Abstract
Synthetic derivatives of the phosphonate antibiotic dehydrophos were tested for antimicrobial activity. Both the phosphonate monomethyl ester and the vinyl phosphonate moiety proved to be important for bacteriocidal activity of the natural product.
The phosphonate tripeptide dehydrophos (1) produced by Streptomycis luridus is a broad spectrum antibiotic against both Gram-positive and Gram-negative bacteria.1 Initially discovered in 1984, the correct structure of dehydrophos was determined in 2007 by comparison with a synthetic sample. The C-terminal residue, which is attached to a glycine–leucine dipeptide, was demonstrated to be the monomethyl ester of 1-aminovinylphosphonate (Fig. 1).2 This unique structure attracted interest in the biosynthesis of dehydrophos3,4 and also raised questions about its mode of action. As part of a program to provide more insight into phosphonate natural products,5 we present structure activity relationship studies on dehydrophos and synthetic analogs to address these questions.
Fig. 1.
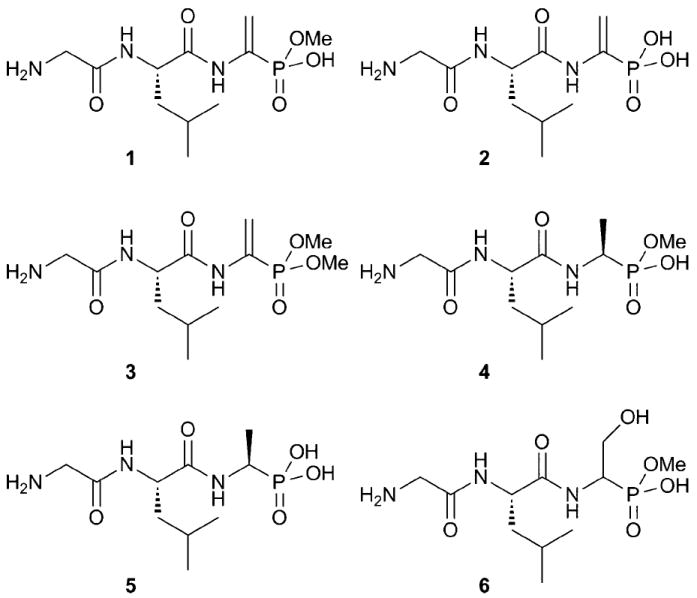
Structures of synthetic compounds 1–6. Note that in aqueous solutions, the anions of 4 and 6 are not chiral at phosphorus.
Many naturally occurring phosphonates are produced as peptides to facilitate uptake by the target organism via nonspecific oligopeptide permeases.6,7 Hydrolysis of the peptide linkages inside the organism then releases the active moiety. Alternatively, for some phosphonate-containing peptides such as K-26, the entire peptide is important for enzyme inhibition.8,9 Dehydrophos has several unusual characteristics. The vinylphosphonate moiety may be important for its mode of action as it represents an electrophilic group that could covalently modify its target. This electrophile would be lost upon peptidase action, because the resulting enamine would hydrolyze. Another unique aspect of the dehydrophos structure is its methyl ester; no other examples have been reported of esterified phosphonate natural products. Methylation of a phosphonate reduces its negative charge and results in a charge distribution closer to that of a tetrahedral intermediate in carboxylate metabolism. Therefore, the phosphonate methyl ester group in dehydrophos may mimic such an intermediate resulting in inhibition of a target enzyme comparable to synthetic phosphonate/phosphonamide containing peptides that were identified as transition state analogue inhibitors of proteases.10 To investigate the importance of the various structural motifs found in dehydrophos, several analogs were prepared in this study.
To test the importance of the methyl ester, the phosphonic acid derivative 2 and its dimethyl analog 3 (Fig. 1) were prepared. N-Cbz protected serine with a tert-butyldimethylsilyl (TBDMS) protected side chain was decarboxylated with lead tetraacetate (Scheme 1).11 The N,O-acetal product was then reacted with trimethylphosphite in the presence of titanium tetrachloride.12 Hydrogenation of the Cbz protecting group followed by solution phase peptide synthesis provided the protected tripeptide. Treatment with TBAF resulted in the alcohol that was activated as the methylsulfonate to introduce the vinyl phosphonate after elimination. Global deprotection with boron tribromide then resulted in desmethyl dehydrophos 2. The dimethyl derivative 3 was prepared similarly (see ESI‡).
Scheme 1.
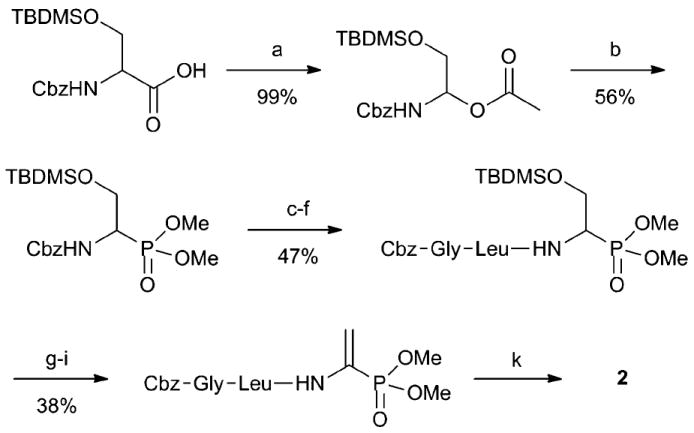
Synthesis of desmethyl dehydrophos 2: (a) Pb(OAc)4, DMF, 0 °C to 25 °C; (b) TiCl4, P(OMe)3, CH2Cl2, −78 °C to 25 °C; (c) Pd/C, H2(g), MeOH, 25 °C; (d) Cbz-Leu-OH, EDC, CH2Cl2, 25 °C; (e) Pd/C, H2(g), MeOH, 25 °C; (f) Cbz-Gly-OH, EDC, CH2Cl2, 25 °C; (g) TBAF, THF, 25 °C; (h) MsCl, NEt3, CH2Cl2, 0 °C to 25 °C; (i) DBU, CH2ClCH2Cl, 84 °C; (k) BBr3, hexanes, toluene 0 °C to 70 °C.
To test the importance of the electrophilic vinylphosphonate, compound 4 (Fig. 1) lacking the vinyl functionality was prepared resulting in a methylated phosphonate analog of L-alanine as the C-terminal residue. N-(Triphenylmethyl) protected dimethyl 1-aminoethyl-phosphonate was synthesised as a racemic mixture as illustrated in Scheme 2.13 After peptide synthesis, the N-Boc protecting group was removed under acidic conditions, and the monomethyl phosphonate ester was isolated after treatment with 10% aqueous NaOH.4 Compound 4 and its diastereomer with the opposite stereochemistry of the aminophosphonate (4b) were subsequently separated by RP-HPLC.
Scheme 2.
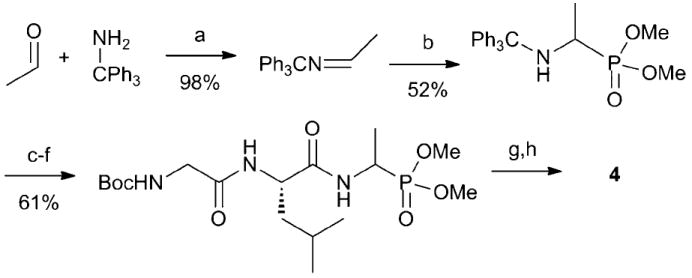
Synthesis of the derivative 4. (a) Toluene, 110 °C; (b) HPO(OMe)2, 100 °C, (c) HCl in MeOH, 25 °C; (d) Boc-Leu-OH, EDC, NMM, CH2Cl2, 25 °C; (e) HCl in MeOH, 65 °C; (f) Boc-Gly-OH, EDC, NMM, CH2Cl2, 25 °C; (g) HCl in MeOH, 65 °C; (h) NaOH, H2O, 25 °C.
In addition, peptide 5 and its epimer at C1 of residue 3 (5b) that contain neither the methyl ester nor the vinyl moiety of the natural product were prepared using boron tribromide to deprotect the tripeptide.4 The resulting two diastereomers were isolated by RP-HPLC, and a crystal structure of stereo-isomer 5 was used to assign the stereochemistry of both compounds.4 Derivatives containing the phosphonate analog of serine (6a and 6b, Fig. 1, epimers at C1 of the methyl phosphonate) were also prepared as they are potential precursors in the biosynthesis of the vinyl group (see ESI‡). Finally, the enantiomer of dehydrophos (ent-1) was synthesised as described previously for 12 to analyse the effect of a d-leucine as the second residue. The introduction of a d-amino acid in dehydrophos was anticipated to affect its bioactivity if the entire peptide interacts with its target. If not, (i.e. if the peptide requires proteolysis for bioactivity) then the d-Leu could still affect bioactivity because the uptake and proteolysis processes may be less effective. Therefore, another diastereomer of 5 with a d-Leu as the second residue (ent-5b) was also prepared. Because compound 5 requires proteolysis for activity (vide infra), introduction of a d-Leu was envisioned to independently report on the importance of stereochemistry in the 2nd position for uptake and proteolysis.
Compounds 1–6 and their stereoisomers were tested for antimicrobial activity with Escherichia coli and Bacillus subtilis ATCC 6633 as indicator strains. Solid agar diffusion bioassays on M9 media resulted in zones of growth inhibition for compounds 1–5 against E. coli (Fig. 2, Table 1). The diastereomer of 4 (4b) showed no activity and 5b demonstrated only a small zone of inhibition. With B. subtilis compounds 1 and 3–5 resulted in a zone of inhibition but not the phosphonic acid 2 (see ESI‡). d-Stereochemistry in the alanine derivatives 4b and 5b resulted in inactive compounds. Bioactivity was also not detected for either diastereomer of the serine derivatives 6 against both E. coli and B. subtilis, and on rich LB media none of the compounds 1–6 showed antimicrobial activity under the tested conditions. This latter observation suggests that some components in LB can overcome the inhibition of the target(s) of these compounds thereby rescuing bacterial growth. IC50 values (growth inhibition) were determined for E. coli in liquid broth (90% M9 and 10% LB media) to quantitatively compare the activities (Table 1).
Fig. 2.
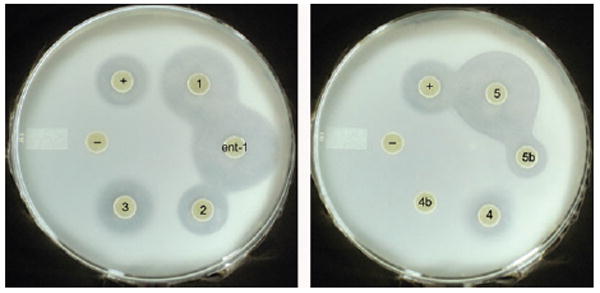
Bioassay against E. coli: (−) negative control (water), (+) positive control (ampicillin), left: compounds 1, ent-1, 2, 3; right: compound 4, 4b, 5 and 5b. See also ESI‡.
Table 1.
Comparison of the dehydrophos analogs 1–6
| Compound | E. colia | B. subtilisa | IC50/μM, E. coli |
|---|---|---|---|
| 1 | ++ | ++ | 181 ± 6 |
| ent-1 | ++ | ++ | 299 ± 18 |
| 2 | + | — | >1000 |
| 3 | + | + | >1000 |
| 4 | + | ++ | >1000 |
| 4b | — | — | ndb |
| 5 | ++ | + | 42 ± 5 |
| 5b | + | — | 121 ± 17 |
| ent-5 | ++ | + | 34 ± 22 |
| ent-5b | + | — | 182 ± 17 |
| 6 | — | — | ndb |
Solid agar diffusion assays: + zone of inhibition, — no activity detected.
nd: not determined.
(1 R)-Aminoethylphosphonate (R-AlaP), the compound that would result from proteolysis of the C-terminal amide bond of 5 has been studied in-depth as the active component of alaphosphin.14-18 It inhibits alanine racemase and therefore bacterial cell wall synthesis. The growth inhibition can be rescued by providing d-alanine and it is known that alaphosphin peptide analogs with an S-AlaP residue are less potent.16 The growth inhibition by compound 5 tested in this study was also rescued by d-alanine and since the diastereomer 5b was less active (E. coli) or not active (B. subtilis), it is very likely that alanine analog 5 of dehydrophos is also hydrolysed inside the cell and inhibits alanine racemase by a mechanism similar to that of alaphosphin. The effect of compounds 1–4 was not rescued by d-alanine, suggesting that their inhibition mechanism is different.
If proteolysis is also required for compounds 1–4, then hydrolysis of the C-terminal amide bond of compound 4 would release the methyl ester of R-AlaP. Previous studies with alanine racemase of B. stearothermophilus showed no inhibition by methyl R-AlaP.19 Nevertheless, activity is detected for tripeptide 4 consistent with a different target for this analog, which is also consistent with the lack of rescue by d-Ala. Compared to dehydrophos, saturation of the double bond in 4 reduces the antimicrobial activity against E. coli substantially (Table 1) demonstrating the importance of the vinyl phosphonate functionality (assuming as in any SAR studies that 4 has the same target as 1). The inactive serine analogs 6 also support this conclusion.
No activity was detected with desmethyl dehydrophos 2 against B. subtilis, and the activity of 2 against E. coli was substantially reduced compared to 1. In liquid broth, no growth inhibition could be detected up to 1 mM whereas the IC50 value for 1 was 180 μM under these conditions (Table 1). Dimethylated dehydrophos (3) resulted in a zone of inhibition in the agar based assays, but no inhibition was detected up to 1 mM in liquid broth (Table 1). Collectively, these observations clearly show that the methyl phosphonate ester is important for the activity of dehydrophos.
The data so far show that both the methyl ester and vinyl groups are important for bioactivity. If intracellular hydrolysis of the C-terminal amide bond of dehydrophos is important for activity, this process would release methyl acetylphosphonate, a phosphonate analog of pyruvate. This compound is known to inhibit pyruvate dehydrogenase and pyruvate oxidase.20 Furthermore, it has been demonstrated that the methyl ester of acetylphosphonate is a stronger inhibitor of pyruvate oxidase at neutral pH than acetylphosphonate, suggesting that the methyl ester might indeed be beneficial to reduce the charge of the phosphonate. Neither acetylphosphonate nor methyl acetylphosphonate showed any antimicrobial activity in our assays, presumably, because they did not enter into the cells.
We attempted to obtain evidence that dehydrophos indeed must be hydrolyzed. Dehydrophos and ent-1 only differ in the stereochemistry of Leu at position 2 and have very similar antimicrobial activities. Compounds 5 and ent-5b also only differ in their stereochemistry at Leu2. Thus, we reasoned that if these latter two compounds, which are known to require hydrolysis to release the active component R-AlaP, also had similar activities, it would provide indirect support for hydrolysis of 1. However, ent-5b proved much less active, and surprisingly, ent-5 containing both a d-Leu and a S-AlaP was significantly more active (Table 1). Therefore, we conclude that the relationship between stereochemistry of the tripeptide and the activity of the permease/protease is complex. Alternatively, ent-5 may have a different mode of action than 5. At any rate, we cannot make any conclusions regarding the need for proteolysis of dehydrophos on the basis of these SAR studies.
In summary, the structure–activity comparisons presented here demonstrate that both the vinyl and methyl ester functionalities are key for dehydrophos bioactivity, and these findings are consistent with pyruvate dehydrogenase/oxidase as its target.
Supplementary Material
Acknowledgments
This work was supported by the NIH (PO1 GM077596 to WAV). MK was supported by a postdoctoral fellowship from the Swiss National Science Foundation.
Footnotes
This article is part of the ‘Enzymes and Proteins’ web-theme issue for ChemComm.
Electronic supplementary information (ESI) available: Experimental details on the syntheses, agar diffusion assays and liquid broth studies are presented. See DOI: 10.1039/c0cc02958k
Notes and references
- 1.Johnson RD, Gordee RS, Kastner RM, Larsen SH, Ose EE. UK 2,127,413. 1984
- 2.Whitteck JT, Ni W, Griffin BM, Eliot AC, Thomas PM, Kelleher NL, Metcalf WW, van der Donk WA. Angew Chem Int Ed. 2007;46:9089. doi: 10.1002/anie.200703810. [DOI] [PubMed] [Google Scholar]
- 3.Circello BT, Eliot AC, Lee JH, van der Donk WA, Metcalf WW. Chem Biol. 2010;17:402. doi: 10.1016/j.chembiol.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Bae B, Kuemin M, Circello BT, Metcalf WW, Nair SK, van der Donk WA. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1006848107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalf WW, van der Donk WA. Annu Rev Biochem. 2009;78:65. doi: 10.1146/annurev.biochem.78.091707.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diddens H, Zahner H, Kraas E, Gohring W, Jung G. Eur J Biochem. 1976;66:11. doi: 10.1111/j.1432-1033.1976.tb10420.x. [DOI] [PubMed] [Google Scholar]
- 7.Kugler M, Loeffler W, Rapp C, Kern A, Jung G. Arch Microbiol. 1990;153:276. doi: 10.1007/BF00249082. [DOI] [PubMed] [Google Scholar]
- 8.Yamato M, Koguchi T, Okachi R, Yamada K, Nakayama K, Kase H, Karasawa A, Shuto K. J Antibiot. 1986;39:44. doi: 10.7164/antibiotics.39.44. [DOI] [PubMed] [Google Scholar]
- 9.Ntai I, Bachmann BO. Bioorg Med Chem Lett. 2008;18:3068. doi: 10.1016/j.bmcl.2007.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett PA, Marlowe CK. Biochemistry. 1983;22:4618. doi: 10.1021/bi00289a002.; Bone R, Sampson NS, Bartlett PA, Agard DA. Biochemistry. 1991;30:2263. doi: 10.1021/bi00222a032.; Fraser ME, Strynadka NCJ, Bartlett PA, Hanson JE, James MNG. Biochemistry. 1992;31:5201. doi: 10.1021/bi00137a016.; Bartlett PA, Giangiordano MA. J Org Chem. 1996;61:3433..
- 11.Corcoran RC, Green JM. Tetrahedron Lett. 1990;31:6827. [Google Scholar]
- 12.Seebach D, Charczuk R, Gerber C, Renaud P, Berner H, Schneider H. Helv Chim Acta. 1989;72:401. [Google Scholar]
- 13.Soroka M, Zygmunt J. Synthesis. 1988:370. [Google Scholar]
- 14.Allen JG, Atherton FR, Hall MJ, Hassall CH, Holmes SW, Lambert RW, Nisbet LJ, Ringrose PS. Nature. 1978;272:56. doi: 10.1038/272056a0. [DOI] [PubMed] [Google Scholar]
- 15.Atherton FR, Hall MJ, Hassall CH, Lambert RW, Lloyd WJ, Ringrose PS. Antimicrob Agents Chemother. 1979;15:696. doi: 10.1128/aac.15.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atherton FR, Hall MJ, Hassall CH, Lambert RW, Ringrose PS. Antimicrob Agents Chemother. 1979;15:677. doi: 10.1128/aac.15.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atherton FR, Hall MJ, Hassall CH, Lambert RW, Lloyd WJ, Ringrose PS, Westmacott D. Antimicrob Agents Chemother. 1982;22:571. doi: 10.1128/aac.22.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atherton FR, Hall MJ, Hassall CH, Lambert RW, Lloyd WJ, Lord AV, Ringrose PS, Westmacott D. Antimicrob Agents Chemother. 1983;24:522. doi: 10.1128/aac.24.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badet B, Inagaki K, Soda K, Walsh CT. Biochemistry. 1986;25:3275. doi: 10.1021/bi00359a029. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien TA, Kluger R, Pike DC, Gennis RB. Biochim Biophys Acta. 1980;613:10. doi: 10.1016/0005-2744(80)90186-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


