Abstract
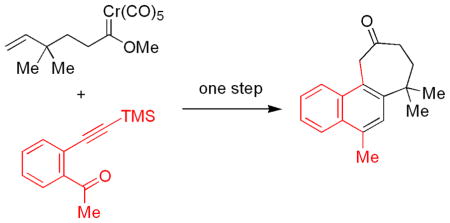
The coupling of pentenylcarbene complexes and 2-alkynylbenzoyl derivatives affords naphthocycloheptanones in a single step involving simultaneous construction of both the seven–membered ring and one of the aromatic rings. Aryl tethered systems undergo intramolecular cyclopropanation.
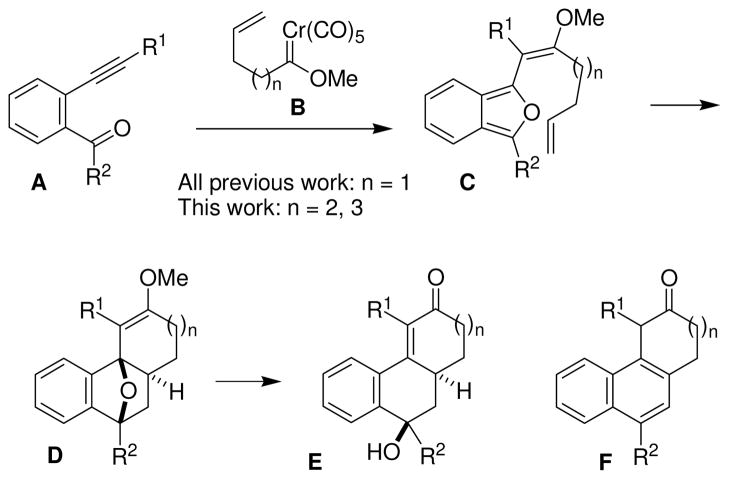
The hydrophenanthrene carbon skeleton (i.e. E and F, Scheme 1) is readily accessed through coupling of 2-alkynylbenzaldehyde derivatives (A) and γ,δ-unsaturated chromium carbene complexes (B, n= 1).1 This net [5+5]-cycloaddition approach to hydrophenanthrenes results from a series of reactions involving (1) regio-and stereoselective carbene-alkyne coupling,2 (2) carbonyl ylide formation,3 (3) isobenzofuran C formation,4 and (5) intramolecular Diels-Alder reaction. Subsequent transformations of the strained and electronically activated oxanorbornenes D leads to the observed products, dihydrophenanthrenes1 F (via dehydration and hydrolysis) or hydrophenanthrenones5 E (via ring opening and hydrolysis). Intramolecular Diels-Alder reactions of isobenzofurans and unactivated alkenes are limited by the instability of isobenzofurans, and nearly all of the examples involve the formation of five- and/or six-membered ring systems.6 Higher yields and a greater substrate range are generally observed with chromiumgenerated systems compared to acid-generated systems,7 however even in the chromium-based systems primarily six-membered ring formation has been documented.8 This manuscript focuses on the formation of seven-membered rings employing this reaction. Furan and/or isobenzofuran Diels-Alder reactions that result in seven-membered and larger rings are comparatively rare.9 Likely complications in this investigation include: (1) competing intramolecular cyclopropanation,10 (2) competing alkene migration,11 and (3) a slower intramolecular Diels-Alder event, thus allowing for competing isobenzofuran decomposition.
Scheme 1.
The coupling of δ,ε-unsaturated carbene complexes (B, n = 2) and alkynylbenzaldehydes would hypothetically lead to seven-membered rings fused to a naphthalene ring system (F, n = 2). Benzo-fused cycloheptanones and their simple derivatives are a very important structural feature in numerous medicinal compounds, including the anti-HIV drug TAK-779,12 intermediates for colchicine synthesis,13 numerous experimental anticancer drugs,14 and treatments for neurodegenerative disorders.15 The title reaction is a two component coupling where a diverse array of synthetic routes exists for both of the coupling partners. The basic coupling process is an intermolecular coupling requiring no pre-organization of the reactants and thus is inherently versatile compared to any process limited to intramolecular systems. In this manuscript, the use of this reaction for the preparation of the more challenging seven-membered ring systems is investigated.
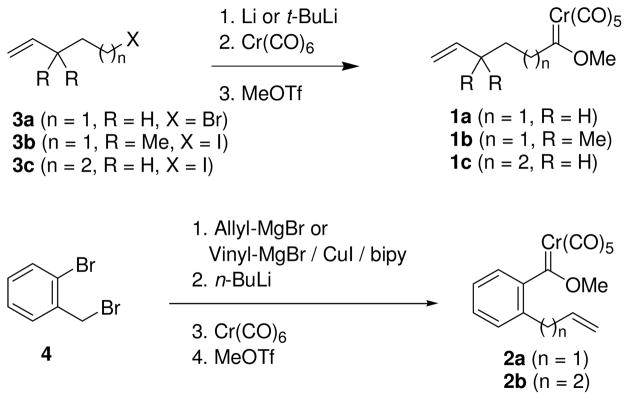
Alkene-containing carbene complexes (1 and 2, Scheme 2) were synthesized from known or commercially-available compounds according to the Fischer synthetic routes depicted in Scheme 2. The organolithium reagents were generated through reaction of the bromides with lithium metal wherever possible due to safety concerns. In more sluggish cases, lithiation of the iodide with t-butyllithium was employed. Competing Bailey cyclization of the organolithium intermediates16 was not a problem under the conditions employed. The aryl-tethered carbene complexes were prepared from 2-bromobenzyl bromide through halogen-metal exchange followed by analogous conversion to the carbene complex. Compound 2a was a very unstable compound and had to be used immediately after its synthesis.
Scheme 2.
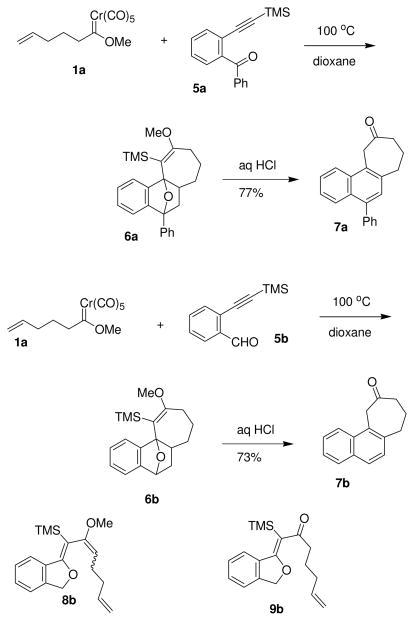
The first reaction examined was the coupling of alkynylbenzophenone derivative 5a (Scheme 3) with carbene complex 1a. The benzophenone derivative 5a had proven to be the most reliable substrate in related [5+5]-cycloaddition processes17 owing to the enhanced stability of arylisobenzofuran intermediates. After treatment of the crude reaction mixture with aqueous HCl, the naphthalene derivative 7a was isolated in 77% yield. The more challenging reaction, coupling of complex 1a alkynylbenzaldehyde derivative 5b, was examined under a variety of conditions. Thermolysis in dioxane followed by treatment with acid led to naphthocycloheptanone 7b in 73% yield. As noted in previous manuscripts, addition of water17 or collidine1 to [5+5]-cycloaddition reactions often led to improved yields of adducts. Addition of either of these additives led to uncyclized compounds tentatively assigned as alkylidenephthalans (8b/9b), which result from a net 1,7-hydride shift of the vinylisobenzofuran intermediate7 and were often produced when a carbene complex incapable of the Diels-Alder step was employed.18 In related six-membered ring-forming processes, competition from this reaction pathway was seldom an issue. A likely explanation for these results is that the seven-membered ring process is slower than the previously-reported six-membered ring-forming process, and the 1,7-hydrogen shift is the preferred fate of the isobenzofuran intermediate. Unfortunately, all attempts to isolate a single derivative of 6b prior to HCl treatment were unsuccessful. This might be attributed to a lack of endo-exo selectivity in the Diels-Alder reaction using the longer-tethered system, or could be due to a mixture of ketones and enol ethers, and their respective protiodesilylation products prior to the hydrolysis step.
Scheme 3.
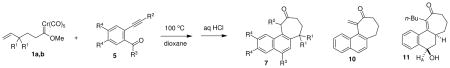
Several different examples are presented in Table 1, which vary in composition of the carbene complex tether, the alkyne substituent, the benzene ring substituents, and the carbonyl group. As noted in Table 1, the reaction in dioxane is unaffected by all of these variables. Silylated alkynes and alkyl-aryl alkynes all produce the naphthocycloheptanone in similar yield, however the yields were slightly lower using a terminal alkyne (entry 4). The simple benzene ring substrates and the one substrate featuring a highly electron-rich aromatic ring (entry 7) work equally effectively. The yields for the carbene complex featuring a gem-dimethyl group in the tether (entries 3, 4, and 8) are equal to that for the carbene complex featuring the unsubstituted tether. It appears as though any steric disadvantage has been offset by a favorable gem dialkyl effect. Use of the propargylic ester 5e (entry 6) led to the elimination product, methylenecycloheptanone 10. A single example afforded the enone-alcohol derivative 11 (entry 5), which only afforded the naphthalene derivative 7e with more vigorous HCl treatment. In compound 11, the hydrogen noted as HA appears as a doublet of doublets (if D2O was added to the chloroform NMR) with coupling constants of 12.0 and 3.7 Hz, which suggests that it is in an axial orientation. The indicated stereochemistry is consistent with complete exo selectivity in the intramolecular Diels-Alder step, even in the longer tethered systems employed in these studies.19
Table 1.
Preparation of naphthocycloheptanones through couplling of δ,ε-unsaturated carbene complexes and 2-alkynylbenzoyl systems.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | reactants | R1 | R2 | R3 | R4 | product | yield % |
| 1 | 1a + 5a | H | TMSa | Ph | H | 7a | 77 |
| 2 | 1a + 5b | H | TMSa | H | H | 7b | 73 |
| 3 | 1b + 5b | Me | TMSa | H | H | 7c | 75 |
| 4 | 1b + 5c | Me | H | H | H | 7c | 62 |
| 5b | 1a + 5d | H | n-Bu | H | H | 11 | 78 |
| 6c | 1a + 5e | H | CH2OAc | H | H | 10 | 74 |
| 7 | 1a + 5f | H | TMSa | H | OMe | 7g | 76 |
| 8 | 1b + 5g | Me | TMSa | Me | H | 7h | 80 |
R2 = H in 7 when R2 = TMS in 5.
The exclusive product was the alcohol derivative 11.
The exclusive product was elimination product 10.
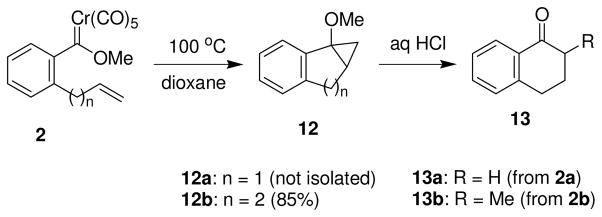
All attempts to make an eight-membered ring using this methodology failed. Reaction of the hexenylcarbene complex 1c with either of the substrates 5a or 5b resulted in products where no Diels-Alder reaction occurred. The crude 1H NMR spectra exhibited substantial absorptions and δ 5.9 and δ 5.0, which is indicative of a monosubstituted alkene group. The reactions employing the arylcarbene complexes 2a and 2b did not result in alkyne-coupled products. Thermolysis of either of carbene complexes with alkyne 5b led to the simple tetralone derivatives 13 (Scheme 4) after HCl treatment. The yields and product distributions are identical, regardless of whether the alkyne is present. A likely mechanism for the formation of these compounds involves intramolecular cyclopropanation10b followed by acid-catalyzed ring opening of the alkoxycyclopropane derivatives.20 In one case the cyclopropane 12b was isolated and leads to α-methyltetralone (13b) upon exposure to acid.
Scheme 4.
In summary, we have presented a versatile two-component coupling approach to form naphthalene-fused cycloheptanones in a net [5+6]-cycloaddition process, where both the seven-membered ring and one of the benzene rings are constructed in a single reaction event. The reaction proceeds in good yields for all systems examined employing sp3 carbons in the tether however aryl-containing tethers undergo rapid intramolecular cyclopropanation in preference to any intermolecular coupling with alkyne groups.
Supplementary Material
Acknowledgments
This work was supported by the SCORE program of NIH (SC1GM083693).
Footnotes
Supporting Information. Complete experimental procedures and photocopies of 1H and 13C NMR spectra for compounds 1, 2, 7, 10, 11, 12b.
References
- 1.Recent examples: Camacho-Davila A, Ranchigoda LSR, Herndon JW. Tetrahedron. 2010;66:4954–4960. doi: 10.1016/j.tet.2010.04.117.Jana GP, Mukherjee S, Ghorai BK. Synthesis. 2010:3179–3187.
- 2.For the most detailed discussion of this issue, see: McCallum JS, Kunng FA, Gilbertson SR, Wulff WD. Organometallics. 1988;7:2346–2360.
- 3.Herndon JW, Wang H. J Org Chem. 1998;63:4564–4565. [Google Scholar]
- 4.For a review on the reactivity of isobenzofurans, see: Friedrichsen W. Adv Heterocycl Chem. 1999;73:1–96.
- 5.For the latest example, see: Menon S, Sinha-Mahapatra DK, Herndon JW. Tetrahedron. 2007;63:8788–8793. doi: 10.1016/j.tet.2007.06.032.
- 6.a Kuninobu Y, Nishina Y, Takai K. Tetrahedron. 2007;63:8463–8468. [Google Scholar]; b Padwa A, Eidell CK. ARKIVOC. 2003;(14):62–76. [Google Scholar]; c Peters O, DeBaerdemaeker T, Friedrichsen WJ. Chem Soc, Perkin Trans. 1999;1:59–69. [Google Scholar]
- 7.For a direct comparison of similar systems, see: Duan SF, Cress K, Waynant K, Ramos-Miranda E, Herndon JW. Tetrahedron. 2007;63:2959–2965. doi: 10.1016/j.tet.2007.01.046.
- 8.In one case, a seven-membered ring forming process was observed using an alternate tethering scheme, however the yield was considerably lower than six-membered ring cases. Luo Y, Herndon JW. Organometallics. 2005;24:3099–3103.
- 9.a Bobeck DR, France S, Leverett CA, Sanchez-Cantalejo F, Padwa A. Tetrahedron Lett. 2009;50:3145–3147. [Google Scholar]; b Padwa A, Lynch SM, Mejia-Oneto JM, Zhang H. J Org Chem. 2005;70:2206–2218. doi: 10.1021/jo047834a. [DOI] [PubMed] [Google Scholar]
- 10.a Alvarez-Toledano C, Rudler H, Daran JC, Jeannin Y. Chem Commun. 1984:574–576. [Google Scholar]; b Söderberg BC, Hegedus LS. Organometallics. 1990;9:3113–3121. [Google Scholar]
- 11.Alvarez-Toledano C, Parlier A, Rudler H, Rudler M, Daran JC, Knobler C, Jeannin Y. J Organomet Chem. 1987;328:357–373. [Google Scholar]
- 12.a Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Izawa Y, Shiraishi M, Arakami Y, Okonogi K, Ogawa Y, Meguro K, Fujino M. Proc Nat Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Westby M, van der Ryst E. Antiviral Chemistry and Chemostherapy. 2005;16:339–354. doi: 10.1177/095632020501600601. [DOI] [PubMed] [Google Scholar]
- 13.van Tamelen EE, Spencer TA, Jr, Allen DS, Jr, Orvis RL. J Am Chem Soc. 1959;81:6341–6342. [Google Scholar]
- 14.a Sriram M, Hall JJ, Grohmann NC, Strecker TE, Wootton T, Franken A, Trawick ML, Pinney KG. Bioorg Med Chem. 2008;16:8161–8171. doi: 10.1016/j.bmc.2008.07.050. [DOI] [PubMed] [Google Scholar]; b Tarnus RC, Defoin A, Albrecht S, Maiereanu A, Faux N, Pale P. Fr Demande. 2008 FR 2908131 A1 20080509. [Google Scholar]
- 15.a Chen YL, Zhang L. PCT Int Appl. 2005 Coden: PIXXD2. [Google Scholar]; b Satoh Y, Hatori C, Ito H. Bioorg Med Chem Lett. 2002;12:1009–1011. doi: 10.1016/s0960-894x(02)00090-2. [DOI] [PubMed] [Google Scholar]; c Itani H, Ito H, Sakata Y, Hatakeyama Y, Oohashi H, Satoh Y. Bioorg Med Chem Lett. 2002;12:799–802. doi: 10.1016/s0960-894x(02)00018-5. [DOI] [PubMed] [Google Scholar]; d Liu Y, Mellin C, Björk L, Svensson B, Csöregh I, Helander A, Kenne L, Andén NE, Hacksell U. J Med Chem. 1989;32:2311–2318. doi: 10.1021/jm00130a015. [DOI] [PubMed] [Google Scholar]; e Hacksell U, Arvidsson LE, Svensson U, Nilsson JLG, Wikström H, Lindberg P, Sanchez D, Hjorth S, Carlsson A, Paalzow L. J Med Chem. 1981;24:429–434. doi: 10.1021/jm00136a012. [DOI] [PubMed] [Google Scholar]
- 16.Bailey WF, Nurmi TT, Patricia JJ, Wang W. J Am Chem Soc. 1987;109:2442–2448. [Google Scholar]
- 17.Li R, Camacho-Davila A, Herndon JW. Tetrahedron Lett. 2005;46:5117–5120. [Google Scholar]
- 18.a Jiang D, Herndon JW. Organic Letters. 2000;2:1267–1269. doi: 10.1021/ol005691i. [DOI] [PubMed] [Google Scholar]; b Duan S, Cress K, Waynant K, Ramos-Miranda E, Herndon JW. Tetrahedron. 2007;63:2959–2965. doi: 10.1016/j.tet.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For a more detailed discussion of this issue see the Supporting Information.
- 20.a Sugimura T, Futagawa T, Yoshikawa M, Tai A. Tetrahedron Lett. 1989;30:3807–3810. [Google Scholar]; b Sugimura T, Futagawa T, Tai A. Tetrahedon Lett. 1988;29:5775–5778. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.