;86:710–25 [PubMed] [Google Scholar]
- 18.Chaitow L. Cranial manipulation — theory and practice. Edinburgh: Churchill Livingston; 2005 [Google Scholar]
- 19.Gehin A. Atlas of manipulative techniques for the cranium and face. Seattle, WA: Eastland Press; 1985 [Google Scholar]
- 20.Pick M. Cranial sutures. Seattle, WA: Eastland Press; 1999 [Google Scholar]
- 21.Laskin D, Temporomandibular disorders: an evidence-based approach to diagnosis and treatment Hanover Park, IL: Quintessence Publishing Co.; 2006 [Google Scholar]
- 22.Bergmann T, Peterson DR, Lawrence DJ. Chiropractic technique. New York: Churchill Livingston; 1993 [Google Scholar]
- 23.Millard FP. Applied anatomy of the lymphatics. Kirksville, MO: International Lymphatic Research Society; 1922 [Google Scholar]
- 24.SORSI Cranial technique participant guide. 3rd ed. Sedan, KS: KS SORSI-SOTO; 1990 [Google Scholar]
- 25.Esposito S, Rigney C. Manual of peripheral technique. Sydney, NSW: Macquarie University; 2004 [Google Scholar]
- 26.Sutherland WG. The cranial bowl. Mankato, MN: Free Press Company; 1939 [Google Scholar]
- 27.Millard FP. Finger surgery in the treatment of the lymphatics of the eye, ear, nose, and throat. Walmsley AG, editor. Applied anatomy of the lymphatics. Kirksville, MO: The Journal Printing Company; 1922 [Google Scholar]
- 28.Simons DG, Travell JG, Simons LS, Cummings BD. Travell & Simons’ myofascial pain and dysfunction: the trigger point manual. 2nd ed. Vol. 1. Baltimore, MD: Lippincott Williams & Wilkins; 1998 [Google Scholar]
- 29.Travell JG, Simons DG. Myofascial pain and dysfunction: trigger point manual. 2nd ed. Vol. 1. Baltimore, MD: Williams & Wilkins; 1999 [Google Scholar]
- 30.Kalamir A, Pollard H, Vitiello A, Bonello R. Manual therapy for temporomandibular disorders: a review of the literature. J Bodyw Move Ther 2007;11:84–90 [Google Scholar]
- 31.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Discord 1992;6:301–55 [PubMed] [Google Scholar]
- 32.Pehling J, Schiffman E, Look J, Shaefer J, Lenton P, Fricton J. Interexaminer reliability and clinical validity of the temporomandibular index: a new outcome measure for temporomandibular disorders. J Orofac Pain 2002;16:296–304 [PubMed] [Google Scholar]
- 33.Dworkin SF, Sherman J, Mancl L, Ohrbach R, LeResche L, Truelove E. Reliability, validity, and clinical utility of the research diagnostic criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain 2002;16:207–20 [PubMed] [Google Scholar]
- 34.Michelotti A, Steenks MH, Farella M, Parisini F, Cimino R, Martina R. The additional value of a home physical therapy regimen versus patient education only for the treatment of myofascial pain of the jaw muscles: short-term results of a randomized clinical trial. J Orofac Pain 2004;18:114–25 [PubMed] [Google Scholar]
- 35.Brown FF, Robinson ME, Riley JL, 3rd, Gremillion HA, McSolay J, Meyers G. Better palpation of pain: reliability and validity of a new pressure pain protocol in TMD. Cranio 2000;18:58–65 [DOI] [PubMed] [Google Scholar]
- 36.Dworkin SF, LeResche L, DeRouen T, von Korff M. Assessing clinical signs of temporomandibular disorders: reliability of clinical examiners. J Prosth Dent 1990;63:574–9 [DOI] [PubMed] [Google Scholar]
- 37.Leher A, Graf K, PhoDuc JM, Rammelsberg P. Is there a difference in the reliable measurement of temporomandibular disorder signs between experienced and inexperienced examiners? J Orofac Pain 2005;19:58–64 [PubMed] [Google Scholar]
- 38.Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, et al. Assessment of pain. Br J Anaesth 2008;101:17–24 [DOI] [PubMed] [Google Scholar]
- 39.Bolton JE. Sensitivity and specificity of outcome measures in patients with neck pain: detecting clinically significant improvement. Spine 2004;29:2410–7 [DOI] [PubMed] [Google Scholar]
- 40.Kropmans TJ, Dijkstra PU, Stegenga B, Stewart R, de Bont LG. Smallest detectable difference in outcome variables related to painful restriction of the temporomandibular joint. J Dent Res 1999;78:784–9 [DOI] [PubMed] [Google Scholar]
- 41.Kropmans TJ, Dijkstra PU, Stegenga B, de Bont LG. Therapeutic outcome assessment in permanent temporomandibular joint disc displacement. J Oral Rehabil 1999;26:357–63 [DOI] [PubMed] [Google Scholar]
- 42.Kropmans TJ, Dijkstra PU, Stegenga B, Stewart R, de Bont LG. Repeated assessment of temporomandibular joint pain: reasoned decision-making with use of unidimensional and multidimensional pain scales. Clin J Pain 2002;18:107–15 [DOI] [PubMed] [Google Scholar]
- 43.Bakke M, Michler L. Temporalis and masseter muscle activity in patients with anterior open bite and craniomandibular disorders. Scand J Dent Res 1991;99:219–28 [DOI] [PubMed] [Google Scholar]
- 44.Marks L, Teng S, Artun J, Herring S. Reaction strains on the condylar neck during mastication and maximum muscle stimulation in different condylar positions: an experimental study in the miniature pig. J Dent Res 1997;76:1412–20 [DOI] [PubMed] [Google Scholar]
- 45.Fujita S, Iizuka T, Dauber W. Variation of heads of lateral pterygoid muscle and morphology of articular disc of human temporomandibular joint: anatomical and histological analysis. J Oral Rehabil 2001;28:560–71 [DOI] [PubMed] [Google Scholar]
- 46.Campbell CD, Loft GH, Davis H, Hart DL. TMJ symptoms and referred pain patterns. J Prosthet Dent 1982;47:430–3 [DOI] [PubMed] [Google Scholar]
- 47.Mahan PE, Wilkinson TM, Gibbs CH, Mauderli A, Brannon LS. Superior and inferior bellies of the lateral pterygoid muscle EMG activity at basic jaw positions. J Prosthet Dent 1983;50:710–8 [DOI] [PubMed] [Google Scholar]
- 48.Murray GM, Phanachet I, Uchida S, Whittle T. The role of the human lateral pterygoid muscle in the control of horizontal jaw movements. J Orofac Pain 2001;15:279–92 [PubMed] [Google Scholar]
- 49.Yang X, Pemu H, Pyhtinen J, Tiilikainen PA, Oikarinen KS, Raustia AM. MRI findings concerning the lateral pterygoid muscle in patients with symptomatic TMJ hypermobility. Cranio 2001;19:260–8 [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Pernu H, Pyhtinen J, Tiilikainen PA, Oikarinen KS, Raustia AM. MR abnormalities of the lateral pterygoid muscle in patients with nonreducing disk displacement of the TMJ. Cranio 2002;20:209–21 [DOI] [PubMed] [Google Scholar]
- 51.Schmidt BL, Pogrel MA, Necoechea M, Kearns G. The distribution of the auriculotemporal nerve around the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86:165–8 [DOI] [PubMed] [Google Scholar]
- 52.de Kanter RJ, Truin GJ, Burgersdijk RC, Van’t Hof MA, Battistuzzi PG, Kalsbeek H, et al. Prevalence in the Dutch adult population and a meta-analysis of signs and symptoms of temporomandibular disorder. J Dent Res 1993;72:1509–18 [DOI] [PubMed] [Google Scholar]
- 53.Karibe H, Goddard G, Gear RW. Sex differences in masticatory muscle pain after chewing. J Dent Res 2003;82:112–6 [DOI] [PubMed] [Google Scholar]
- 54.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil 2008;89:16–23 [DOI] [PubMed] [Google Scholar]
- 55.Simons DG. New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil 2008;89:157–9 [DOI] [PubMed] [Google Scholar]