Abstract
The authors report the case of a 68-year-old woman with secondary adenocarcinoma of the lungs from an unknown primary. Erlotinib was started which produced symptoms suggestive of uveitis. Erlotinib was stopped and restarted a month later at a lower dose, which resulted in severe bilateral anterior uveitis. The uveitis settled after stopping erlotinib and treatment with topical steroids and cycloplegics. To the best of the authors’ knowledge, this is the first case of erlotinib-related anterior uveitis.
Background
This is the first reported case of uveitis associated with erlotinib. If not recognised this may lead to inappropriate treatment (before an ophthalmologist review, as in our case) and may lead to visual loss.
Case presentation
A 68-year-old woman was referred to us with symptoms of bilateral red and sore eyes for a period of 3 weeks. Ocular history included left amblyopia but no known history of uveitis. Significant medical history included secondary lung carcinoma with unknown primary. Recent chemotherapy had yielded suboptimal response after which oral erlotinib (tarceva) 150 mg was started. She developed sore and red eyes bilaterally. She was treated with topical chloramphenicol 0.5% four times a day on a presumed diagnosis of conjunctivitis but did not respond to treatment. Two weeks later, she developed fever which was attributed to erlotinib and this drug was then stopped. Patient described improvement in red and sore eyes while off medication. Oral erlotinib was restarted a month later at a reduced dosage of 100 mg per day. She developed bilateral sore eyes again 2 days after starting oral erlotinib at the lower dose. At this stage an ophthalmic opinion was sought.
Her visual acuity was recorded as 6/12 OD and 6/36 OS (amblyopic eye). Slit-lamp examination showed bilateral non-granulomatous anterior uveitis with posterior synaechae in both eyes (figure 1). Posterior segment examination was unremarkable.
Figure 1.
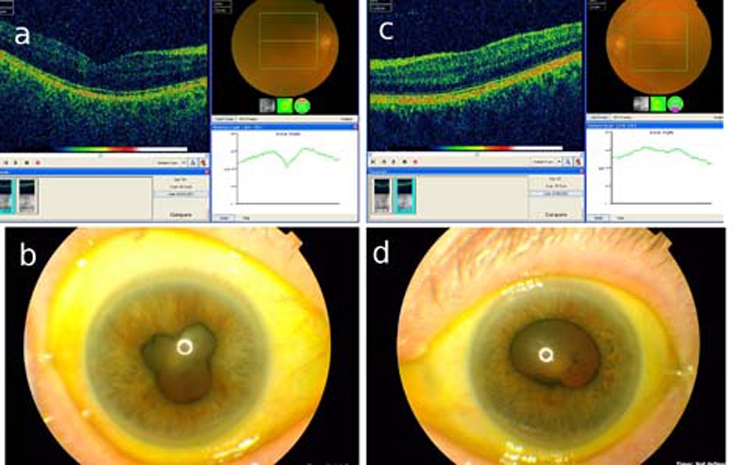
Photograph showing ocular coherence tomography and retinal photograph ((A) right eye, (C) left eye). Also showing posterior synaechiae ((B) right eye, (D) left eye). Note hazy retinal photograph in OCT inset due to uveitis.
Investigations
Ocular coherence tomography did not reveal any macular oedema attributable to uveitis (figure 1). Routine blood examination was negative.
Treatment
Erlotinib was discontinued and topical guttae prednisolone 1% (predforte) one hourly, guttae cyclopentolate 1% three times a day and betnesol ointment were started. This was reduced to prednisolone eye drops four times a day after a week and stopped after tapering.
Outcome and follow-up
The uveitis responded well, posterior synaechae resolved and symptoms improved.
Discussion
Erlotinib (tarceva) is an epidermal growth factor receptor tyrosine kinase inhibitor which is licensed for use in advanced non-small cell carcinoma of the lung.1 Known ocular side effects are ocular surface disorders, conjunctivitis, trichomegaly and keratoconjunctivitis.2–4 The drug is reported to be associated with trichomegaly and corneal epithelial defects. In our case, the diagnosis was missed by general physicians as the symptoms were thought to have been caused by conjunctivitis or ocular surface irritation and treated with topical chloramphenicol. A slit-lamp examination by ophthalmologist was necessary to clinch the underlying diagnosis. The close temporal relationship of symptoms with the onset of ocular symptoms indicates the likely association of erlotinib with bilateral uveitis. In this case, erlotinib was restarted at a lower dose, despite which the patient developed uveitis. This may suggest development of an idiosyncratic reaction causing uveitis rather than a dose dependent effect.
As erlotinib is a relatively new drug introduced into clinical practice, the potential adverse effects should be monitored. As best as the authors know, uveitis is not a side effect of this medication.5 We suggest caution when patients complain of sore eyes, photophobia or decreased vision or symptoms suggestive of uveitis when using this medication. An ophthalmology consultation should be sought if symptoms do not correspond to ocular surface disease or the symptoms do not respond to treatment.
Learning points.
-
▶
Ocular surface disorders may be more common with erlotinib; however, physicians should keep a high index of suspicion when symptoms do not correspond to ocular surface problems.
-
▶
We suggest caution when patients complain of sore eyes, photophobia or decreased vision or symptoms suggestive of uveitis when using this medication.
-
▶
This is the first reported case of uveitis associated with erlotinib. An ophthalmology consultation should be sought if symptoms do not correspond to ocular surface disease or the symptoms do not respond to treatment.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Tarceva Erlotinib http://www.tarceva.net/portal/eipf/pb/tarceva/erlotinib/home?_requestid=855968 (accessed 3 March 2011).
- 2.Márquez G, Herrera-Acosta E, Vidal I, et al. A case of trichomegaly of the eyelashes and facial hypertrichosis induced by erlotinib (Tarceva). Int J Dermatol 2009;48:97–8 [DOI] [PubMed] [Google Scholar]
- 3.Johnson KS, Levin F, Chu DS. Persistent corneal epithelial defect associated with erlotinib treatment. Cornea 2009;28:706–7 [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos R, Chasapi V, Bachariou A. Trichomegaly induced by erlotinib. Orbit 2008;27:329–30 [DOI] [PubMed] [Google Scholar]
- 5.Tarceva Erlotinib Tablets http://www.tarceva.com/patient/considering/effects.jsp (accessed 3 March 2011).


