Abstract
Therapy of intracellular pathogens can be complicated by drug toxicity, drug resistance and the need for prolonged treatment regimens. One approach which has shown promise is immunotherapy. Leishmaniasis, a vector-borne disease ranked among the six most important tropical infectious diseases by the WHO, has been treated clinically with crude or defined vaccine preparations or cytokines such as IFN-γ and GM-CSF in combination with chemotherapy. We have attempted to develop an improved and defined immunotherapeutic using a mouse model of cutaneous leishmaniasis. We hypothesized that immunotherapy may be improved by using TLR synergy to enhance the parasite-specific immune response. We formulated L110f, a well-established Leishmania poly-protein vaccine candidate, in conjunction with either monophosphoryl lipid A (MPL), a TLR 4 agonist, or CpG, a TLR 9 agonist, or a combination of these and evaluated anti-leishmania immune responses in absence or presence of active disease. Only mice treated with L110f + MPL-CpG were able to induce a strong effective T cell response during disease and subsequently cured lesions and reduced parasite burden when compared to mice treated with L110f and either single adjuvant. Our data help to define a correlate of protection during active infection and indicate TLR synergy to be a potentially valuable tool in treating intracellular infections.
Introduction
Leishmania are obligate intracellular parasites that induce chronic infections in which these protozoa may establish itself in part through immunomodulation of the host. Depending on the species, infection may cause lesions of skin (cutaneous leishmaniasis; CL) or mucosa (mucosal leishmaniasis; ML) or the most fatal outcome, wherein parasite disseminates in the spleen and liver causing visceral leishmaniasis (VL; Kala-Azar) (1). Post-Kala-Azar Dermal Leishmaniasis or PKDL, is yet another disease caused by persistence of Leishmania donovani parasites in the skin following apparently successful treatment of VL (2, 3). Chemotherapy with antimony containing drugs, such as Pentostam and Glucantime or anti-fungal and anti-protozoan drugs such as Amphotericin B and Miltefosine have showed some success but drug-resistant strains are on the rise (4–6).
To date, only two vaccines have been licensed for use in humans, one for prophylaxis using live parasites in Uzbekistan and the second for immunotherapy, that used killed parasites in Brazil (no longer manufactured). While both these vaccines have shown varied success, reports of adverse reactions at injection site and quality control issues are significant (7–10). Second-generation vaccines using live genetically modified parasites, or bacteria or viruses containing Leishmania genes, recombinant or native fractions have been known since the 1990s (11, 12). A number of groups have reported on the use of TLR agonists as adjuvants with recombinant proteins against this disease. Agonists of TLRs 4 (MPL and GLA), 7 (Imiquimod), and 9 (CpG) have all shown to be successful in different animal models of leishmaniasis (13–20).
LEISH-F1 (also known as L110f) in combination with the adjuvant MPL, is the first defined vaccine candidate for leishmaniasis to be tested in both animals and human clinical trials (12, 14, 15, 21, 22). This vaccine has been shown to induce strong Th1 responses that can help eliminate parasite upon infection (14, 21). Recently, Seder and co-workers used this antigen (Ag) to establish a protective correlate for prophylaxis. Optimal formulation and delivery of this Ag, was shown to induce a multi-functional CD4 T cell subset that secreted IFN-γ, TNF-α and IL-2, found to be crucial for parasite clearance upon infection (14, 20, 23). However, less is known regarding the ability to generate effective T cell responses in the face of clinical or subclinical infection, an important issue for individuals in endemic areas. There is still no approved “second-generation” vaccine for therapy of leishmanial infection, even though immunotherapy against Leishmaniae has been practiced for more than a century now (24). Use of heat killed Leishmania as an immunotherapeutic vaccine, while approved in Brazil is still not widely accepted (11, 12). The undefined nature of this product coupled with reports of adverse effects, have made it difficult to standardize formulation and define protective correlates during infection.
We previously used a defined Ag preparation to successfully treat drug-refractory ML patients (25, 26). Patients were treated with a combination of 4 leishmanial proteins (3 of which have been fused to create L110f) adjuvanted with GM-CSF. In this study we used L110f to optimize adjuvants in the vaccine formulation and define immune correlates for protection during active infection. We explored the use of TLR synergy during active disease in mice, using a high dose infection model. Our studies show that while administration of L110f with either TLR4 or TLR9 agonist induce high levels of multi-functional effector T cells in the absence of infection they fail to do so during active infection. The combination of these agonists, however, overcomes this defect. Further, we also show the importance of antigen during immunotherapy, in the absence of which treatment with adjuvants alone may exacerbate disease.
Materials & Methods
Mice
Female Balb/C mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained in specific pathogen-free conditions in the animal facilities of IDRI, Seattle, WA. All mice entered experiments at 6–8 weeks of age. All animal procedures were approved by the institutional animal care and use committee.
Parasites & Infection
L. major clone V1 (MHOM/IL/80/Friedlin) parasites were grown as previously described (21). 1–2*105 highly infective-stage metacyclic promastigotes were isolated from stationary cultures (5–7 day-old) by negative selection using peanut agglutinin (Vector Labs, Burlingame, CA) and injected sub-cutaneously (s.c) in the left footpad. The evolution of the lesion was monitored weekly by measuring foot pad thickness using metric caliper (Mitutoyo Measuring Instruments, Aurora, IL). To determine parasite concentrations, footpads were weighed and homogenized using 20 ml M199 medium/gram of tissue. Two fold serial dilutions of the homogenized tissue suspensions were then plated in 96-well plates and incubated at 26°C for 7–10 days. Wells were examined for viable and motile promastigotes and the highest dilution that was positive for parasites was considered to be the parasite concentration per milligram of tissue. The total parasite burden was calculated using the weight of the footpad.
Immunizations
5 μg of recombinant L110f protein (IDRI, Seattle, WA) was mixed with either 20 μg either GLA-SE (IDRI), MPL-SE® (GSK Biologicals, Hamilton, MT) or 50 μg CpG ODN 1826 (Coley Pharmaceuticals, Ottawa, ON, Canada) that was formulated in a stable emulsion as reported earlier (27). For the vaccine-containing adjuvant combo, L110f was mixed with CpG that had been previously mixed in MPL-SE® (MPL-CpG) or GLA-SE (GLA-CpG). All mice were treated 7 days post-infection, by s.c. injection of 0.1 ml vaccine at the base of the tail at weekly intervals.
Cell preparations
For Bone Marrow Dendritic cells (BMDCs), Balb/C bone marrow cells were collected by flushing the femur and tibia with RPMI-1640 (Invitrogen, Carlsbad, CA) and cultured in 100 mm Petri dishes (VWR, West Chester, PA ) as previously described for 10 days in the presence of GM-CSF (28).
Single cell suspensions were prepared from individual spleens and pooled draining lymph nodes (dLN; inguinal for vaccine and popliteal for parasite). Spleens and dLN were disrupted between frosted slides and erythrocytes removed from spleens by lysis in 1.66% NH4 Cl solution. Mononuclear cells were enumerated using either a hemocytometer or a ViaCount assay with a PCA system (Guava Technologies, Hayward, CA).
Flow cytometry
Single cell suspensions from individual spleens and pooled dLNs were isolated after 3 or 5 doses of vaccine and cultured at 1×106 cells/well cells in duplicate or triplicate in a 96-well U-bottom plate (Corning Incorporated, Corning, NY) in RPMI-1640 supplemented with 5% heat-inactivated FCS and 50,000 Units penicillin/streptomycin (Invitrogen). Cells were cultured in the presence of 10 μg/ml L110f and GolgiStop for 12–14 hrs (eBioscience, San Diego, CA). Cells were then fixed for 10 min with cytofix/cytoperm (BD Biosciences, San Jose, CA), washed in PBS containing 0.1% BSA, incubated with Fc block (eBioscience) for 15 min at 4 °C, and stained with fluorochrome-conjugated mAb anti-CD3, CD4, CD44, IFN--γ, TNF-α, IL-2 (eBioscience) in 1× Perm/Wash buffer (BD Biosciences) for 30 min at 4 °C. Later cells were washed twice in 1x Perm/Wash buffer, suspended in PBS, and analyzed on a modified 3-laser LSRII flow cytometer (BD Biosciences). Viable lymphocytes were gated by forward and side scatter, and 50,000 CD3+CD4+ events were acquired for each sample and analyzed with Flowjo software (Treestar, Ashland, OR).
Cytokine ELISAs
BMDCs were harvested from 10-day old bone marrow cultures and stimulated at 5 × 104 cells per well in triplicate with titrating concentrations of either an aqueous formulation of MPL (MPL-AF: GSK Biologicals) or GLA (GLA-AF: IDRI) or CpG ODN 1826 (Coley Pharmaceuticals) or a mixture of both. Culture supernatants were collected after 16–24 hrs and assayed for IL-12p70 production by ELISA, according to the manufacturer’s instructions (eBioscience).
Single cell suspensions from either pooled dLNs or individual spleens were cultured at 2 × 105 cells per well in triplicate or duplicate in a 96-well U-bottomed plate (Corning Incorporated) in RPMI-1640 supplemented with 5% heat-inactivated FCS and 50,000 Units penicillin/streptomycin (Invitrogen). Cells were cultured in the presence of 10 μg/ml L110f. Culture supernatants were harvested after 72 h and cytokine content assayed for IFN-γ and IL-13 production by ELISA, according to the manufacturer’s instructions (eBioscience).
Luminex Assay
BMDCs were harvested from 10-day old bone marrow cultures and stimulated with titrating concentrations of either MPL-AF (GSK Biologicals) or CpG ODN 1826 (Coley Pharmaceuticals) or a mixture of both. Culture supernatants were collected at 16–24 hrs and assayed for the presence of a panel of chemokines and cytokines using the Procarta Cytokine Assay Kit (Panomics, Fremont, CA) according to the manufacturer’s instructions. The assay was run on the Luminex 200 instrument (Luminex corp, Austin, TX) and analytes were quantified using Masterplex software (Mirai Bio, South San Franciso, CA)
Relative Cytokine Gene Expression by Real-Time PCR
Infected hind foot pad tissues (obtained using a sterile 2 mm biopsy punch, Miltex Inc.) and popliteal lymph nodes were collected, immediately immersed in a suitable volume of RNA later™ (QIAGEN, Valencia, CA) and stored at −20C until use. Samples were homogenized using the QIAGEN TissueLyser for 2 cycles at 25 Hz (1.5 min/cycle). Total RNA was isolated using the RNeasy Fibrous Tissue Kit. Post elution, RNA samples were treated with Turbo DNase (Ambion, Austin, TX) and purified using the RNeasy MinElute Cleanup Kit. RNA concentrations were ascertained using the ND-100 NanoDrop Spectrophotometer (Nanodrop Technologies Inc, Wilmington, DE), volume adjusted to parity and quality assessed on a 1.2% agarose gel. First-strand cDNA synthesis was performed with 2 μg of RNA in a total reaction volume of 20 μl using the High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Ambion). The cDNA was diluted to a final concentration of 10 ng/ul (original input RNA). 10 μl of cDNA dilution was amplified with TaqMan® Universal PCR Master Mix and primer/probes (Applied Biosystems, Foster City, CA) on a LightCycler 480 system (Roche Applied Science, Indianapolis, IN). Amplification conditions consisted of an initial pre-incubation at 95°C for 10 min, followed by amplification of the target DNA for 50 cycles of 95°C for 15 s and 60°C for 1 min. The expression levels of genes of interest were normalized to β-actin levels. The results are expressed in fold change over saline control. Negative controls were also included and contained all the elements of the reaction mixture except template DNA. Universal precautions and one-way flow of DNA extraction and amplification were used to prevent contamination.
Statistics
The p-values were determined using Student’s t-test.
Results
TLR 4 agonist, MPL synergizes with CpG to enhance IL-12p70 production
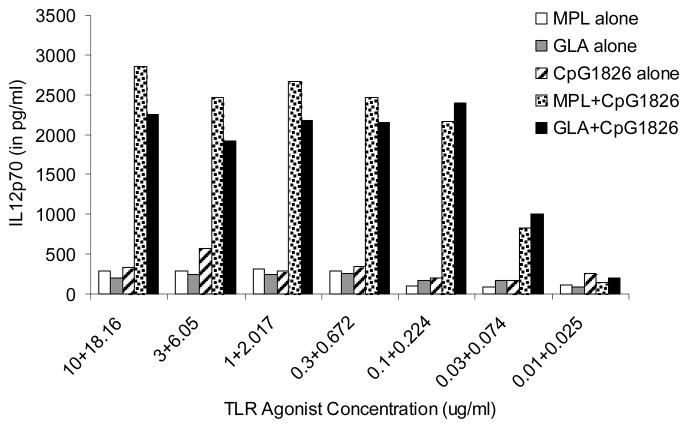
The L. major murine infection model has been widely used to define T helper (Th) responses in vivo (29). It is established that IL-12 production plays a critical role in both the induction and magnitude of a primary Th1 response, and is crucial for protection against leishmania disease progression (29–31). It has been reported previously that TLR4 agonists, MPL and GLA can induce dendritic cells to produce high levels of pro-inflammatory cytokines including IL-12 (32–34). Further, using this agonist as an adjuvant in a prophylactic setup with the polyprotein Ag L110f has been shown to induce a protective response against the parasite (12, 14, 21). To optimize formulations for therapeutic use, we explored other TLR agonists known to induce or enhance the production of IL-12, either alone or in combination with MPL. Activation of TLRs 4 and 9 with LPS and CpG was shown to act synergistically and enhance IL-12 production by dendritic cells (35). To investigate whether MPL could synergize similarly with a TLR 9 agonist we cultured BMDCs with titrating concentrations of MPL in presence or absence of CpG. We also tested the synthetic TLR4 agonist, GLA, a glucopyranosyl lipid A (33, 34). Combination of these agonists were synergistic as was seen with LPS and CpG in previous reports, with > 10 fold increase in the levels of IL-12p70 in BMDC cultures (35) (Fig. 1).
Figure 1. TLR4 agonist, MPL and its synthetic analog, GLA synergize with TLR9 agonist CpG to enhance IL-12 production.
Balb/C BMDCs were cultured with titrating concentrations of either TLR4 agonists (MPL or GLA) or TLR 9 agonist (CpG) or a combination of both. Superantants were collected after 24 hrs and assayed for IL-12p70. Data is representative of 3 independent experiments.
The Immunotherapy Model
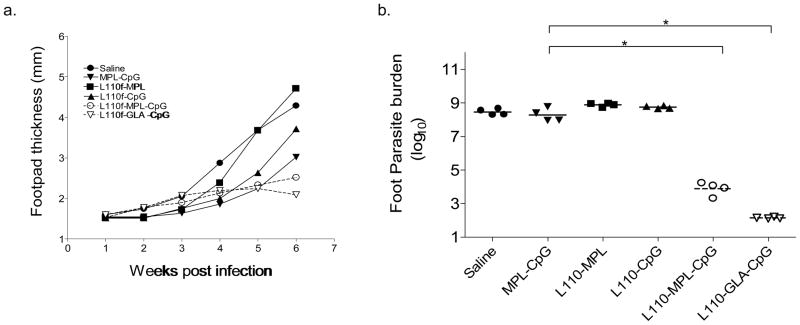
We have previously shown that prophylactic immunization of naïve animals with L110f and MPL can protect against L. major and L. infantum infection (14, 21). To identify factors that were crucial in a therapeutic vaccine which could be administered during active infection, we developed a high-dose infection model wherein Balb/C mice were infected in the footpad with a virulent strain of L. major. As seen in Fig. 2. a, infection with this dose induced a rapid increase in foot pad parasite numbers with accompanying swelling reaching greater than 4 mm in size within 6 weeks of infection. Infected animals were immunized on a weekly basis beginning 7 days-post infection with either L110f-MPL, L110f-CpG, or the vaccine-containing adjuvant combo (L110f-MPL-CpG; L110f-GLA-CpG). As a control, mice were injected with either saline or adjuvants alone.
Figure 2. The immunotherapy model.
Balb/c mice were infected in the hind-footpads with 1–2*105 metacyclic promastigotes of L. major. One week post-infection, mice were immunized, weekly with either control saline or various vaccine formulations and foot pads measured every week prior to vaccination (a). Footpads were harvested at the end of the experiment by limiting dilution to estimate parasite burden (b). Footpad lesion sizes of saline treated (top) vs. vaccine-containing adjuvant combo treated (bottom) are shown (c).* p values <0.0001
Data is representative of 4 independent experiments.
There was a dramatic lessening of disease progression as seen by reduction of footpad swelling in mice treated with L110f and the adjuvant combination containing both TLR agonists (Fig. 2. a). Calculation of parasite burden in these animals 6 weeks later showed a significant decrease in animals that had received the vaccine-containing adjuvant combo (p value<0.0001; Fig. 2. b). These results clearly showed that only this vaccine was capable of inhibiting disease progression and corresponding parasite burden.
Understanding the influence of infection on the immune system
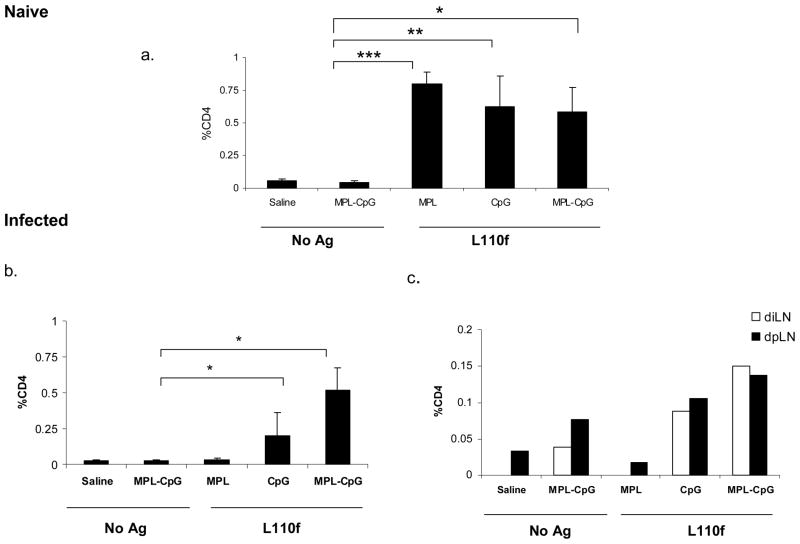
The inability to alter course of infection using therapeutic vaccination with a single adjuvant suggested that the presence of the parasite may be affecting the generation of an effective immune response. To address this, we evaluated immune responses against these Ag/adjuvant(s) both during and in the absence of infection. Naïve or infected mice were euthanized either after 3 or 5 doses of vaccine and cells stimulated ex-vivo and evaluated for Ag-specific IFN-γ secreting CD4 T cells. On comparing immune responses after 3 doses of vaccine (at week 4), we found that the frequency of IFN-γ secreting CD4 T cells was similar between all groups that had received Ag and was mainly restricted to the spleen in the absence of infection (Fig. 3. 1. a). During infection however, we observed a distinct decrease in the overall frequency of these cells. This was especially seen with L110f-MPL, which failed to generate Ag-specific CD4 T cells in the spleen during infection (Fig. 3. 1. b). We also found that while the majority of Ag-specific IFN-γ secreting CD4 T cells was present in the spleen, a small frequency was also observed in both parasite-draining (popliteal) and vaccine-draining (inguinal) lymph nodes (LN; Fig. 3. 1. c). This selective recruitment of Ag-specific T cells to the parasite-draining LN was seen in groups that received L110f-CpG or the vaccine-containing adjuvant combo, and only slightly enhanced in the latter. We did not observe any significant changes in the CD8 T cell subset during infection (Supplementary Fig. 1).
Figure 3. Effect of ongoing infection on the immune response.
1. Naïve (a) or Infected (b&c) Balb/C mice were immunized weekly and harvested 1 week post third immunization. Individual spleens and pooled draining LNs were stimulated ex vivo for 12 hours and analyzed by ICS for activated CD44hiIFN-γ++CD4 T cells. Percent CD4 T cells positive for CD44 and IFN-γ are plotted. *p values ≤ 0.04, ** p values ≤ 0.06, *** p values ≤ 0.001. Data is representative of 2 independent experiments.
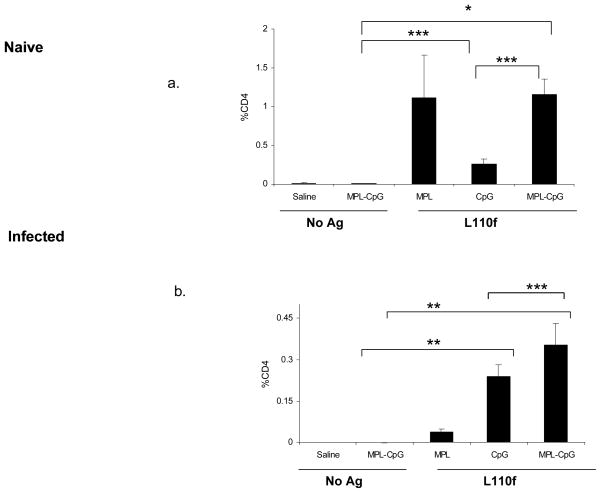
2. Naïve (a) or Infected (b) Balb/C mice were immunized weekly and harvested 1 week post the fifth immunization. Spleens were stimulated ex vivo for 12 hours and analyzed by ICS for activated CD44hiIFN-γ++CD4 T cells. Percent CD4 T cells positive for CD44 and IFN-γ are plotted. * p values <0.005; **p values <0.001;***p value < 0.05; Data is representative of 3 independent experiments.
After 5 doses of vaccines at week 6 the CD4 response was markedly reduced in mice that received L110f-CpG in comparison to mice immunized with vaccine-containing adjuvant combo which showed a much higher frequency of activated Ag-specific CD4 T cells (p value<0.005 versus adjuvants alone; p value ≤0.01 versus L110f-CpG). (Fig. 3. 2. a). We failed to see a statistically significant number of activated T cells in either draining LNs during infection at this time with most Ag-specific CD4 T cells being found in the spleen (Fig. 3. 2. b). Further analysis for T cell activation clearly showed that the vaccine-containing adjuvant combo was more successful in activating T cells during infection in comparison to other vaccines (p value<0.005 versus L110f-MPL; p value<0.05 versus L110f-CpG).
High ratio of Terminal Effector cells to Multi-functional cells help control parasite burden
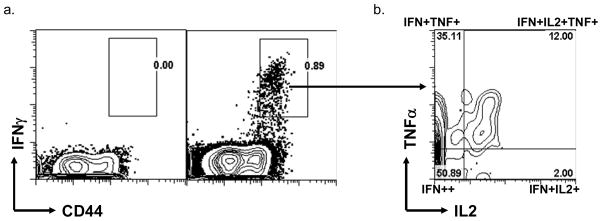
Our data showed that, both L110f-CpG and the vaccine-containing adjuvant combo to be capable of inducing Ag-specific Th cell activation during infection. Yet only the latter managed to control the disease, leading us to question the quality of these activated T cells. Recently Darrah et al. (20, 23) reported multi-functional CD4 T cells that produce IFN-γ, TNF-α, and IL-2, to be a protective correlate for vaccines based on Th1 responses. To determine whether this was a protective correlate in our system, we analyzed our activated T cell subset for both TNF-α, and IL-2 expression (Fig. 4. 1. a&b).
Figure 4. Higher ratio of Terminal effectors to multi-functional T cells needed during active infection.
1. Gating strategy to identify Multi-functional T cells versus Terminal effectors: Using Multi-parameter flow cytometry, CD44hiIFN-γ++CD4 T cells were further analyzed for IL-2 and TNF-α production.
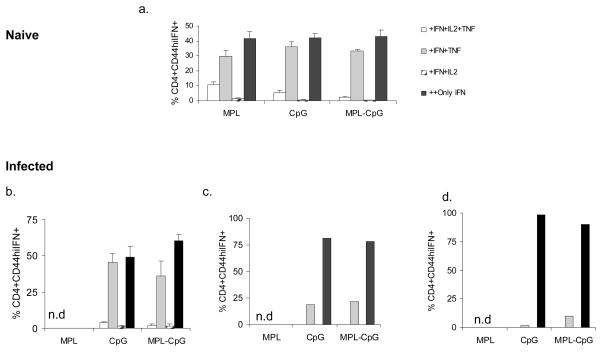
2. Naïve mice were immunized weekly with L110f in combination with different adjuvant(s) and harvested 1 week post third immunization and splenocytes stimulated ex vivo for 12 hours and analyzed by ICS (a). Infected mice were immunized weekly and harvested 1 week post third immunization and splenocytes stimulated ex vivo for 12 hours and analyzed by ICS (b). Cells from pooled inguinal LNs (c) and pooled popliteal LNs (d) of infected mice were also stimulated ex vivo for 12 hours and analyzed by ICS. Percent CD44hiIFN-γ++CD4 T cells positive for IFN-γ and/or IL-2 and/or TNF-α are plotted for all organs. n.d.-none detected. Data is representative of 2 independent experiments.
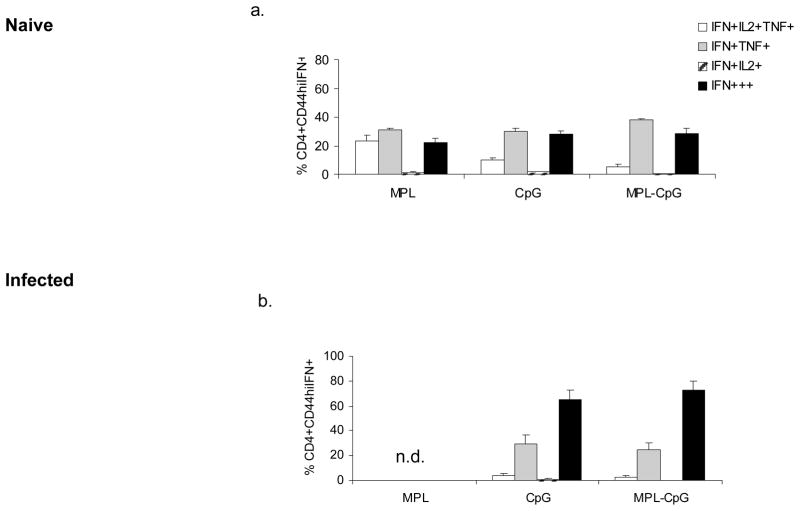
3. Naïve (a) or Infected (b) Balb/C mice were immunized weekly with L110f in combination with different adjuvant(s) and harvested 1 week post fifth immunization. Splenocytes were stimulated ex vivo for 12 hours and analyzed by ICS for activated CD44hiIFN-γ++CD4 T cells. Percent CD44hiIFN-γ++CD4 T cells positive for IFN-γ and/or IL-2 and/or TNF-α are plotted. n.d.-none detected. Data is representative of 3 independent experiments.
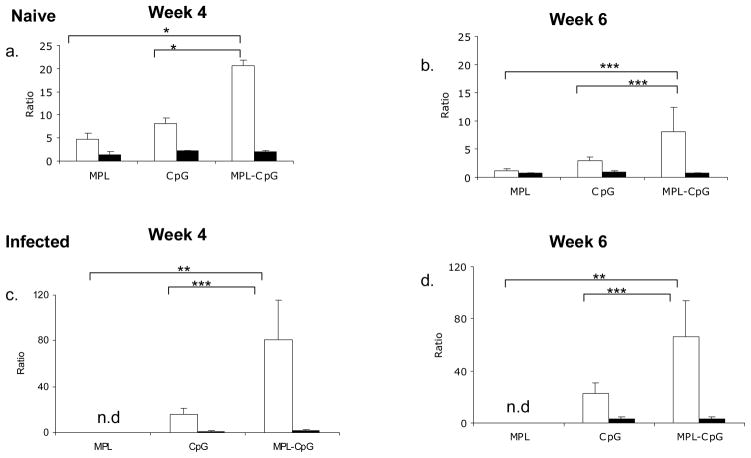
4. Flowdata from Week 4 were reanalyzed for to calculate ratios of terminal effectors (Only IFN-γ++) to bifunctional T cells (IFN-γ +TNF-α+; closed bars) or multifunctional T cells (IFN-γ+TNF-α+IL-2+; open bars) in the absence (a) or presence of infection (c). reanalyzed for to calculate ratios of terminal effectors (Only IFN-γ++) to bifunctional T cells (IFN-γ+TNF-α+;closed bars or multifunctional T cells (IFN-γ+TNF-α+IL-2+; open bars) in the absence (b) or presence of infection (d). * p value < 0.01; ** p value ≤ 0.05; ***p value < 0.1; n.d.-none detected. Data is representative of 2 independent experiments.
As reported earlier a significant proportion of the IFN-γ producing cells generated in the absence of infection were multifunctional, especially in animals treated with L110f-MPL. Week 4 analyses showed that this vaccine induced the highest frequency (~10% of CD44hi IFN-γ++) of these cells (14, 20). Notably, the group treated with the vaccine-containing adjuvant combo induced the lowest frequency of this subset even in the absence of infection (~2% of the IFN-γ-producers; Fig. 4. 2. a). Analyses for generation of multifunctional subsets during infection at week 4, showed greater than 50% of the IFN-γ producers to be terminal effectors (only IFN-γ+++). The remainder of the population was mostly double positive for both IFN-γ and TNF-α with a distinct lack of IL-2 producers (Fig. 4. 2. b–d).
Analyses of the IFN-γ producers in both the absence and presence of infection at week 6 showed a trend similar to that seen earlier at week 4 (Fig. 4. 3. a&b). The complete lack of IL-2 producers, while not surprising was rather striking in these animals. The fact that animals which had received L110f-CpG generated a response similar to animals that were treated with vaccine-containing adjuvant combo, lead us to reanalyze our data and calculate the ratio of these subsets with respect to IFN-γ producers. As seen in Fig. 4. 4. (a&b), uninfected mice that received the vaccine-containing adjuvant combo showed a higher frequency of terminal effectors in comparison to multi-functional subset. More importantly this ratio was maintained even when this vaccine was administered during active infection (Fig. 4. 4. c&d). These data showed generation of terminal effectors to be an effective correlate of protection for an immunotherapeutic vaccine.
Controlling the cytokine milieu during infection
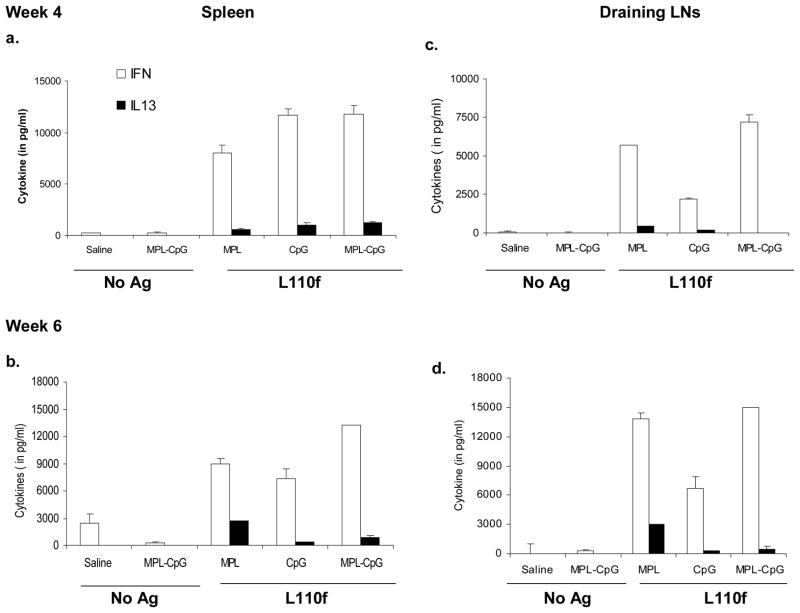
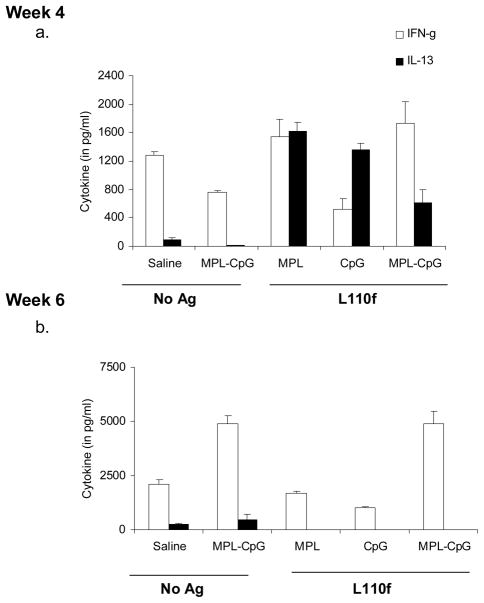
L. major is a protozoan known to take advantage of the Th2 response in the Balb/c model (36). Strategies that promote a Th1 response during infection in this model have been shown to reduce disease drastically (12, 13, 37). Our data showed that the vaccine-containing adjuvant combo induced high frequencies of terminal effectors early on during infection (Fig. 4. 2. b-d). To examine the effect of this subset on the surrounding cytokine milieu, we analyzed the cytokine response of these animals by stimulating them with Ag ex vivo. Assays for IFN-γ and IL-13 production on uninfected animals at both weeks 4 and 6 showed that all groups that received Ag and adjuvant induced a strong Th1 response with a high ratio of IFN-γ/IL-13 producers in both spleen (Fig. 5. 1. a&b) and dLNs (Fig. 5. 1. c&d). Assays for other Th2 cytokines such as IL-4, IL-5, and IL-10 were mostly negative in these cultures (data not shown). To test the effect of these terminal effectors during infection, we mainly focused on the parasite-draining LNs. Interestingly, while we did detect the presence of the latter cytokines (IL-4, IL-5 &IL-10) in the presence of infection the most striking aspect, was the high ratio of IFN-γ/IL-13 producers in the parasite draining popliteal LNs (Fig. 5. 2. a&b; data not shown). Examination of serum Ig responses for IgG1/IgG2a against L110f showed a similar trend with animals that received vaccine-containing adjuvant combo showing a dominant Th1-dependent IgG2a response during infection (data not shown). These data indicated that vaccine-containing adjuvant combo treatment induced a much stronger Th1 response during infection, when compared to the groups that received L110f-MPL or L110f-CpG, and thus far more capable of curing infection.
Figure 5. Control of cytokine milieu using vaccine-containing adjuvant combo during infection.
1. Naïve Balb/C mice were immunized weekly and spleens harvested 1 week post-third (a) or -fifth immunization (b) stimulated ex-vivo for 72 hours. Supernatants were collected and assayed for cytokines, IFN-γ and IL-13. Vaccine draining inguinal LNs (pooled) were also harvested and assayed similarly at either 1 week post- third (c) or -fifth immunization (d). Data is representative of 2 independent experiments.
2. Infected Balb/C mice were immunized weekly and draining popliteal LNs (pooled) were harvested 1 week post- third (a) or -fifth immunization (b) stimulated ex-vivo for 72 hours and supernatants were assayed for cytokines, IFN-γ and IL-13. Data is representative of 2 independent experiments.
TLR Synergy induced chemokines may help promote disease clearance
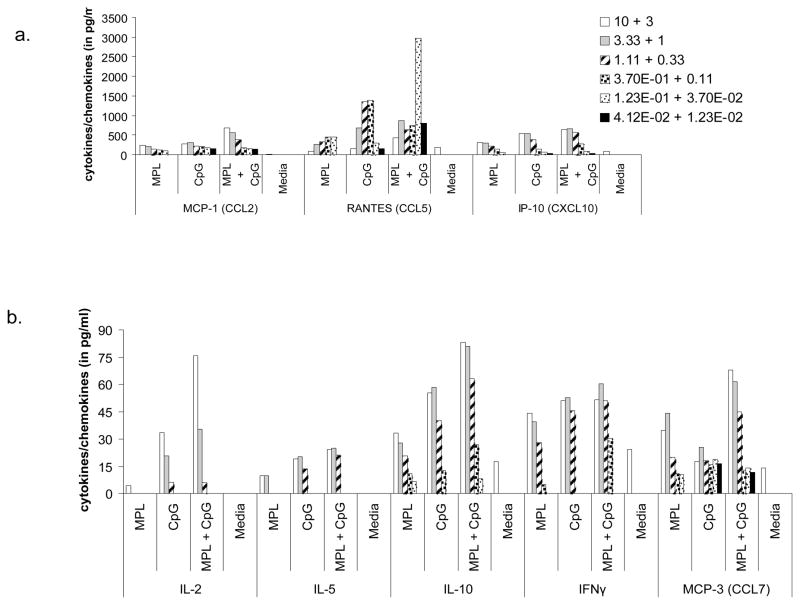
It was apparent from our data that the vaccine-containing adjuvant combo induced a strong Th1 response which contributed to inhibiting disease progression in these animals, but questions remained regarding the ability of these cells to actually respond to L. major infection (Fig. 2). Several reports have shown that L. major modulates the immediate environment so as to only recruit Th2 cells to the site of infection by virtue of CCL7 induction (38–40). This chemokine while being able to bind multiple receptors (CCR1, 2 &3), expressed on DCs, monocytes and T cells, can recruit Th2 cells specifically by virtue of the latter differentially expressing CCR3. To address whether TLR synergy may enhance specific chemokines to help Th1 recruitment, we analyzed supernatants from BMDCs that had been cultured with either TLR4 or TLR9 agonists or a mixture of both on a multiplex platform for production of several cytokines and chemokines. As shown in Fig. 6. a, RANTES (CCL5) a chemokine involved in Th1 cell recruitment was synergistically enhanced in cells that were cultured with both agonists. Notably, any increase seen in CCL7 production by these DCs was additive if not non-existent (Fig. 6. b). This clearly, showed that combining these two agonists was extremely beneficial in not just triggering a Th1 response but also possibly in helping these cells migrate to the site of infection.
Figure 6. IL-12-induced chemokine, RANTES (CCL5) may help recruit Ag-specific T cells.
Balb/c BMDCs were stimulated with titrating concentrations of MPL ± CpG (μg/ml) for 24 hrs. Supernatants were assayed for a series of Th1/Th2 cytokines (a) and chemokines (a&b) using a Multiplex platform. Data is representative of 2 independent experiments.
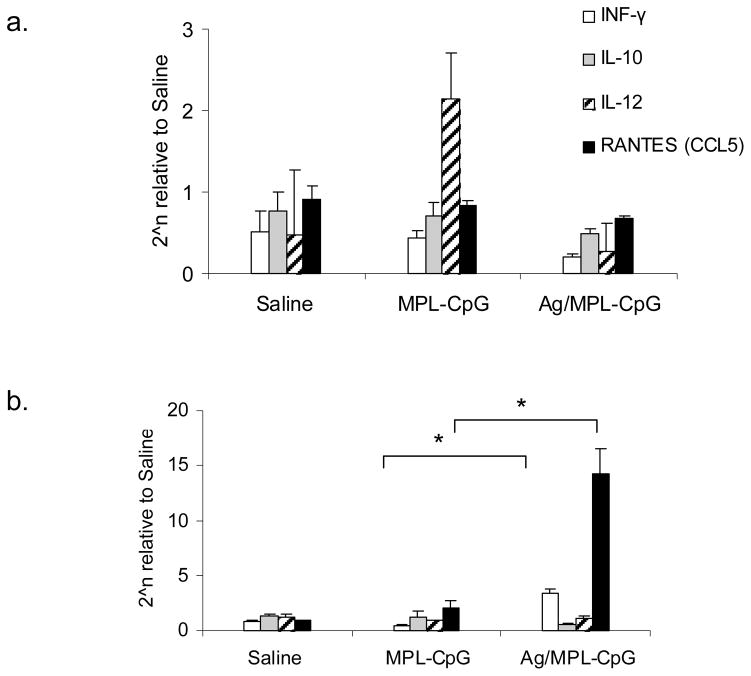
To confirm if this was true in vivo, we also analyzed the parasite-draining popliteal LNs (dLN) and foot pads from mice that had been infected and treated with either adjuvant alone or the vaccine containing adjuvant combo for the expression of IL-12, IFN-γ, IL-10, and RANTES mRNA. Interestingly, IL-12 expression was significantly higher in the dLNs of mice that had received adjuvants alone compared to the group that received the vaccine-containing adjuvant combo (Fig. 7. a). In contrast, analyses of infected footpads from the latter group revealed high levels of RANTES mRNA (~7 fold; p value<0.05) and IFN-γ (~8 fold; p value<0.05) being expressed in this group compared to the footpads of the adjuvants alone group (Fig. 7. b). Even though the adjuvants had induced high levels of IL-12, they failed to induce RANTES at the site of infection. These data showed the importance of Ag in ‘directing’ activated T cells to the site of infection during immunotherapy.
Figure 7. IL-12-induced chemokine, RANTES (CCL5) is expressed in footpads of mice treated with vaccine-containing adjuvant combo.
Infected Balb/C mice were immunized weekly and parasite-draining popliteal LNs (a) and footpads (b) were harvested week post- third immunization for RNA isolation. Samples were assayed for IL-12, IFN-γ, IL-10 and RANTES (CCL5) by RT-PCR. *p value < 0.05; Data is representative of 2 independent experiments.
Discussion
Over the past decade, we have come to understand the role played by the host innate immune response in initiating and directing adaptive immunity (41, 42). New insights into the role of the innate immune system in inducing IL-12, a crucial cytokine in initiating Th1 responses has simplified vaccine design to a great extent (30, 43). The protozoan parasite Leishmania is one for which immune control mechanisms are fairly well understood but improved therapies are needed. Anti-leishmania drugs such as Pentostam, Ambisome, and Paramomycin, have been only partially successful. (6). First reported in 1912, immunotherapy was largely abandoned with the introduction of antimony till the 1970s, when drug-resistance and toxicity came into fore (8, 10, 24, 44). The initial studies used an active approach with live or killed parasite which while successful, has fallen out of favor due to reports of adverse reactions at injection site and quality control issues (8, 10, 11, 24). A similar approach with heat-killed parasite and BCG vaccination has met with varied success but is also falling out of favor for similar reasons (44). In 1993, Badaro & Johnson showed passive immunotherapy using a combination of IFN-γ and drug to be successful in drug refractory visceral leishmaniasis in humans (45). Further, administration of IL-12 has also proven curative in mouse models of CL (30, 46). Alternatively, anti-IL-4 antibody therapy has been shown to be successful in treating borderline infection in mice (47). Recently, our group advanced this approach with the successful use of defined antigens with the immune modulator GM-CSF to treat patients with drug refractory mucosal leishmaniasis (26).
In the present study we focused on MPL and CpG mainly due to their history of effectiveness in leishmaniasis (12, 14, 18–21). These agonists induce a variety of cytokines, including IL-12 and have both been used individually with great success in several vaccine studies especially in prophylaxis (14, 20, 21). Infection of TLR4−/− mice, have shown these animals to be far more susceptible to disease than wild type animals (48). Signaling through TLR4 has been shown to control IL-10 and MCP1 expression and elevate Th1 cytokine, IFN-γ during L. major infection (48, 49). Further, the TLR 4 agonist MPL has also been shown to establish a strong Th1 response that protects against disease (12, 14, 21). Similarly, expression of its receptor, TLR9 on dermal DCs has made CpG an attractive target for both prophylaxis and immunotherapy (50). Flynn et al. reported injection of CpG ODN in the site of infection 3 days before and after challenge enhanced host resistance to CL and decreased lesion severity (19). Also, Li et al. (2004) reported that gene therapy with an unmethylated plasmid expressing IL-18 that controlled disease by targeting TLR9 (51). The protective effect is explained by the fact that all vaccine approaches that result in early accumulation of IFN-γ producers at the lesion site have always been successful in Leishmania (12, 21, 46, 47, 52). The lack of effectiveness of these agonists (when used individually) in our study could be explained by the delay in this process. It should also be noted that groups that have used CpG successfully in immunotherapy started treatment shortly after infection, unlike the approach used in the present study (18, 19).
In this study we questioned whether these TLR agonists could control disease if used in a therapeutic vaccine aimed at areas that are endemic for disease. We developed a high-dose infection model wherein all mice, if not treated, would succumb to infection. Our results clearly showed that both of these agonists, which have been shown to be successful individually during prophylaxis by virtue of their inducing multi-functional T cells, were ineffective when used therapeutically (Figs. 2 & 4). Combination of these two agonists in the vaccine mixture however increased efficacy. Our data showed that TLR synergy helped initiate a strong Th1 response during disease by increasing IL-12 production. Further, analysis of our data revealed that this was at least in part, to the high frequencies of IFN-γ++ “terminal effectors” generated by this vaccine that presumably helped decrease parasite burden in these animals. These results also raise an argument about using multi-functional T cells as a protective correlate for vaccines against leishmaniasis when analyzed in the context of a therapeutic setting.
Our data show that triggering more than one TLR could be an effective approach to optimize immune therapy. Lanzavechhia and co-workers recently raised the possibility of using this synergistic TLR stimulation in ‘real life’, to mimic pathogens which contain several TLR agonists that trigger TLRs in different cellular compartments (35). In agreement, Bagchi et al. (2007), demonstrated that MyD88-associated and TRIF-associated TLR agonists can synergize to induce high levels of pro-inflammatory cytokines by virtue of signaling through these two different pathways simultaneously (53). It is now clear that MyD88-associated TLRs 2, 5, 7 and 9 can cooperate with TRIF-associated TLRs 3 and 4 to enhance pro-inflammatory cytokines. This synergistic activation has been shown to enhance NK, Th, and CTL responses in vitro and in vivo (35, 53–55). More interestingly, Whitmore et al. (2004) showed this synergistic activity (between TLR3 and 9) to be beneficial in anti-tumor therapy in a mouse tumor model. Mice treated with CpG and Poly I:C (a TLR 3 agonist) showed enhanced anti-tumor activity compared to treatment with either agonist alone (56). Combining the MyD88-independent TLR4 agonist, MPL, with CpG, turned out to be an ideal combination in our study. As reported earlier with LPS, combination of either LPS-derivative MPL® or the synthetic MPL analog, GLA with the TLR 9 agonist, CpG induced high levels of IL-12 in BMDC cultures. Further this adjuvant combination also inhibited disease progression when administered with Ag L110f during active disease (Fig. 2).
Importantly, our study highlighted the role played by Ag in immunotherapy. Animals that received adjuvants alone failed to control parasite burden even though they did show a slight delay in footpad increase in some experiments (Fig. 2. a&b; data not shown). It was clear in our study that the presence of Ag in the vaccine was as crucial as TLR synergy to direct activated T cells to the site of infection. Previous studies that have used adjuvant alone to induce immunomodulation in CL have encountered similar results and had to rely on parallel treatments with leishmanicidal drugs. Imiquimod, a TLR 7 agonist, was only partially successful when applied as a topical cream on the lesion (Aldara@; 5% Imiquimod) in mice (13). This was mainly due to the fact that the response was localized and did not prevent proliferation of the parasite. Use of this cream in conjunction with meglumine antimonite or Leshcutan (Paramomycin ointment) has been more successful and results comparable to those observed with combination therapy with IL-12 or IFN-γ (37, 57–60). Unfortunately, these treatments are neither affordable nor feasible. Drug-induced toxicities associated with leishmanicidal drugs include hepatotoxicity, cardiotoxicity and pancreatitis (6, 11). Our study proposes a new approach to the issue by targeting the parasite with an Ag that helps direct the activated T cells to the site of infection.
Lastly, we show that this targeting of Ag-specific T cells to the site of infection, is aided by TLR synergy. It is clear now that several pathogens resident in the tissue may divert the centrally generated cytokine repertoire (61). Recent work by Fowell and colleagues showed that L. major modulates the local milieu so as to only attract Th2 cells to the site and thus evade the immune system (39). This is accomplished by inducing CCL7, a chemokine known to attract only Th2 cell and actively suppressing RANTES expression at the site of infection. Recently, da Costa Santiago et al. (2004) showed the presence of RANTES to correlate with protection against L. major infection and its blockade to increase susceptibility to disease (38). Our in vitro data showed that TLR synergy enhances RANTES expression and possibly aids activated T cells to migrate to the site of infection (Fig. 6). Interestingly, while both CCL7 and IL-10 were induced when stimulated with either CpG or MPL alone, we failed to see synergy on stimulation with both these agonists together. Our in vivo data confirmed that TLR synergy that we had observed in vitro was operational during infection (Fig. 6 & 7). The fact that mice treated with adjuvants alone failed to induce RANTES at the site of infection despite inducing high levels of IL-12 in the dLNs also showed Ag to be a crucial factor in this process. While we were surprised to see low levels of IL-12 being produced in the dLNs compared to the footpad in the group that received vaccine containing the adjuvant-combo, this could be explained by the differential kinetics of the immune response between the two groups. Studies that dissect the early kinetics of the immune response should be more helpful in explaining this process.
Our findings show the benefits of assessing immune function in disease before designing vaccines against them. We show that TLR synergy can be exploited to its full use in disease settings to induce optimal immune responses.
Acknowledgments
We would like to thank Drs. Tom Vedvick, Chris Fox and the formulation group for providing adjuvants and Drs. Rhea Coler, Yasuyuki Goto, Sylvie Bertholet, and Randall Howard for helpful comments and suggestions. We would like to thank Dr. Karen Cowgill for helping us gain access to old manuscripts and Crystal Turnbull for help formatting the manuscript for publication.
This work was supported in part by grant R01-AI025038 from the National Institutes of Health and Grants 31929 and 42387 from the Bill & Melinda Gates Foundation.
References
- 1.2004 Scientific working group on Leishmaniasis Meeting report; 2–4 February 2004; Geneva, Switzerland. http://apps.who.int/tdr/publications/tdr-research-publications/swg-report-leishmaniasis/pdf/swg_leish.pdf. [Google Scholar]
- 2.Ghalib H, Modabber F. Consultation meeting on the development of therapeutic vaccines for post kala azar dermal leishmaniasis. Kinetoplastid biology and disease. 2007;6:7. doi: 10.1186/1475-9292-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. The Lancet infectious diseases. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 4.Firdous R, Yasinzai M, Ranja K. Efficacy of glucantime in the treatment of Old World cutaneous leishmaniasis. International journal of dermatology. 2009;48:758–762. doi: 10.1111/j.1365-4632.2009.04072.x. [DOI] [PubMed] [Google Scholar]
- 5.Choudhury K, Zander D, Kube M, Reinhardt R, Clos J. Identification of a Leishmania infantum gene mediating resistance to miltefosine and SbIII. International journal for parasitology. 2008;38:1411–1423. doi: 10.1016/j.ijpara.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Ouellette M, Drummelsmith J, Papadopoulou B. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist Updat. 2004;7:257–266. doi: 10.1016/j.drup.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Noazin S, Modabber F, Khamesipour A, Smith PG, Moulton LH, Nasseri K, Sharifi I, Khalil EA, Bernal ID, Antunes CM, Kieny MP, Tanner M. First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine. 2008;26:6759–6767. doi: 10.1016/j.vaccine.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 8.Dubovsky P. Vaccinotherapy in Cutaneous Leishmaniais. Tropical Diseases Bulletin. 1943;40:297. [Google Scholar]
- 9.Genaro O, de Toledo VP, da Costa CA, Hermeto MV, Afonso LC, Mayrink W. Vaccine for prophylaxis and immunotherapy, Brazil. Clinics in dermatology. 1996;14:503–512. doi: 10.1016/0738-081x(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt CL. The present and future of vaccination for cutaneous leishmaniasis. Progress in clinical and biological research. 1980;47:259–285. [PubMed] [Google Scholar]
- 11.Palatnik-de-Sousa CB. Vaccines for leishmaniasis in the fore coming 25 years. Vaccine. 2008;26:1709–1724. doi: 10.1016/j.vaccine.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Coler RN, Reed SG. Second-generation vaccines against leishmaniasis. Trends in parasitology. 2005;21:244–249. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Buates S, Matlashewski G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. The Journal of infectious diseases. 1999;179:1485–1494. doi: 10.1086/314782. [DOI] [PubMed] [Google Scholar]
- 14.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infection and immunity. 2007;75:4648–4654. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertholet S, Goto Y, Carter L, Bhatia A, Howard RF, Carter D, Coler RN, Vedvick TS, Reed SG. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27:7036–7045. doi: 10.1016/j.vaccine.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos-Neto A, Porrozzi R, Greeson K, Coler RN, Webb JR, Seiky YA, Reed SG, Grimaldi G., Jr Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infection and immunity. 2001;69:4103–4108. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed SG, Coler RN, Campos-Neto A. Development of a leishmaniasis vaccine: the importance of MPL. Expert review of vaccines. 2003;2:239–252. doi: 10.1586/14760584.2.2.239. [DOI] [PubMed] [Google Scholar]
- 18.Walker PS, Scharton-Kersten T, Krieg AM, Love-Homan L, Rowton ED, Udey MC, Vogel JC. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6970–6975. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn B, Wang V, Sacks DL, Seder RA, Verthelyi D. Prevention and treatment of cutaneous leishmaniasis in primates by using synthetic type D/A oligodeoxynucleotides expressing CpG motifs. Infection and immunity. 2005;73:4948–4954. doi: 10.1128/IAI.73.8.4948-4954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Seder MRA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 21.Coler RN, Skeiky YA, Bernards K, Greeson K, Carter D, Cornellison CD, Modabber F, Campos-Neto A, Reed SG. Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infection and immunity. 2002;70:4215–4225. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velez ID, Gilchrist K, Martinez S, Ramirez-Pineda JR, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Kahn SJ, Beckmann AM, Cowgill KD, Reed SG, Piazza FM. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 23.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 24.Row R. The curative value of Leishmania culture “vaccine” in oriental sore. The British Medical Journal. 1912;9:540–541. doi: 10.1136/bmj.1.2671.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badaro R, Lobo I, Munos A, Netto EM, Modabber F, Campos-Neto A, Coler RN, Reed SG. Immunotherapy for drug-refractory mucosal leishmaniasis. The Journal of infectious diseases. 2006;194:1151–1159. doi: 10.1086/507708. [DOI] [PubMed] [Google Scholar]
- 26.Badaro R, Lobo I, Nakatani M, Muinos A, Netto EM, Coler RN, Reed SG. Successful use of a defined antigen/GM-CSF adjuvant vaccine to treat mucosal Leishmaniasis refractory to antimony: A case report. Braz J Infect Dis. 2001;5:223–232. doi: 10.1590/s1413-86702001000400008. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, Reed SG, Coler RN. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009;27:3063–3071. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of immunological methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 29.Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 30.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science (New York, NY) 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 31.Park AY, Hondowicz BD, Scott P. IL-12 is required to maintain a Th1 response during Leishmania major infection. J Immunol. 2000;165:896–902. doi: 10.4049/jimmunol.165.2.896. [DOI] [PubMed] [Google Scholar]
- 32.Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infection and immunity. 2003;71:2498–2507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, Chesko J, Coler RN, Friede M, Reed SG, Vedvick TS. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids and surfaces. 2009 doi: 10.1016/j.colsurfb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, Mompoint F, Vedvick TS, Bertholet S, Coler RN, Reed SG. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–5963. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 35.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature immunology. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott P, Pearce E, Cheever AW, Coffman RL, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunological reviews. 1989;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 37.Nabors GS, Afonso LC, Farrell JP, Scott P. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and Pentostam. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santiago HC, Oliveira CF, Santiago L, Ferraz FO, de Souza DG, de-Freitas LA, Afonso LC, Teixeira MM, Gazzinelli RT, Vieira LQ. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infection and immunity. 2004;72:4918–4923. doi: 10.1128/IAI.72.8.4918-4923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katzman SD, Fowell DJ. Pathogen-imposed skewing of mouse chemokine and cytokine expression at the infected tissue site. The Journal of clinical investigation. 2008;118:801–811. doi: 10.1172/JCI33174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filipe-Santos O, Pescher P, Breart B, Lippuner C, Aebischer T, Glaichenhaus N, Spath GF, Bousso P. A dynamic map of antigen recognition by CD4 T cells at the site of Leishmania major infection. Cell host & microbe. 2009;6:23–33. doi: 10.1016/j.chom.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual review of immunology. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 42.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunology today. 1993;14:335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 44.Convit J, Castellanos PL, Rondon A, Pinardi ME, Ulrich M, Castes M, Bloom B, Garcia L. Immunotherapy versus chemotherapy in localised cutaneous leishmaniasis. Lancet. 1987;1:401–405. doi: 10.1016/s0140-6736(87)90116-4. [DOI] [PubMed] [Google Scholar]
- 45.Badaro R, Johnson WD., Jr The role of interferon-gamma in the treatment of visceral and diffuse cutaneous leishmaniasis. The Journal of infectious diseases. 1993;167(Suppl 1):S13–17. doi: 10.1093/infdis/167.supplement_1.s13. [DOI] [PubMed] [Google Scholar]
- 46.Gurunathan S, Prussin C, Sacks DL, Seder RA. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nature medicine. 1998;4:1409–1415. doi: 10.1038/4000. [DOI] [PubMed] [Google Scholar]
- 47.Uzonna JE, Bretscher PA. Anti-IL-4 antibody therapy causes regression of chronic lesions caused by medium-dose Leishmania major infection in BALB/c mice. European journal of immunology. 2001;31:3175–3184. doi: 10.1002/1521-4141(200111)31:11<3175::aid-immu3175>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 48.Kropf P, Freudenberg MA, Modolell M, Price HP, Herath S, Antoniazi S, Galanos C, Smith DF, Muller I. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infection and immunity. 2004;72:1920–1928. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antoniazi S, Price HP, Kropf P, Freudenberg MA, Galanos C, Smith DF, Muller I. Chemokine gene expression in toll-like receptor-competent and -deficient mice infected with Leishmania major. Infection and immunity. 2004;72:5168–5174. doi: 10.1128/IAI.72.9.5168-5174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clinical and experimental immunology. 2007;147:176–183. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Ishii K, Hisaeda H, Hamano S, Zhang M, Nakanishi K, Yoshimoto T, Hemmi H, Takeda K, Akira S, Iwakura Y, Himeno K. IL-18 gene therapy develops Th1-type immune responses in Leishmania major-infected BALB/c mice: is the effect mediated by the CpG signaling TLR9? Gene therapy. 2004;11:941–948. doi: 10.1038/sj.gt.3302240. [DOI] [PubMed] [Google Scholar]
- 52.Zhang WW, Matlashewski G. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infection and immunity. 2008;76:3777–3783. doi: 10.1128/IAI.01527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 54.Vanhoutte F, Paget C, Breuilh L, Fontaine J, Vendeville C, Goriely S, Ryffel B, Faveeuw C, Trottein F. Toll-like receptor (TLR)2 and TLR3 synergy and cross-inhibition in murine myeloid dendritic cells. Immunology letters. 2008;116:86–94. doi: 10.1016/j.imlet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 55.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–550. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 56.Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BR. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer research. 2004;64:5850–5860. doi: 10.1158/0008-5472.CAN-04-0063. [DOI] [PubMed] [Google Scholar]
- 57.Arevalo I, Ward B, Miller R, Meng TC, Najar E, Alvarez E, Matlashewski G, Llanos-Cuentas A. Successful treatment of drug-resistant cutaneous leishmaniasis in humans by use of imiquimod, an immunomodulator. Clin Infect Dis. 2001;33:1847–1851. doi: 10.1086/324161. [DOI] [PubMed] [Google Scholar]
- 58.El-On J, Bazarsky E, Sneir R. Leishmania major: in vitro and in vivo anti-leishmanial activity of paromomycin ointment (Leshcutan) combined with the immunomodulator Imiquimod. Experimental parasitology. 2007;116:156–162. doi: 10.1016/j.exppara.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Murray HW, Berman JD, Wright SD. Immunochemotherapy for intracellular Leishmania donovani infection: gamma interferon plus pentavalent antimony. The Journal of infectious diseases. 1988;157:973–978. doi: 10.1093/infdis/157.5.973. [DOI] [PubMed] [Google Scholar]
- 60.Miranda-Verastegui C, Tulliano G, Gyorkos TW, Calderon W, Rahme E, Ward B, Cruz M, Llanos-Cuentas A, Matlashewski G. First-Line Therapy for Human Cutaneous Leishmaniasis in Peru Using the TLR7 Agonist Imiquimod in Combination with Pentavalent Antimony. PLoS neglected tropical diseases. 2009;3:e491. doi: 10.1371/journal.pntd.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nature reviews. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]