Abstract
The aim of this work is to evaluate the influence of MgO, SrO and SiO2 doping on mechanical and biological properties of β-tricalcium phosphate (β-TCP) to achieve controlled resorption kinetics of β-TCP system for maxillofacial and spinal fusion application. We prepared dense TCP compacts of four different compositions, i) pure β-TCP, ii) β-TCP with 1.0 wt. % MgO + 1.0 wt. % SrO, iii) β-TCP with 1.0 wt. % SrO + 0.5 wt. % SiO2, and iv) β-TCP with 1.0 wt. % MgO + 1.0 wt. % SrO + 0.5 wt. % SiO2, by uniaxial pressing and sintering at 1250 °C. β phase stability is observed at 1250 °C sintering temperature due to MgO doping in β-TCP. In vitro mineralization in simulated body fluid (SBF) for 16 weeks shows excellent apatite growth on undoped and doped samples. Strength degradation of TCP samples in SBF is significantly influenced by both dopant chemistry and amount of dopant. Compressive strengths for all samples show degradation in SBF over the 16 week time period with varying degradation kinetics. MgO/SrO/SiO2 doped sample shows no strength loss, while undoped TCP shows the maximum strength loss from 419 ±28 MPa to 158 ±28 MPa over the 16 week study. In case of MgO/SrO doped TCP, strength loss is slow and gradual. TCP doped with 1.0 wt. % MgO and 1.0 wt. % SrO shows excellent in vivo biocompatibility when tested in male Sprague-Dawley rats for 16 weeks. Histomorphology analysis reveals that MgO/SrO doped TCP promoted osteogenesis by excellent early stage bone remodeling as compared to undoped TCP.
Keywords: β-tricalcium phosphate, MgO-SrO-SiO2, doping, in vitro degradation, in vivo osteogenesis
1. Introduction
β-tricalcium phosphate [β-TCP, β-Ca3(PO4)2] is one of the most attractive biomaterials for bone repair since it shows an excellent biological compatibility, osteoconductivity, and resorbability [1–2]. In many applications, the resorption properties make TCP an ideal bone substitute, where the ceramic supplies temporary support, which eventually degrades and is replaced by natural tissue [3–4]. Among several clinical applications of TCP, posterior spinal fusion and craniomaxillofacial applications have gained significant attention [5]. TCP ceramics, with improved mechanical properties and controlled resorbability, can assist in designing optimal biodegradable bone substitutes for spinal fusion, and craniomaxillofacial applications. Use of such bone substitutes also avoids second site surgery that is needed due to autograft harvesting.
Synthesis of cation-substituted TCP ceramics has been the subject of immense interest owing to the critical roles played by these ions in the biological activity of bone [6–8]. In our earlier studies [2, 9–12], we demonstrated that addition of dopants such as NaF, TiO2, SiO2, CaO, ZnO and Ag2O improve mechanical properties of TCP without altering its inherent biocompatibility. In recent years, in vitro and in vivo studies clearly indicated beneficial effects of strontium (Sr) on bone formation [13–15]. Our previous work demonstrated that the presence of Sr in calcium phosphate (CaP) promotes osteoblast function and subsequent bone formation [16, 17]. Sr at low dose enhances the replication of preosteoblastic cells, and simulates bone formation. It has also been demonstrated that Sr suppresses bone resorption and maintains bone formation. In contrast, a high dose of Sr induces defective bone mineralization and alters the mineral profile [18]. CaP ceramics with 1.0% Sr has been reported to be the optimal proportion for most favorable osteoblastic cell growth [13].
Magnesium (Mg) is also undoubtedly one of the most important bivalent ions associated with biological apatites [19–21]. Substitution of Mg in CaPs has received much attention due to its impending role in the qualitative changes in bone matrix, its indirect influence on mineral metabolism, promoting catalytic reactions, and controlling biological functions [22]. Silicon (Si) is an important element that influences bone formation and calcification. Si has been reported to stimulate cellular activities, such as proliferation and differentiation of osteoblast-like cells, mineralization of human osteoblasts and osteogenic differentiation of mesenchymal stem cells [23–25]. The importance of Si for bone formation has been demostrated by indicating the presence of up to 0.5 wt% Si in the osteoid of the bones of young mice and rats [26]. Extensive research has also been conducted on high Si content biomaterials such as bioglass [27–29].
Considering the beneficial effects of Sr, Mg and Si in bone formation, the aim of the present study was to evaluate the influence of Sr2+, Mg2+ and Si4+ on the physical, mechanical, and biological properties of TCP. SrO, MgO and SiO2 were used as dopants with commercially pure TCP. We fabricated doped TCP samples via solid-state reaction, and studied the phase composition, microstructure and mechanical properties of doped samples. We evaluated early stage osteogenesis and bone remodeling in vivo using a defect model in the distal femur of male Sprague-Dawley rats over a 16 weeks period.
2. Materials and Methods
2.1. Materials
High purity (99.99%) β-TCP powder was obtained from Berkeley Advanced Biomaterials Inc. (Berkeley, CA), with an average particle size of 550 nm. High purity strontium oxide (SrO, 99.9% purity) was purchased from Aldrich (MO, USA). Magnesium oxide (MgO, Puratronic, 99.998%) and silicon dioxide (SiO2, Puratronic, 99.999% purity) were procured from Alfa Aesar (MA, USA). All other chemicals were of analytical grade and used without further purification.
2.2. Sample preparation
Based on our preliminary studies and previous work [2, 10, 17], four compositions of TCP were processed for this study: (i) Pure TCP; and TCP doped with (ii) 1.0 wt. % MgO + 1.0 wt. % SrO; (iii) 1.0 wt. % SrO + 0.5 wt. % SiO2 and (iv) 1.0 wt. % MgO + 1.0 wt. % SrO + 0.5 wt. % SiO2. Samples were prepared by mixing 50 g TCP nanopowder and appropriate amounts of dopants in a 250 mL polypropylene Nalgene bottles containing 75 mL of anhydrous ethanol and 100 g of 5 mm diameter zirconia milling media. The mixtures were then milled for 6 h at 70 rpm to minimize agglomerate formation, and to increase the homogeneity of the powder. After milling, powder was dried in an oven at 60 °C for 72 h.
Dried powder was pressed to disc (12 mm diameter and 2.5 mm thickness) and cylindrical (6.5 mm diameter and 12 mm long) shapes using a uniaxial press, at 145 MPa and 58 MPa, respectively. Disc samples were used for density, dissolution and mineralization studies, and cylindrical compacts were used for mechanical property evaluation. Five samples were made from individual compositions for each type of analysis. Green compacts were then cold isostatically pressed at 414 MPa for 5 min at room temperature followed by sintering at 1250 °C for 2 h in a muffle furnace. The apparent density of each composition was determined by the Archimede’s method and reported as relative density after normalizing to the theoretical density of TCP, i.e., 3.07 g/cm3.
2.3. Microstructure and phase analysis
Surface morphologies of disc compacts of all compositions were observed under a scanning electron microscope (SEM) (FEI Inc., OR, USA) following gold sputter-coating (Technics Hummer V, CA, USA). Average grain size was determined from SEM images via a linear intercept method using the equation G = (L/N) C, where G is the average grain size (μm), L is the test line length (cm), N is the number of intersections with grain boundaries along test line L; and C is the conversion factor (μm/cm) of the picture on which the test lines were drawn as obtained from the scale bar. Phase analysis of sintered undoped and doped TCP samples was carried out by X-ray diffraction (XRD) using a Philips PW 3040/00 Xpert MPD system (Philips, Eindhoven, the Netherlands) and Cu Kα radiation with a Ni-filter. Each run was performed with 2θ values of 10° to 60° at a step size of 0.02° and a count time of 0.5 s per step.
2.4 Mineralization study in SBF
In order to evaluate the in vitro bioactivity and strength degradation behavior of undoped and doped TCP samples, disc compacts of all compositions were immersed in a simulated body fluid (SBF) buffered at pH 7.40 with tris(hydroxymethyl)aminomethane/HCl. As has been frequently reported [30], the SBF is an acellular solution with an ionic composition (in units of mM, 142 Na+, 5.0 K+, 1.5 Mg2+, 2.5 Ca2+, 103.0 Cl−, 27.0 HCO3−, 1.0 HPO42− and 0.5 SO42−) almost equal to that of human plasma and buffered at a similar pH. The samples were exposed to the SBF solution under static condition in a thermostat at 37 °C for 2, 4, 8, 12, and 16 weeks, respectively. For each time period six samples of each composition were used. Over the course of the study, the SBF was changed every 3 days. A portion of the used SBF fluid from a set of samples was taken for Ca2+ concentration evaluation using a Shimadzu AA-6800 atomic absorption spectrophotometer (AAS) (Shimadzu, Kyoto, Japan). At each time point, samples exposed to SBF were taken out, washed with distilled water and dried at 65 °C in an oven for 7 days. Once dried, weight of the samples was carefully recorded and compared with their dry weight before immersion. Surface microstructures of these samples were observed using SEM to determine mineralization at the top layer. Attenuated total reflection infrared (ATR-IR) analysis was done on disc surfaces using a Fourier transform infrared spectrometer (FTIR, Nicolet 6700, ThermoFisher, Madison, WI) to confirm the presence of apatite in the mineralized layer. The samples were placed on an ATR diamond crystal and pressed with an indenter so that the samples could remain in contact with the ATR diamond crystal. Before each measurement, a background FTIR spectrum was taken and deducted from the sample spectra. All spectra were collected in the 400–2000 cm−1 wavenumber range.
2.5. Mechanical strength analysis
Compressive strength of undoped and doped TCP was determined using a screw-driven universal testing machine (AG-IS, Shimadzu, Japan) with a constant crosshead speed of 0.33 mm/min. Compressive strength was calculated based on the maximum load recorded and sample dimension. Compressive strength was tested on six samples for each composition.
2.6. In vivo study
2.6.1. Implant preparation
Implants for the in vivo study using rats were prepared by uniaxially pressing approximately 0.20 g of each powder at 25 MPa in a steel mold with an internal diameter of 3.75 mm. Samples were then isostatically pressed and sintered using the same technique as before. Implants of undoped TCP were used as a control. The TCP doped with Sr/Mg was fabricated because of its superior mechanical properties.
2.6.2. Surgery and implantation procedure
For each implant composition group (n=4), Sprague-Dawley rats (280–300 grams, Charles Rivers Laboratories International, Inc., Wilmington, MA, USA) were used. Prior to surgery, the rats were housed in individual cages with alternating 12-hour cycles of light and dark in temperature and humidity controlled rooms. Following acclimatization, all animals underwent bilateral surgery to create a cortical defect in the distal femur (3 mm diameter). Rats were anesthetized using IsoFlo® (isoflurane, USP, Abbott Laboratories, North Chicago, IL, USA) coupled with an oxygen (Oxygen USP, A-L Compressed Gases Inc., Spokane, WA, USA) regulator, and monitored by pedal reflex and respiration rate to maintain proper surgical anesthesia. The defect was created in the distal femur by means of a 3 mm drill bits. The cavity was rinsed with physiological saline to wash away remaining bone fragments. Each animal received a control implant, and in addition, a doped implant in the contralateral leg. Following implantation, undyed braided-coated polyglycolic acid synthetic absorbable surgical suture (Surgical Specialties Corporation, Reading, PA, USA) was used for stitching. Disinfectant was applied to the wound site to prevent infection. At 4, 8, 12 and 16 weeks post-surgery, rats were euthanized by overdosing with halothane in a bell jar, followed by administration of a lethal injection of potassium chloride (70%) into the heart. At necropsy, samples harvested were preserved at −20°C for future analysis. All experimental procedures were performed according to the protocol approved by the Institutional Animal Care and Use Committee of Washington State University.
2.6.3. Histomorphology
For histomorphological analysis, bone-implant specimens were fixed in 10% buffered formalin solution and dehydrated in a graduated ethanol (70%, 95%, and 100%) series. After embedding samples in Spurr’s resin, each undecalcified implant block was sectioned perpendicular to the implant surface using a low speed diamond blade. After polishing, the sections were stained by Masson Goldner’s trichrome stain and observed under light microscope [Olympus BH-2, Olympus America Inc., USA].
2.7. Statistical analysis
Data for relative density, grain size and Ca2+ concentration are presented as mean ± standard deviation. Statistical analysis was performed on weight change and compressive strength results using student’s t-test, and P value < 0.05 was considered significant.
3. Results
3.1. Phase identification
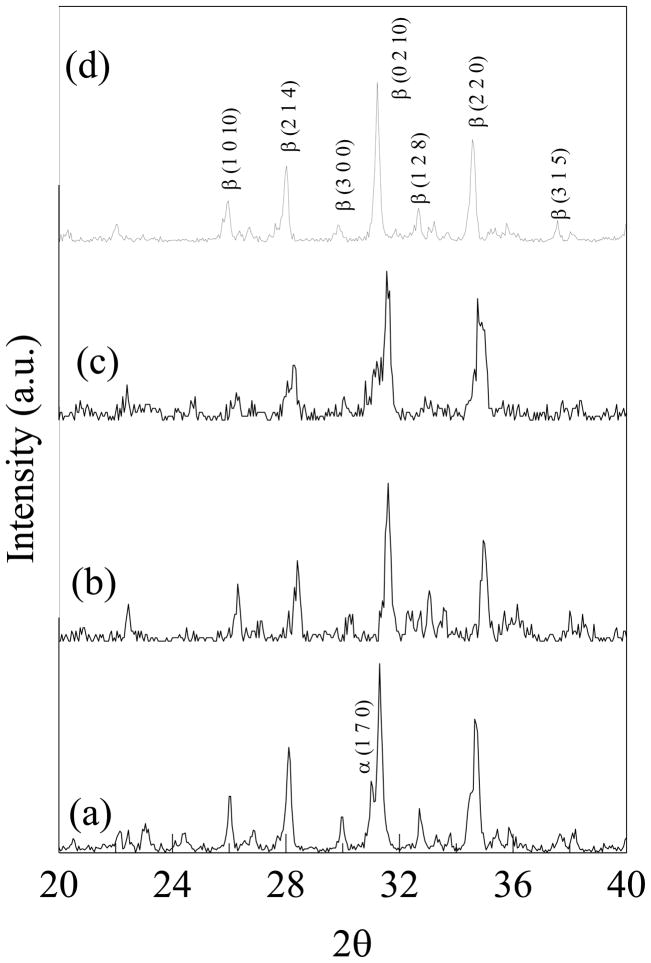
The XRD spectra of samples sintered at 1250 °C is shown in Figure 1. The spectra of undoped TCP (JCPDS No 09-169) showed the presence of some α-TCP peaks indicating β to α-TCP (JCPDS No 09-0348) phase transformation during densification. However, no α-TCP peaks were detected in the spectra of doped samples containing MgO, and all the peaks observed were of β-TCP. In case of TCP doped with SrO/SiO2, weak α-TCP peaks were observed compared to undoped TCP.
Figure 1.
X-ray powder diffraction patterns of (a) TCP, (b) TCP-MgO/SrO, (c) TCP-SrO/SiO2 and (d) TCP-MgO/SrO/SiO2.
3.2. Densification and microstructure
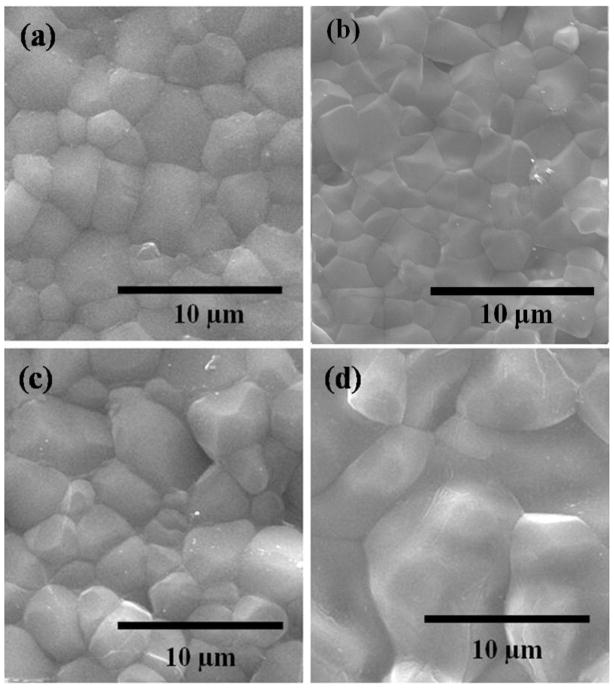
Figure 2 shows surface morphology of samples sintered at 1250 °C. Microstructures of all the three compositions revealed a highly dense structure. Some glassy phase formation was observed in the case of TCP samples with SrO/SiO2 and MgO/SrO/SiO2. Mean grain diameters measured from the SEM micrographs were 2.62 ±0.14 μm, 2.33 ±0.18 μm, 3.35 ±0.3 μm and 5.43 ±0.55 μm for undoped, MgO/SrO, SrO/SiO2 and MgO/SrO/SiO2 doped TCP samples, respectively, as shown in Table 1. The relative density of undoped, MgO/SrO, SrO/SiO2 and MgO/SrO/SiO2 doped TCP were found to be 96.2 ±1.2%, 97.7 ±0.7%, 95.5 ±1.0%, and 95.9 ±1.0%, respectively (Table 1). No significant change in density is observed due to these dopant additions. However, presence of SrO/SiO2 and MgO/SrO/SiO2 decreased the density of TCP compacts.
Figure 2.
SEM micrographs showing the grain size of (a) TCP (2.62 ± 0.14 μm), (b) TCP-MgO/SrO (2.33 ± 0.18 μm), (c) TCP-SrO/SiO2 (3.35 ± 0.3 μm), and (d) TCP-MgO/SrO/SiO2 (5.43 ± 0.55 μm), sintered at 1250 °C.
Table 1.
Relative density and grain size of undoped and doped samples
| Composition | Relative Density (%) | Grain Size (μm) |
|---|---|---|
| Undoped TCP | 96.2 ± 1.2 | 2.62 ± 0.14 |
| 1 wt. % Sr + 1 wt. % Mg doped TCP | 97.7 ± 0.7 | 2.33 ± 0.18 |
| 1 wt. % Sr + 0.5 wt. % Si doped TCP | 95.5 ± 1.0 | 3.35 ± 0.3 |
| 1 wt. % Sr + 1 wt. % Mg + 0.5 wt % Si doped TCP | 95.9 ± 1.0 | 5.43 ± 0.55 |
3.3. Mineralization in SBF
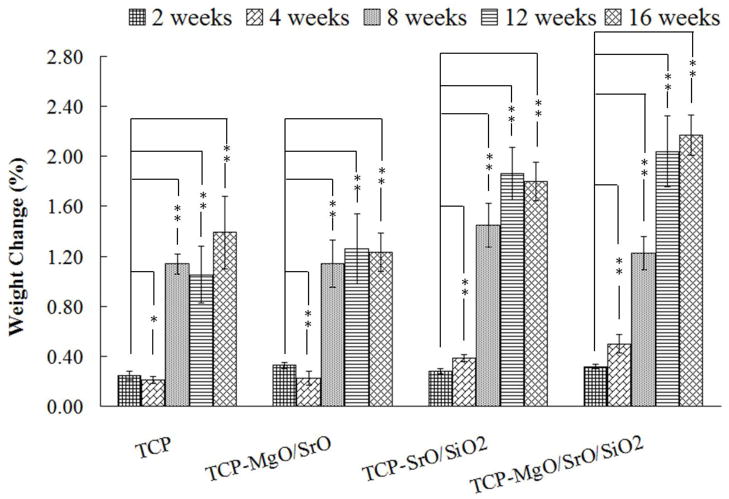
Degradation kinetics of doped and undoped samples were evaluated in SBF. Figure 3 shows the weight change of the of undoped TCP and TCP doped with MgO/SrO, SrO/SiO2 and MgO/SrO/SiO2 at 2, 4, 8, 12 and 16 weeks in SBF. All samples gained weight during these time periods suggesting apatite formation on the surfaces. The highest weight gain of 2.17 ±0.16% was observed for TCP-MgO/SrO/SiO2, while TCP-MgO/SrO showed the lowest weight gain, 1.23 ±0.15%. The weight gain observed for TCP and TCP-SrO/SiO2 were 1.39 ±0.29% and 1.80 ±0.17%, respectively. A correlation between weight gain by apatite formation and sintered density was observed. Samples with low sintered density, and therefore possibly with high porosity, showed more weight gain by apatite formation.
Figure 3.
Weight change as a function of time for TCP, TCP-MgO/SrO, TCP-SrO/SiO2 and TCP-MgO/SrO/SiO2 in simulated body fluid (**P < 0.05, *P > 0.05; n = 6).
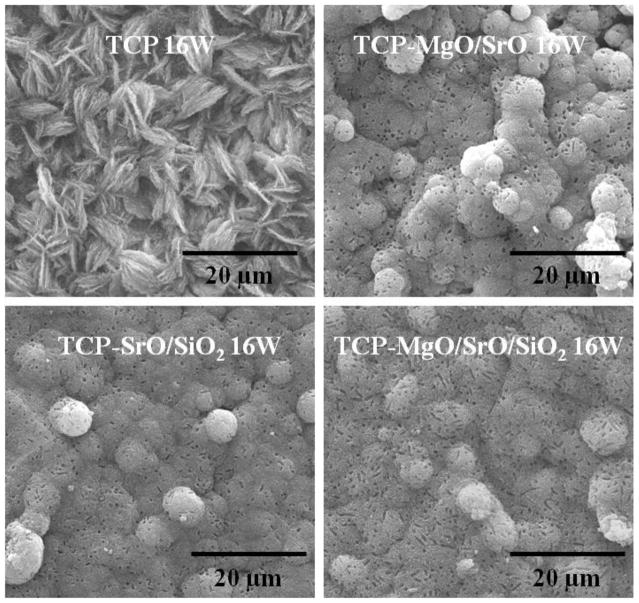
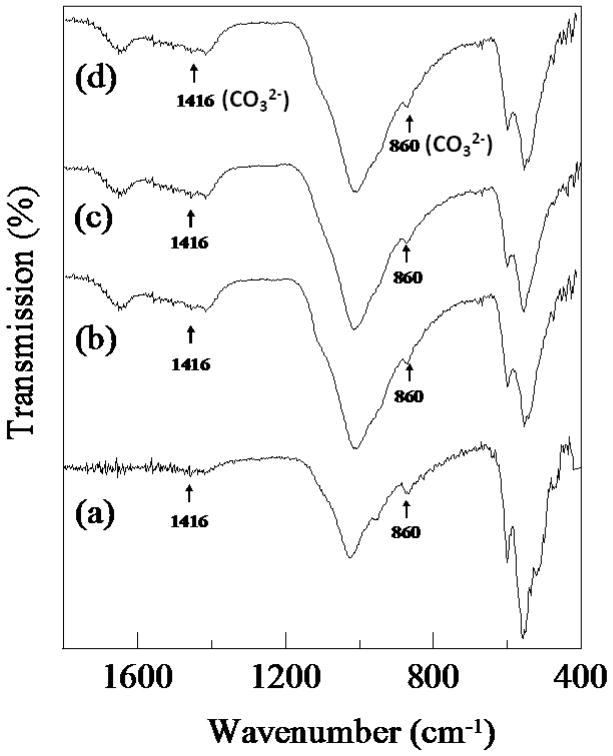
SEM micrographs of the samples after immersion in SBF for 8 weeks are shown in Figure. 4. Significant growth of the apatite layer was observed on the surfaces of both TCP and doped samples. The morphology of apatite crystallites formed on undoped and doped TCP was quite different. Doped samples showed complete coverage of the surface by aggregated granules of apatite. These individual granules are flower-shaped and composed of large number of flake-like crystallites. Nucleation of apatite on the doped samples started on these individual granules and formed a dense compact layer by growing bigger with time [31]. The surface of undoped TCP did not show any such specific sites of initial nucleation, rather the whole surface was covered uniformly with plate-like apatite crystallites. The formation of apatite on the surface of the samples was confirmed by ATR-IR analysis. IR spectra of undoped and doped TCP samples before and after soaking in SBF for 16 weeks are shown in Figure 5. All samples soaked for 16 weeks in SBF showed bands of CO32− at 1417 and 879 cm−1, confirming the presence of hydroxy-carbonate apatite [32, 33].
Figure 4.
SEM micrographs of TCP, TCP-MgO/SrO, TCP-SrO/SiO2 and TCP-MgO/SrO/SiO2 in simulated body fluid after 16 weeks.
Figure 5.
ATR-IR of TCP, TCP-MgO/SrO, TCP-SrO/SiO2 and TCP- MgO/SrO/SiO2 at 16 weeks in simulated body fluid.
3.4. Ca2+ concentration
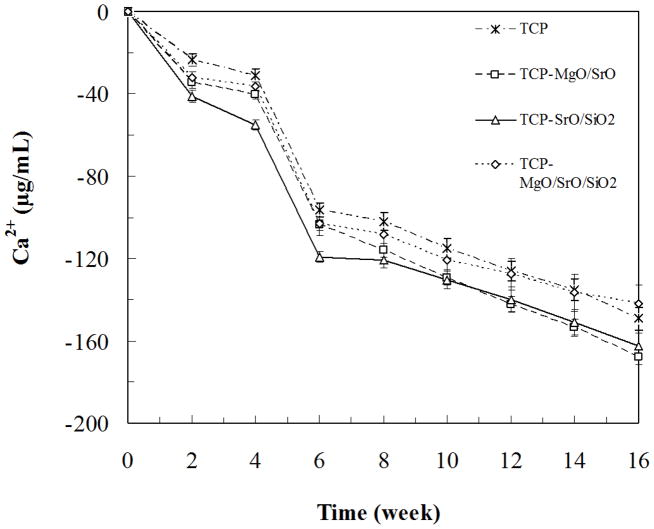
The Ca2+ concentration of SBF after soaking samples at each time point is shown in Figure 6. A decrease in Ca2+ indicates adsorption of Ca2+ on to the surface from SBF. Adsorption of Ca2+ was high in the initial 6 weeks on both undoped and doped TCP samples. Significant adsorption of Ca2+ on the surface of samples was observed up to 8 weeks, which remained steady till the end of the 16-week study.
Figure 6.
Ca2+ release as a function of time from TCP, TCP-MgO/SrO, TCP-SrO/SiO2 and TCP-MgO/SrO/SiO2 in simulated body fluid.
3.5. Strength Degradation in SBF
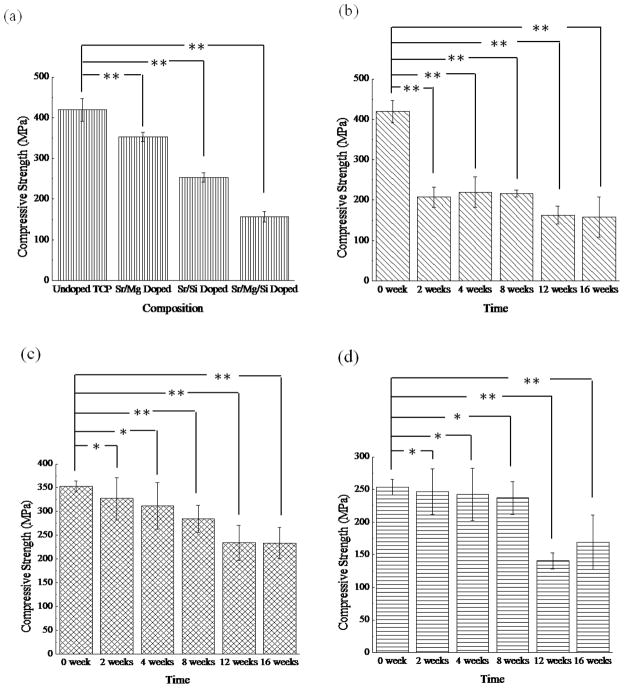
Effect of dopants on strength degradation rate and mineralization was studied in vitro by soaking samples in SBF for 16 weeks. Figure 7 shows the compressive strength of undoped and TCP doped with MgO/SrO, SrO/SiO2 and MgO/SrO/SiO2 compacts soaked for 0, 2, 4, 8, 12 and 16 weeks in SBF. High initial compressive strengths of 419 ±28 MPa and 352.7 ± 11.6 MPa were observed for undoped and MgO/SrO doped TCP samples. However, a lower strength of 157 ±13 MPa was observed for MgO/SrO/SiO2 doped TCP. A significant drop in strength from 419 ±28 MPa to 207 ±25 MPa was observed for undoped TCP for the initial 2 weeks followed by a small decrease to 158 ±50 MPa till 16 weeks of SBF study. However, MgO/SrO doped TCP showed a slow and gradual decrease in strength from 353 ±12 MPa to 234 ±32 MPa over the 16-week period. In case of MgO/SrO/SiO2 doped TCP the strength remained steady over the entire study period, while SrO/SiO2 doped TCP exhibited steady strength till 8 weeks followed by a drop to 141 ±12 MPa.
Figure 7.

Variation in compressive strength due to dopants (a), and compressive strength as a function of time for TCP (b), TCP-MgO/SrO (c), TCP-SrO/SiO2 (d) and TCP-MgO/SrO/SiO2 (e) in simulated body fluid (**P < 0.05, *P > 0.05; n = 6).
3.7. In vivo study
3.7.1. Histomorphology
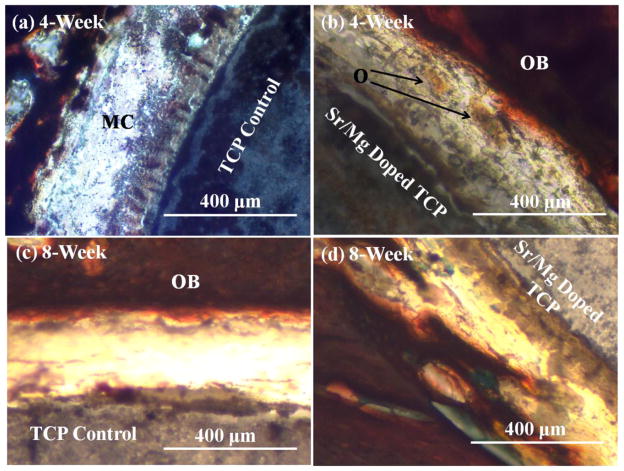
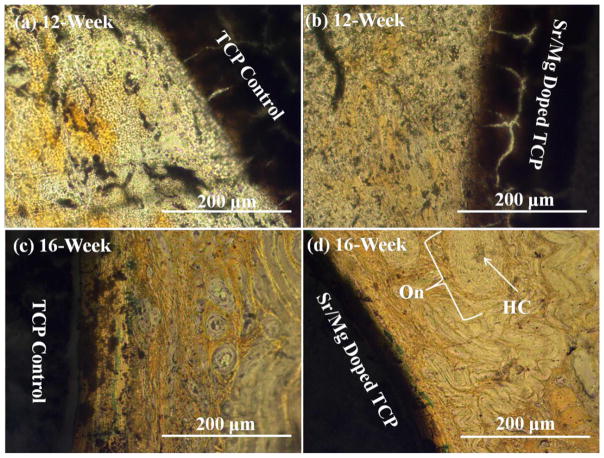
Histological evaluation at the bone–implant interface was performed at 4, 8, 12 and 16 weeks, respectively, to evaluate the influence of SrO and MgO on biocompatibility and new bone formation. Figure 8 shows the presence of a fibrous interzone (FIZ) enriched with mesenchymal cells (MC) and collagen between the implant (undoped and MgO/SrO doped) and the cortical bone. Figure 8a shows no obvious osteoid-like new bone formation starting after 4 weeks in the fibrous interzone (FIZ) between host bone and undoped TCP, while Figure 8b shows osteoid formation initiation in the FIZ of β-TCP–MgO/SrO implant and host bone. More pronounced osteoid formation was also observed after 8 weeks in the FIZ of MgO/SrO doped TCP as shown in Figure 8c than the FIZ of undoped TCP, as shown in Figure 8d. Figure 9 presents the bone remodeling after 12 and 16 weeks. A uniform bone remodeling initiation was observed after 12-week with Sr/Mg doped TCP, Figure 9b, as compared to undoped TCP, Figure 9a. Bone remodeling was clearly observed by many osteon formations with haversian system. After 16 weeks, osteon-rich FIZ was observed for both MgO/SrO doped and undoped β-TCP as shown in Figure 9c & 9d. A uniform and compact interface was observed between MgO/SrO doped implant and newly remodeled bone compared to undoped TCP. Increased new bone formation and enhanced bone remodeling in the FIZ of doped samples indicate an early stage of healing for doped β-TCP as compared to pure β-TCP.
Figure 8.
Photomicrograph of β-TCP implants (a) & (c), and β-TCP-MgO/SrO implants (b) & (d) showing the development of new bone formation and bone remodeling after in the fibrous interzone of the implant and the adjacent bone 4 and 8 weeks. Masson Goldner’s trichrome staining of transverse section. O: osteoid, OB: old bone, MC: mesanchymal cell.
Figure 9.
Photomicrograph of β-TCP implants (a) & (c), and β-TCP-MgO/SrO implants (b) & (d) showing the development of new bone formation and bone remodeling in the fibrous interzone (FIZ) of the implant and the adjacent bone after 12 and 16 weeks. Masson Goldner’s trichrome staining of transverse section. On: Osteon, HC: Haversian Canal.
4. Discussion
This study demonstrates the significance of the presence of MgO, SrO and SiO2 in TCP on phase stability, microstructure, densification, in vitro degradation kinetics and in vivo osteogenesis. The major phase of TCP remains unchanged even after the addition of MgO, SrO and SiO2. The presence of some α-TCP in TCP and TCP-SrO/SiO2 sintered samples is due to phase transition as β-TCP starts transforming to α-TCP above 1125°C [34]. TCP samples containing MgO displayed no α-TCP peaks as the addition of MgO to TCP increases the β- to α-transformation temperature [17, 35]. Glassy phase formation and increased grain size are observed in TCP-SrO/SiO2 and TCP-MgO/SrO/SiO2 sintered samples due to the presence of SiO2. This is probably caused by the segregation of some SiO2 in the grain boundary region [36] Higher density of TCP-MgO/SrO in comparison to undoped and SrO/SiO2 doped TCP is hypothesized because of the absence of α-TCP. Low density for TCP-MgO/SrO/SiO2 is attributed to increased grain size of TCP due to doping.
Influence of dopants on mineralization and the rate of strength degradation were studied in vitro by soaking the samples in SBF for up to 16 weeks. The in vitro strength degradation rate and mineralization study reveal the significant influence of doping on TCP. Samples of all compositions gained weight during this time period. Weight change in ceramics is primarily affected by two mechanisms: (i) degradation or dissolution of the material in SBF, and (ii) formation of hydroxyl carbonate apatite [2, 9]. Our results reveal that apatite formation is dominated over dissolution for all samples. The SEM micrographs of all TCP sample surfaces confirm the formation of apatite layer. Analysis of Ca2+ concentration during the SBF study reveals that adsorption process dominates over dissolution process over the entire 16-week period. Ca2+ adsorption helps form a Ca2+ rich amorphous calcium phosphate (ACP) [37]. Ca2+ rich ACP favors binding specifically with the PO43− in the SBF solution to form a Ca-deficient ACP. Ca2+ deficient ACP is thermodynamically stabilized by transforming into a crystalline phase of apatite as solubility of apatite is lower than any other CaP in aqueous media. Apatite grows spontaneously in SBF which is supersaturated with apatite forming ions [38]. Trace ions (Na+, K+, Mg2+, CO32− etc.) are also incorporated into apatites during its spontaneous growth in SBF [38]. The significant weight gain at 16 weeks and CO32− peaks at 1417 and 879 cm−1 in the FTIR spectra confirms the influence of high Ca2+ adsorption and bone mineral like hydroxy-carbonate apatite formation on the surface of the samples [39].
Compressive strength obtained for undoped TCP samples, 419 ±28 MPa, sintered at 1250 °C are within the values reported in literature, 319–687 MPa [40–42]. Wide variation in reported compressive strength of β-TCP is due to synthesis route, particle size, density and application of different processing parameters. Reported high-end values are obtained from 99.7% dense sintered compacts, which are typically processed via hot isostatic pressing [42]. A decrease in initial compressive strength of TCP is observed due to the addition of SrO/MgO, SrO/SiO2 and Sr/MgO/SiO2 in TCP. Although β-TCP is biodegradable, questions have been raised regarding the extent and rate of degradation and associated structural stability. The high degradation rate of β-TCP makes it difficult to match with the new bone growth rate [43]. Thus, there is a need to control degradation kinetics of β-TCP. In this study, strength degradation rate in SBF reveals two general trends: strength degradation and no strength degradation. During the first 2-week time periods, more than 50% strength loss in SBF is observed for undoped TCP, while significant strength loss is observed for MgO/SrO and SrO/SiO2 doped TCP after 8 and 12 weeks, respectively. No significant strength loss is observed even after 16 weeks for MgO/SrO/SiO2 doped TCP. In vitro degradation of CaP ceramics is governed by many factors, such as, chemistry, degree of crystallinity and porosity [2, 44].
Our results indicate that both dopant chemistry and amount can significantly influence initial strength as well as in vitro strength degradation behavior of β-TCP. High initial compressive strength for MgO/SrO doped TCP compared to SrO/SiO2 and MgO/SrO/SiO2 doped TCP is due to high initial density achieved by combined substitution of Sr and Mg in the unit cell of TCP. Combined substitution of Sr and Mg causes reduction in the unit cell parameters of the crystal lattice [17]. Si4+ is not substituted for Ca2+ in the crystal lattice of TCP; rather it is substituted for P5+ [45, 46]. The substitution of P5+ by Si4+ addresses the increased grain size due to the presence of SiO2 as ionic radius of Si4+ (2.71 Å) is higher than that of P5+ (1.28 Å). Our results reveal that combined substitution of metal ions in the crystal lattice of TCP is not the only factor that controls final density and compressive strength. Grain size also plays a significant role towards final density and compressive strength of CaP ceramics. Previously we have reported that for CaPs, compressive strength decreases with increasing grain size [47]. The same trend is also observed here for the doped TCP samples. Ca2+ substitution by Sr2+ results in increased dissolution of CaPs [48]. Substitution of P5+ by Si4+ also increases the solubility of TCP by introducing crystal defects [46] This explains a large significant drop in compressive strength of SrO/SiO2 doped TCP after 12 weeks immersion in SBF. Presence of Mg ions retards dissolution of CaP [49, 50]; hence gradual strength degradation is observed for TCP-MgO/SrO. No in vitro strength degradation for TCP-MgO/SrO/SiO2 is probably due to the coexistence of Mg and Si. It has been shown that coexistence of Mg and Si in alumina ceramic enhances their solubility in the bulk than the grain boundary [36]. Thus, increased Mg substitution in the crystal lattice of TCP due to the presence of Si might have caused increased structural stability. As a result no strength degradation for TCP-MgO/SrO/SiO2 is observed. These results show that although pure TCP has the highest initial compressive strength, it suffers with a sudden drop in strength degradation at the very initial stage. Among the three doped TCP samples in this study, the highest initial compressive strength as well as no sudden drop in the compressive strength is also observed for MgO/SrO doped TCP. Thus, the high initial strength and gradual strength degradation kinetics of MgO/SrO doped TCP make it suitable for many clinical applications, where the mechanical support is gradually transferred to the newly grown bone. Thus, based on these in vitro strength degradation kinetics data and previously reported in vitro and in vivo data from our lab [17], we choose to further investigate the role of MgO/SrO doped TCP for early stage in vivo osteogenesis and bone remodeling, and compare that to pure TCP as control in the cortical defect of rat distal femur model.
Ideal implant materials should have good biocompatibility and strong bonding ability with the host bone, which can reduce healing time and improve patient’s mobility. Our in vivo results show that MgO/SrO doped β-TCP favored formation of new bone tissue. High osseous activity at the bone and doped implant interface indicates faster bone growth and remodeling. The gap between implant and host bone, fibrous interzone (FIZ), is filled with fibroblast-like cells, mesenchymal cells (MCs) and collagen protein. Proliferation and differentiation of mesenchymal stromal cells into osteoblast cells takes place followed by bone matrix deposition. These processes lead to osteoid formation [51]. Early stage osteoid formation in the FIZ of MgO/SrO doped TCP confirms faster bone repairing process than pure TCP. This result is in line with our previous findings on in vivo study using rat distal femur model for osteocalcin and type I collagen content [17]. Osteon formation is an indication for osteoid turning into laminar and osteonic bone [52]. FIZ between MgO/SrO doped-implant and host bone completely filled with osteons after 16 weeks clearly demonstrates the presence of MgO and SrO in β-TCP is beneficial for early stage osteoconduction and osteogenesis.
Our results show that MgO/SrO doped β-TCP can reduce the time for early stages of bone formation. Further, slow degradation of MgO/SrO doped implant than pure TCP will help to maintain its strength over a longer time period and hence facilitate strong bone-material integration. Improved mechanical property, slow and controlled in vitro strength degradation, and early stage in vivo osteogenesis exhibited by SrO/MgO doped TCP make it a potential candidate for hard tissue repair.
Conclusions
This study examined the influence of MgO, SrO and SiO2 dopants in β-TCP on the mechanical properties and in vitro degradation kinetics in simulated body fluid over 16-week time period. Undoped and MgO/SrO, SrO/SiO2 and MgO/SrO/SiO2 doped TCP samples showed sintered density of 96.2 ±1.2%, 97.7 ±0.7%, 95.5 ±1.0%, and 95.9 ±1.0% and compressive strength of 419 ±28 MPa, 341 ±30 MPa, 253 ±12 MPa and 157 ±13 MPa, respectively. Both undoped and doped TCP samples showed weight gain in SBF revealing good apatite growth. Compressive strength measurements were carried out at different time periods after soaking in SBF. Undoped and SrO/SiO2 doped TCP exhibited a sudden drop in compressive strength, while MgO/SrO/SiO2 doped TCP retained its strength during 16 week time period in SBF. Only MgO/SrO doped TCP exhibited a gradual decrease in compressive strength, from 353 ±12 MPa to 234 ±32 MPa, and still retained significant strength after 16 weeks. MgO/SrO doped TCP was used for in vivo study in rat distal femur for 4, 8, 12, and 16 weeks with undoped TCP as control. MgO/SrO doped TCP showed improved early stage osteoconduction by initiating osteoid formation, and enhanced osteogenic capability by increased number osteon formation in the FIZ as compared to undoped TCP. These results suggest that MgO/SrO doped TCP is an excellent potential candidate for bone tissue engineering applications.
Acknowledgments
The authors would like to acknowledge financial support from the National Institutes of Health, NIBIB (Grant # NIH-R01-EB-007351, program manager Dr. Albert Lee). The authors would also like to thank J. Takemoto, M. Roy and Sanjib Mukherjee for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kondo N, Ogose A, Tokunaga K, Ito T, Arai K, Kudo N. Bone formation and resorption of highly purified β-tricalcium phosphate in the rat femoral condyle. Biomaterials. 2005;26:5600–8. doi: 10.1016/j.biomaterials.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay A, Bernard S, Xue W, Bose S. Calcium phosphate-based resorbable ceramics: Influence of MgO, ZnO, and SiO2 dopants. J Am Ceram Soc. 2006;89:2675–2688. [Google Scholar]
- 3.Tang R, Wu W, Haas M, Nancollas GH. Kinetics of Dissolution of β-Tricalcium Phosphate. Langmuir. 2001;17:3480–3485. [Google Scholar]
- 4.Kannan S, Lemos A, Rocha JHG, Ferreira JMF. Characterization and mechanical performance of the Mg-stabilized β-Ca3(PO4)2 prepared from Mg-substituted Ca-deficient apatite. J Am Ceram Soc. 2006;89:2757–2761. [Google Scholar]
- 5.Guo X, Lee L, Law, Chow H, Rosier R, Cheng C. Recombinant human bone morphogenetic protein-4 (rhBMP-4) enhanced posterior spinal fusion without decortication. J Orthop Res. 2002;20:740–746. doi: 10.1016/S0736-0266(01)00167-X. [DOI] [PubMed] [Google Scholar]
- 6.Pietak AM, Reid JW, Stott MJ, Sayer M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28:4023–4032. doi: 10.1016/j.biomaterials.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Obadia L, Deniard P, Alonso B, Rouillon T, Jobic S, Guicheux J, Julien M, Massiot D, Bujoli B, Bouler JM. Effect of Sodium Doping in β-tricalcium phosphate on its structure and properties. Chem Mater. 2006;18:1425–33. [Google Scholar]
- 8.Kalita S, Bose S, Hosick H, Bandyopadhyay A. CaO P2O5 Na2O-based sintering additives for hydroxyapatite (HAp) ceramics. Biomaterials. 2004;25:2331–2339. doi: 10.1016/j.biomaterials.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Seeley Z, Bandyopadhyay A, Bose S. Influence of TiO2 and Ag2O addition on mechanical properties of tricalcium phosphate. J Biomed Mater Res. 2007;82A:113–121. doi: 10.1002/jbm.a.31077. [DOI] [PubMed] [Google Scholar]
- 10.Xue W, Dahlquist K, Banerjee A, Bandyopadhyay A, Bose S. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J Mater Sci Mater Med. 2008;19:2669–2677. doi: 10.1007/s10856-008-3395-4. [DOI] [PubMed] [Google Scholar]
- 11.Seeley Z, Bandyopadhyay A, Bose S. Tricalcium phosphate based resorbable ceramics: Influence of NaF and CaO addition. Mater Sci & Eng C. 2008;28:11–17. [Google Scholar]
- 12.Bandyopadhyay A, Withey EA, Moore J, Bose S. Influence of ZnO doping in calcium phosphate ceramics. Mater Sci & Eng C. 2007;27:14–17. [Google Scholar]
- 13.Qiua K, Zhaob XJ, Wana CX, Zhao CS, Chen YW. Effect of strontium ions on the growth of ROS17/2.8 cells on porous calcium polyphosphate scaffolds. Biomaterials. 2006;27:1277–1286. doi: 10.1016/j.biomaterials.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Marie PJ, Ammann P, Boivin G, Rey C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif Tissue Int. 2001;69:121–129. doi: 10.1007/s002230010055. [DOI] [PubMed] [Google Scholar]
- 15.Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P, Tsouderos Y, Delmas PD, Christiansen C. Incorporation and distribution of strontium in bone. Bone. 2001;28(4):446–453. doi: 10.1016/s8756-3282(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 16.Xue W, Moore JL, Hosick HL, Bose S, Bandyopadhyay A, Lu WW, Cheung KMC, Luk KDK. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J Biomed Mater Res. 2006;79A:804–814. doi: 10.1002/jbm.a.30815. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee SS, Tarafder S, Davies NM, Bandyopadhyay A, Bose S. Understanding the Influence of MgO and SrO Binary Doping on Mechanical and Biological Properties of β-TCP Ceramics. Acta Biomater. 2010;6:4167–4174. doi: 10.1016/j.actbio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Hashizume M, Yamaguchi M. Stimulatory effect of β-alanyl-histidinato zinc on cell proliferation is dependent on protein synthesis in osteoblastic MC3T3-E1 cells. Mol Cell Biochem. 1993;122:59–64. doi: 10.1007/BF00925737. [DOI] [PubMed] [Google Scholar]
- 19.Bertoni E, Bigi A, Cojazzi G, Gandolfi M, Panzavolta S, Rover N. Nanocrystals of Magnesium and fluoride substituted hydroxyapatite. J Inorg Biochem. 1998;72:29–35. doi: 10.1016/s0162-0134(98)10058-2. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki M. Crystallographic properties of heterogeneous Mg containing fluoridated apatites synthesized with a two-step supply system. Biomaterials. 1995;16:703–707. doi: 10.1016/0142-9612(95)99698-l. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki Y, Yoshida Y, Okazaki M, Shimazu A, Uchida T, Kubo T, Akagawa Y, Hamada Y, Takahashi J, Matsura N. Synthesis of functionally graded MgCO3 apatite accelerating osteoblast adhesion. J Biomed Mater Res. 2002;62:99–105. doi: 10.1002/jbm.10220. [DOI] [PubMed] [Google Scholar]
- 22.Mayer I, Schlam R, Featherstone JDB. Magnesium-containing carbonate apatites. J Inorg Biochem. 1997;66:1–6. doi: 10.1016/s0162-0134(96)00145-6. [DOI] [PubMed] [Google Scholar]
- 23.Gomes P, Botelho C, Lopes M, Santos J, Fernandes M. Effect of silicon-containing hydroxyapatite coating on human in vitro osteoblastic response. Bone. 2009;44 (supplement 2):S267. [Google Scholar]
- 24.Jones JR, Tsigkou O, Coates EE, Stevens MM, Polak JM, Hench LL. Extracellular matrix formation and mineralization on a phosphate free porous bioactive glass scaffold using primary human osteoblast (HOB) cells. Biomaterials. 2007;28:1653–1663. doi: 10.1016/j.biomaterials.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Reffitt DM, Ogston M, Jugdaohsingh R, Cheung HF, Evans BA, Thompson RP, Powell JJ, Hampson GN. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–135. doi: 10.1016/s8756-3282(02)00950-x. [DOI] [PubMed] [Google Scholar]
- 26.Carlise E. Si: An essential element for the chick. Science. 1972;178:619–621. doi: 10.1126/science.178.4061.619. [DOI] [PubMed] [Google Scholar]
- 27.Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Ionic Products of Bioactive Glass Dissolution Increase Proliferation of Human Osteoblasts and InduceInsulin-like Growth Factor II mRNA Expressionand Protein Synthesis. Biochemical and Biophysical Res Com. 2000;276:461–465. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Vega JM, Saiz E, Tomsia AP, Marshall GW, Marshall SJ. Bioactive glass coatings with hydroxyapatite and Bioglass® particles on Ti-based implants. 1. Processing Biomaterials. 2000;21:105–111. doi: 10.1016/s0142-9612(99)00131-3. [DOI] [PubMed] [Google Scholar]
- 29.Jones JR, Tsigkou O, Coates EE, Stevens MM, Polak JM, Hench LL. Extracellular matrix formation and mineralization on a phosphate-free porous bioactive glass scaffold using primary human osteoblast (HOB) cells. Biomaterials. 2007;28:1653–1663. doi: 10.1016/j.biomaterials.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Xin R, Leng Y, Chen J, Zhang Q. A comparative study of calcium phosphate formation on bioceramics in vitro and in vivo. Biomaterials. 2005;26:6477–6486. doi: 10.1016/j.biomaterials.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Landi E, Tampieri A, Celotti G, Langenati R, Sandri M, Sprio S. Nucleation of biomimetic apatite in synthetic body uids: dense and porous scaffold development. Biomaterials. 2005;26:2835–2845. doi: 10.1016/j.biomaterials.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Fleet ME. Infrared spectra of carbonate apatites: ν2 –region bands. Biomaterials. 2009;30:1473–1481. doi: 10.1016/j.biomaterials.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Kannan S, Goetz-Neunhoeffer F, Neubauer J, Pina S, Torres PMC, Ferreira JMF. Synthesis and structural characterization of strontium- and magnesium-co-substituted β-tricalcium phosphate. Acta Biomater. 2010;6:571–576. doi: 10.1016/j.actbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Enderlea R, Götz-Neunhoeffera F, Göbbelsa M, Müllerb FA, Greilb P. Influence of magnesium doping on the phase transformation temperature of β-TCP ceramics examined by Rietveld refinement. Biomaterials. 2005;26:3379–3384. doi: 10.1016/j.biomaterials.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Gavrilov KL, Bennison SJ, Mikeska KR, Levi-setti R. Grain boundary chemistry of alumina by high-resolution imaging SIMS. Acta Mater. 1999;47:4031–4039. [Google Scholar]
- 37.Beruto DT, Mezzasalma SA, Capurro M, Botter R, Cirillo P. Use of a-tricalcium phosphate (TCP) as powder and as an aqueous dispersion to modify processing, microstructure, and mechanical properties of polymethylmethacrylate (PMMA) bone cements and to produce bone-substitute compounds. J Biomed Mater Res. 2000;49:498–505. doi: 10.1002/(sici)1097-4636(20000315)49:4<498::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Kim HM, Himeno T, Kokubo T, Nakamura T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials. 2005;26:4366–4373. doi: 10.1016/j.biomaterials.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Yan H, Zhang K, Blanford CF, Francis LF, Stein A. In vitro hydroxycarbonate apatite mineralization of CaO-SiO2 sol-gel glasses with a three-dimensionally ordered macroporous structure. Chem Mater. 2001;13:1374–1382. [Google Scholar]
- 40.Chen B, Jiang D, Zhang J, Dong M, Lin Q. Gel-casting of _-TCP using epoxy resin as a gelling agent. J Euro Cer Soc. 2008;28:2889–2894. [Google Scholar]
- 41.Akao M, Aoki H, Kato K, Sato A. Dense polycrystalline β-tricalcium phosphate for prosthetic applications. J Mate Sci. 1982;17:343–346. [Google Scholar]
- 42.Jarcho M, Salsbury RL, Thomas MB, Doremus RH. Synthesis and fabrication of β-TCP phosphate (whitlockite) ceramics for potential prosthetic applications. J Mater Sci. 1979;14:142–150. [Google Scholar]
- 43.Kalita SJ, Bhatt HA, Dhamne A. MgO-Na2O-P2O5-Based Sintering Additives for Tricalcium Phosphate Bioceramics. J Am Cer Soc. 2006;89:875–881. [Google Scholar]
- 44.Guo D, Xu K, Han Y. The in situ synthesis of biphasic calcium phosphate scaffolds with controllable compositions, structures, and adjustable properties. J Biomed Mater Res. 2009;88A:43–52. doi: 10.1002/jbm.a.31844. [DOI] [PubMed] [Google Scholar]
- 45.Langstaff SD, Sayer M, Smith TJN, Pugh SM. Resorbable synthetic bone grafts formed from a silicon stabilized calcium phosphate bioceramic. Mater Res Soc Symp Proc. 1999;550:1727–41. [Google Scholar]
- 46.Pietak AM, Reid JW, Stott MJ, Sayer M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28:4023–4032. doi: 10.1016/j.biomaterials.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Bose S, Dasgupta S, Tarafder S, Bandyopadhyay A. Microwave-processed nanocrystalline hydroxyapatite: Simultaneous enhancement of mechanical and biological properties. Acta Biomater. 2010;6:3782–3790. doi: 10.1016/j.actbio.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo D, Xu K, Zhao X, Han Y. Development of a strontium-containing hydroxyapatite bone cement. Biomaterials. 2005;26:4073–4083. doi: 10.1016/j.biomaterials.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Renaudin G, Laquerrière P, Filinchuk Y, Jallot E, Nedelec JM. Structural characterization of sol gel derived Sr-substituted calcium phosphates with anti-osteoporotic and anti-inflammatory properties. J Mater Chem. 2008;18:3593–3600. [Google Scholar]
- 50.Yin X, Calderin L, Stott MJ, Sayer M. Density functional study of structural, electronic and vibrational properties of Mg- and Zn-doped tricalcium phosphate biomaterials. Biomaterials. 2002;23:4155–4163. doi: 10.1016/s0142-9612(02)00199-0. [DOI] [PubMed] [Google Scholar]
- 51.Perrien DS, Brown EC, Aronson J, Skinner RA, Montague DC, Badger TM, Lumpkin CK., Jr Immunohistochemical Study of Osteopontin Expression During Distraction Osteogenesis in the Rat. The J Histochem Cytochem. 2002;50:567–574. doi: 10.1177/002215540205000414. [DOI] [PubMed] [Google Scholar]
- 52.Locke M. Structure of Long Bones in Mammals. J Morphol. 2004;262:546–565. doi: 10.1002/jmor.10282. [DOI] [PubMed] [Google Scholar]