We agree with the authors of this important piece that many self-report measures of medication adherence have lacked adequate reliability and strong evidence of validity. Limitations in adherence measurement constitute a major problem in this important area of investigation. In a rush to document the effectiveness of their interventions, or to identify correlational or causal elements in the adherence puzzle, too many researchers have simply constructed their own adherence measures or modified existing ones, failing to apply even rudimentary techniques of psychometric analysis and measurement development. Rarely are the standard expectations for measurement development in the field of psychology ever applied to adherence assessment, although patient adherence is essentially a behavioral phenomenon. In a 2004 meta-analysis of 569 studies measuring adherence [1], inadequate self-report assessments (such as single-item measures and retrospective estimates) were often identified. Comparing self-report measures of adherence with other approaches (such as pill counts, electronic measures, surrogate reports, chemical markers, and prescription refills), however, shows that they are not inflated and, although still problematic in many cases, have generally fared well in adherence measurement [1]. There have been some notable developments in the literature recently, among them a U.C. Davis study focusing on detailed psychometrics of adherence measurement [2] and the work discussed below.
There are potential advantages of robust self-report adherence assessment instruments; medication adherence is a behavior about which people should be able to report. Self-report is potentially the most accurate record of what a given patient has done—if the patient can remember taking the medication and is motivated to be absolutely truthful about what is remembered. Thus, the appropriate context of measurement (e.g., a supportive health professional) and strategies to enhance recall are essential to the accurate assessment of adherence behavior.
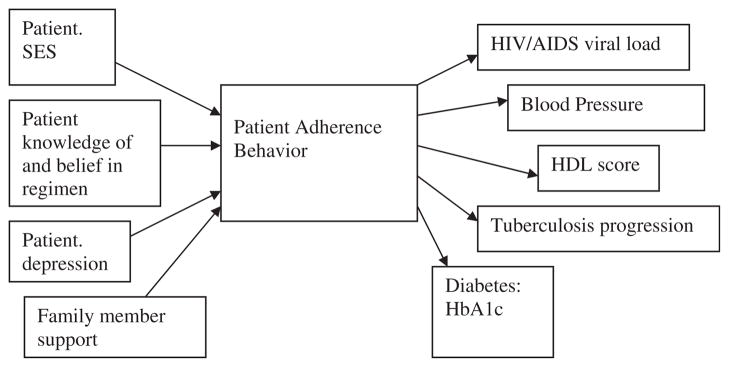
One of us (M.R.D.) holds that adherence assessment should always focus squarely on medication adherence as a behavior—not on its predictors (causal indicators) or its consequences (effect indicators). The assessment of medication adherence behavior is potentially even easier than of other health behaviors: Was the pill swallowed or not? It is not necessary to assess the quality of the behavior (such as with exercise) or the exact amount and type (such as of food). Of course, if we know that certain causal indicators increase the risk of nonadherence, their measurement can help clinically to raise red flags, point to likely nonadherence, and/or explain a health outcome that is suboptimal. But ideally, research on self-reported adherence should focus research attention on the development, reliability assessment, and validation of new self-report measures of adherence behavior or the further development of older methods [2]. When developing new self-report measures, key issues should involve the specific questions that are asked, the response options that are offered, and the methods of administration that are used. The focus should be on ways to improve the accuracy of responses by improving patient memory and creating an environment in which patients are truthful and motivated to provide the most precise and accurate information about their adherence [3]. Measurement should be direct and behaviorally focused. This perspective disagrees with the authors that adherence assessment should involve both causal and effect indicators: “Direct measurement may be undesirable because it does not provide information on why people are not taking their medications as prescribed, which may be important for designing interventions” (page 5). Instead, as illustrated in Fig. 1, adherence behavior should be placed in the center of the conceptual framework, preceded by specific determinants of adherence, and followed by specific physiological and health outcomes.
Fig. 1.
Conceptual model showing examples of causal determinants of adherence behavior leading to health outcomes.
Another perspective (D.E.M) is based on research showing that over 50% of patients identify forgetting, or having problems remembering, as their main reason for not taking their medication [4,5]. In 1986, Hopkins-based researchers developed a simple 4-item scale (now known as the Morisky Medication Adherence Scale-4 items, MMAS-4) to measure self-reported medication-taking behavior among outpatients diagnosed with essential hypertension [6–9]. This scale showed moderate reliability as well as good sensitivity and moderate specificity in identifying nonadherent individuals and validity relationships with health outcomes, as indicated by criterion-related validity, both concurrent and predictive. An updated version of this scale was developed in 2008, consisting of an 8-item measure with a reliability of 0.83 and good concurrent and predictive validity [10]. This measure has been found to positively correlate with pharmacy fills [11]. Compared with the 8-item scale, the 4-item scale has been more effectively used in the office setting in which the health care provider can identify and counsel nonadhering patients with adherence-enhancing recommendations that are specific to the cause of the patient’s nonadherence. The ease of a dichotomous response, with avoidance of acquiescence response set (a “yes” bias), allows visit-based interpretation. If forgetting is the primary reason for nonadherence, the health care provider can recommend strategies such as placing medication nearby a tooth brush or hygiene kit to serve as a behavioral prompt. Individuals who forget to bring their medication along when traveling can place their medications in a pill container in a purse or hygiene kit. These helpful recommendations have demonstrated significant improvements in adherence behavior among patients with chronic and long-term infectious diseases, such as tuberculosis, human immunodeficiency virus/acquired immune deficiency syndrome, and diabetes [12–14]. Interesting results and studies using the MMAS scales have been shown to have high discriminant validity using the short form of the Marlow–Crowne assessment for social desirability (a common concern for many investigators using self-reported measures).
We have several areas of agreement with the authors and with each other. We agree that research should be longitudinal and (using effective measurement strategies) examine the effects of changes in health status and treatment regimens, the degree of stability of adherence behaviors, and all clinical issues in the patient’s disease condition. This would require interdisciplinary teams (e.g., physicians to assess clinical parameters and behavioral scientists to assess behavior effectively). We also agree that adherence measurement should not be dichotomous but rather use continuous measurement. As with many physiological outcome measures, rounding off can place individuals from a nondisease state to a disease state or from an adherence to a nonadherence label, and the robustness of continuous measurement allows greater opportunity to identify adherence-outcome relationships [15].
We suggest a few other important issues in adherence measurement. Focusing on a particular disease and a particular regimen is very important (e.g., medication for hypertension) to hold constant a number of relevant clinical factors. What we learn about adherence in one disease condition or treatment regimen might not be generalizable to others; adherence researchers should study many different conditions and regimens and assess the similarities and differences in findings.
Finally, we are in greatest agreement with the authors and with each other that the effective measurement of patient medication adherence using patient self-report is both valuable and achievable. Reliable, valid, and clinically useful self-report measures can be developed and supported with convincing effectiveness research [2,6,9,10].
References
- 1.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–9. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 2.Jerant A, DiMatteo R, Arnsten J, Moore-Hill M, Franks P. Self-report adherence measures in chronic illness: retest reliability and predictive validity. Med Care. 2008;46:1134–9. doi: 10.1097/MLR.0b013e31817924e4. [DOI] [PubMed] [Google Scholar]
- 3.Hays R, DiMatteo MR. Key issues and suggestions for patient compliance assessment: sources of information, focus of measures, and nature of response options. J Compliance Health Care. 1987;2:37–53. [Google Scholar]
- 4.Reynolds NR, Testa MA, Marc LG, Chesney MA, Neidig JL, Smith SR, et al. Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: a multicenter, cross-sectional study. AIDS Behav. 2004;8:141–50. doi: 10.1023/B:AIBE.0000030245.52406.bb. [DOI] [PubMed] [Google Scholar]
- 5.Haugbolle LS, Sorensen EW, Henriksen HH. Medication- and illness-related factual knowledge, perceptions and behaviour in angina pectoris patients. Patient Educ Couns. 2002;47:281–9. doi: 10.1016/s0738-3991(01)00229-4. [DOI] [PubMed] [Google Scholar]
- 6.Green LW, Levine DM, Wolle J, Deeds SG. Development of randomized patient education experiments with urban poor hypertensives. Patient Couns Health Educ. 1979;1:106–11. doi: 10.1016/s0738-3991(79)80027-0. [DOI] [PubMed] [Google Scholar]
- 7.Levine DM, Green LW, Deeds SG, Chwalow AJ, Russell RP, Finlay J. Health education for hypertensive patients. JAMA. 1979;241:1700–3. [PubMed] [Google Scholar]
- 8.Lewis FM, Morisky DE, Flynn BS. A test of the construct validity of health locus of control and home assistance: their effects on self-reported compliance for hypertensive patients. Health Educ Monogr. 1978;6:138–48. doi: 10.1177/109019817800600105. [DOI] [PubMed] [Google Scholar]
- 9.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence and long-term predictive validity of blood pressure control. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Morisky DE, Ang A, Krousel-Wood M, Ward H. Predictive validity of a medication adherence measure for hypertension control. J Clin Hypertens. 2008;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Krousel-Wood MA, Islam T, Webber LS, Morisky DE, Muntner P. Concordance of self-reported medication adherence by pharmacy fill in patients with hypertension. Am J Manag Care. 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Morisky DE, Malotte CK, Choi P, Davison P, Rigler S, Sugland B, et al. A patient education program to improve adherence rate with antituberculosis drug regimens. Health Educ Q. 1990;17:253–68. doi: 10.1177/109019819001700303. [DOI] [PubMed] [Google Scholar]
- 13.DiIorio C, McCarty F, Resnicow K, Holstad MM, Soet J, Yeager K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20:273–83. doi: 10.1080/09540120701593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babamoto KS, Sey KW, Camilleri AJ, Karlan VJ, Catalasan J, Morisky DE. Improving diabetes care and health measures among hispanics using community health workers: results from a randomized controlled trial. Health Educ Behav. 2009;36:113–26. doi: 10.1177/1090198108325911. [DOI] [PubMed] [Google Scholar]
- 15.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]