Abstract
Calcified amorphous tumour (CAT) of the heart is a rarely reported non-neoplastic cardiac mass. The authors report a 69-year-old female with long-standing severe asthma and on home oxygen, who presented with a 2 cm mobile mass in the left ventricular outflow tract and symptoms of left heart failure and stroke. During minimal access cardiac surgery, a CAT was found attached to the base of the mitral valve. The tumour was removed and the patient had an uneventful postoperative course. The authors present their experience with this patient and review the current literature on this rare kind of tumour.
Background
Left-sided tumours of the heart are known to cause symptoms of left heart failure and arterial embolism. Hence, resection using open heart surgery is the treatment of choice.1 However, in patients with severe respiratory disease, median sternotomy and cardiac surgery using cardiopulmonary bypass may not be feasible due to its very high perioperative risk of respiratory failure.
A calcified amorphous tumour (CAT) of the heart is an extremely rare non-neoplastic cardiac mass. It was initially described in 1997 and only a handful of cases have been published so far.2
We report our experience with a patient presenting with a CAT and severe respiratory disease who underwent surgical removal using minimal access cardiac surgery, and review the current literature.
Case presentation
A 69-year-old female presented to King’s College Hospital with acute dyspnoea and pulmonary oedema, without signs of infection. Her coronary risk factors included hypertension, type II diabetes and myocardial infarction 10 years ago. Given her history of coronary artery disease and a positive troponin test (1.1 μg/l), the episode was initially diagnosed as an ischaemic coronary event. Other relevant comorbidities were severe chronic obstructive pulmonary disease with bronchial asthma requiring home oxygen and polymyalgia rheumatica, both involving long-term steroid use of prednisolone (20–50 mg) according to symptoms. Her lung function test showed a severe obstruction with a forced expiration over 1 s of 900 ml (50% of the predicted), without effect to bronchodilators. Her arterial blood gas on room air was pCO2 6.0 kPa and pO2 7.7 kPa and biochemistry assays, including serum calcium and parathormone levels were within normal limits. There was no evidence of deranged renal function and ECG on initial presentation showed sinus rhythm with no evidence of cardiac ischaemia or left venticle dysfunction.
Investigations
Transthoracic echocardiography (TTE) after initial admission highlighted a mobile mass of 2 cm in length in her left ventricular outflow tract (LVOT) (figure 1). Three-D transoesophageal echocardiography (3-D TOE) showed the mass attached around the basal part of the A2 scallop of her mitral valve. The mass moved freely across the aortic valve during systole. The clinical presentation of the patient and the morphology of the mass were consistent with a provisional diagnosis of an intracardiac tumour rather than vegetation, and the probable diagnosis of a papillary fibroelastoma was made. The left ventricular ejection fraction was 55% and the posterior mitral valve leaflet showed some restriction resulting in mild mitral regurgitation.
Figure 1.
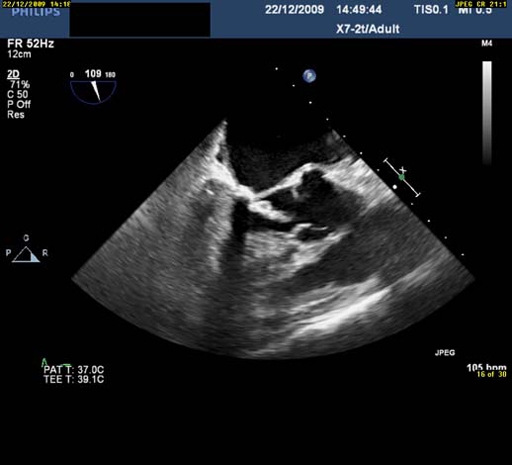
Echocardiography showing the mobile mass in the left ventricular outflow tract and prolapsing into the open aortic valve.
As part of her preoperative diagnostics, the patient underwent a coronary angiogram which revealed an occluded right coronary artery (RCA). After discussion at our multidisciplinary cardiac meeting she was offered high-risk cardiac surgery using a limited sternotomy. However, due to improvements of her symptoms and the very high predicted perioperative risks she faced, the patient turned down the option of any surgical treatment at that time.
In the following 4 months she had further four admissions with dyspnoea and also suffered a left-sided cerebral infarct. After complete neurological investigations this event was thought to be most likely of embolic nature caused by the intracardiac mass. Her neurological symptoms resolved over a period of 1 week, and she was now willing to consent for cardiac surgery. Given her lack of coronary symptoms and her respiratory condition, the decision was made not to revascularise the RCA.
Treatment
The operation was performed through an L-shaped limited sternotomy into the 4th right intercostal space, under 3-D TOE guidance. After the patient was placed on cardiopulmonary bypass using a two-stage cannula, she was cooled down to 32ºC and cardioplegia was given directly into the aortic root. The ascending aorta was opened through a transverse incision 1 cm above the RCA ostium and the tumour approached through the aortic valve. Examination of the calcified tumour showed that it was attached to the anterior mitral valve ring. A shave excision of the mass was possible and the tumour was therefore removed without the valve requiring any repair.
Outcome and follow-up
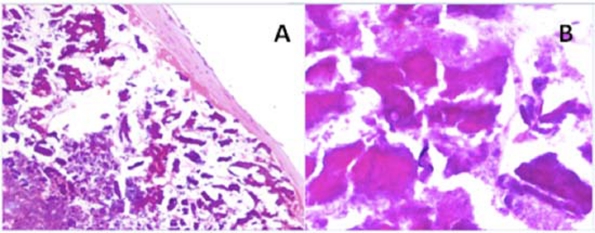
The patient made an uneventful recovery from surgery and was discharged home on the seventh postop day. Histological investigation of the mass showed no malignant cells and the final diagnosis of a CAT tumour was made (figure 2). At 3 months follow-up, her TTE was satisfactory; however, the patient continues to suffer from dyspnoea on moderate exertion.
Figure 2.
(A) Normal and (B) ×4 magnification shows the calcified amorphous tumour mass with large area of calcification.
Discussion
CAT was first described as a distinct pathological entity in 1997 when Reynolds et al published a series of 11 cases from the Mayo Clinic between 1965 and 1997.2 Grossly, the lesions were firm, yellow-white, and partially calcified, and arose in any of the four chambers. Microscopically, all lesions were characterised by nodular calcium in a background of degenerating blood elements and chronic inflammation. The initiating factors leading to fibrin aggregation and for the thrombotic material to undergo dystrophic calcification rather than the usual process of organisation, is not clear. However, the fibrin on the surface is a potential source of emboli.
Since then, there have been another eight cases reported in the literature.3–9 Six of these patients had CAT involving the myocardium of the heart,3–8 whereas the other two where mobile masses arising from the mitral valve and tricuspid valve respectively, as in our case.4 9 The patients had initially presented with syncopy, dyspnoea, chest pain and arrhythmia, whereas in one patient it was a coincidental finding on echo while investigating sepsis.
The differential diagnosis of a cardiac tumour includes a variety of neoplastic and non-neoplastic processes. Cardiac valve tumours are rare, accounting for only 8% of cardiac tumours and of these, papillary fibroelastomas are the most predominant. Although TTE is sufficient for the diagnosis of most cardiac tumours, small or multiple tumours may be missed and TOE is recommended. Following 3-D TOE, the main differential diagnosis in our case was papillary fibroelastoma as 90% of these tumours affect the heart valves with 35% of these affecting the mitral valve.10 Also 90% of these tumours are solitary, as was the case here, and their calcification has also been reported in the literature.11 However, histologically these tumours have a connective tissue core of collagen, elastin and smooth muscle cells covered with endothelium, none of these features were observed in our case. Myxomas can show prominent areas of calcification and have also been reported to affect the mitral valve.12 But on echocardiogram they are typically found in the left atria attached to the septum by a stalk and histologically there should still have been some areas of classic myxoma present showing the polygonal myxoma cells.
Therefore, as the mass was composed of amorphous material with areas of fibrosis and heavy calcification, histologically, the differential diagnosis became one of non-neoplastic causes such as vegetation. The mass could be a sequelae of some past infection with the infected mass subsequently becoming calcified. However, our patient gave no history of infective endocarditis or rheumatic fever and the changes on the mitral valve were of ischaemic nature and explained with the occlusion of the RCA. Therefore, taking the history and histological features in combination confirmed our diagnosis of CAT.
The location of the cardiac tumour is of importance because of its potential to embolise. Right-sided cardiac tumours remain predominantly asymptomatic until they become large enough to interfere with intracardiac blood flow, alter haemodynamic function or induce arrhythmias. Chronic, repeating pulmonary embolisation may lead to significant hypoxemia and severe pulmonary hypertension. Left-sided tumours may result in arterial embolism, resulting in stroke, myocardial infarction, mesenteric ischaemia, renal infarction or limb ischaemia. Depending on their size and mobility, the cardiac tumour can also give rise to obstruction of left ventricular filling during diastole, or LVOT obstruction, resulting in left heart failure and possible pulmonary oedema. The CAT reported here was unique in that it was freely mobile across the aortic valve.
The surgical resection of a symptomatic or asymptomatic benign cardiac mass offers good long-term survival.1 Left-sided tumours should be resected even in asymptomatic patients to prevent sudden death and arterial emboli. Surgical excision of a CAT is usually sufficient to manage the tumour as it is benign and complete surgical excision of the mass should be curative. Incomplete resection resulting in recurrence has been reported in the literature for these tumours and therefore their surgical removal requires due diligence.5
However, severe comorbidities such as severe respiratory disease, as in our patient, may increase the predicted operative mortality such that on balance any surgical intervention is deemed not to be feasible. Clearly in our case, the patient was extremely lucky to fully recover from her stroke after she had initially turned down the option of surgical resection of the mass. Nevertheless, our experience with her postoperative course demonstrates that using modern surgical techniques, in the form of minimal access cardiac surgery, reduces perioperative risk allowing for surgical intervention in high-risk patients who would otherwise have been deemed inoperable in the past.
Learning points.
-
▶
The surgical resection of a symptomatic or asymptomatic benign cardiac mass offers good long-term survival.1
-
▶
Left-sided tumours should be resected even in asymptomatic patients to prevent sudden death and arterial emboli.
-
▶
Minimal access cardiac surgery reduces perioperative risk allowing for surgical intervention in high-risk patients who would otherwise have been deemed inoperable in the past.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Elbardissi AW, Dearani JA, Daly RC, et al. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation 2008;118(14 Suppl):S7–15 [DOI] [PubMed] [Google Scholar]
- 2.Reynolds C, Tazelaar HD, Edwards WD. Calcified amorphous tumor of the heart (cardiac CAT). Hum Pathol 1997;28:601–6 [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez-Barrios A, Muriel-Cueto P, Lancho-Novillo C, et al. Calcified amorphous tumor of the heart. Rev Esp Cardiol 2008;61:892–3 [PubMed] [Google Scholar]
- 4.Morishima A, Sasahashi N, Ueyama K. [Calcified amorphous tumors with excision in hemodialysis patients: report of 2 cases]. Kyobu Geka 2006;59:851–4 [PubMed] [Google Scholar]
- 5.Fealey ME, Edwards WD, Reynolds CA, et al. Recurrent cardiac calcific amorphous tumor: the CAT had a kitten. Cardiovasc Pathol 2007;16:115–18 [DOI] [PubMed] [Google Scholar]
- 6.Lewin M, Nazarian S, Marine JE, et al. Fatal outcome of a calcified amorphous tumor of the heart (cardiac CAT). Cardiovasc Pathol 2006;15:299–302 [DOI] [PubMed] [Google Scholar]
- 7.Ho HH, Min JK, Lin F, et al. Images in cardiovascular medicine. Calcified amorphous tumor of the heart. Circulation 2008;117:e171–2 [DOI] [PubMed] [Google Scholar]
- 8.Habib A, Friedman PA, Cooper LT, et al. Cardiac calcified amorphous tumor in a patient presenting for ventricular tachycardia ablation: intracardiac echocardiogram diagnosis and management. J Interv Card Electrophysiol 2010;29:175–8 [DOI] [PubMed] [Google Scholar]
- 9.Flynn A, Mukherjee G. Calcified amorphous tumor of the heart. Indian J Pathol Microbiol 2009;52:444–6 [DOI] [PubMed] [Google Scholar]
- 10.Ngaage DL, Mullany CJ, Daly RC, et al. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg 2005;80:1712–18 [DOI] [PubMed] [Google Scholar]
- 11.Paelinck B, Vermeersch P, Kockx M. Calcified papillary fibroelastoma of the tricuspid valve. Acta Cardiol 1998;53:165–7 [PubMed] [Google Scholar]
- 12.Martìn-Suàrez S, Botta L, Dell’Amore A, et al. Mitral valve myxoma involving both leaflets. Cardiovasc Pathol 2007;16:189–90 [DOI] [PubMed] [Google Scholar]