Abstract
Rationale
Bone marrow (BM)-derived mesenchymal stem cells (MSCs) hold great promise for cardiovascular cell therapy owing to their multipotency and culture-expandability.
Objective
The aim of the study was to investigate whether MSCs can treat experimental acute myocardial infarction (MI) and diabetic neuropathy.
Methods and Results
We isolated mononuclear cells from mouse BM and cultured MSCs in a conventional manner. Flow cytometry analyses of these cultured cells at passage four showed expression of typical MSC markers such as CD44 and CD29, but not hematopoietic markers such as c-kit, flk1 and CD34. To determine the therapeutic effects of MSCs, we injected MSCs into the periinfarct area after ligation of the left anterior descending coronary arteries of mice, and as separate experiments injected the same batch of MSCs into hindlimb muscles of mice with diabetic neuropathy. During the follow-up at 4–8 weeks after cell transplantation, growing tumors were observed in 30% of hearts in the MI model, and in 46% of hindlimbs in the diabetic neuropathy model. Histologic examination of the tumors revealed hypercelluarity, pleomorphic nucleoli, cytologic atypia and necrosis, and positive staining for α-smooth muscle actin, indicative of malignant sarcoma with myogenic differentiation. Chromosomal analysis of these MSCs showed multiple chromosomal aberrations including fusion, fragmentation, and ring formation.
Conclusions
Genetically unmodified MSCs can undergo chromosomal abnormalities even at early passages and form malignant tumors when transplanted in vivo. These results suggest that careful monitoring of chromosomal status is warranted when in vitro expanded MSCs are used for cell therapy such as for MI.
Keywords: Bone marrow, mesenchymal stem cells, malignant tumors, transplantation, ischemia
Introduction
Isolated from various sources including bone marrow (BM), cartilage, adipose tissue, amniotic fluids and umbilical cord blood,1–3 mesenchymal stem cells (MSCs) have drawn much attention for cell therapy because they can be easily expanded in vitro and differentiate into tissues of mesenchymal origin, including muscle, fibroblast, bone, tendon, ligament, and adipose tissue.4 Indeed, MSCs have been used for regenerating damaged tissues of noncardiac disorders such as stroke, Crohn’s disease, osteogenesis imperfecta, and graft versus host disease.5–8 Furthermore, MSCs have been used to regenerate cardiovascular tissues by virtue of their capability to transdifferentiate into cardiomyocytes and vessel-like cells.9–16 One pilot study reported that intracoronary injection of autologous MSCs could improve global and regional LV function and reduce the size of the perfusion defect in patients with acute MI.17 However, recent studies argued against the transdifferentiation potential of MSCs and proposed that the main mechanism of MSCs on cardiovascular disease may be paracrine action.15, 18–22 Accordingly, in this study, we designed experiments to elucidate the mechanism of BM-derived MSCs (BM-MSCs) in myocardial regeneration or repair.
Furthermore, we have attempted to treat diabetic peripheral neuropathy with BM-MSCs. Although diabetic neuropathy is the most common complication of diabetes, no effective therapy has been developed.23 Diabetic neuropathy is pathophysiologically associated with destruction of the vasa nervorum and loss of myelinated neurons.24 Recently we have reported that endothelial progenitor cells and BM-derived mononuclear cells exert favorable therapeutic effects on diabetic neuropathy through their angiogenic and neurotrophic effects.24 Therefore, we sought to utilize MSCs for treating diabetic peripheral neuropathy based on the angiogenic effects of MSCs.
On the other hand, concerns have been raised about the safety of MSCs for clinical use, with studies reporting the potential risk of in vitro expanded MSCs to develop tumors upon transplantation. Human MSCs derived from adipose tissues became immortalized and spontaneously transformed after long-term culture, possibly due to augmented chromosome instability associated with dysregulation of telomere activity and cell cycle related genes.25 Mice injected with these MSCs developed tumors in multiple organs. Similarly, mouse BM-MSCs can become transformed after long-term culture and induce tumors in mice.26–28 Chromosome instability, upregulated c-Myc expression and elevated telomerase activity were suggested as contributing factors for acquiring malignancy in mouse BM-MSCs. In addition, the finding that a subpopulation of BM cells recruited to the stomach undergoing chronic infection became malignant and contributed to gastric cancer over time also suggests a tendency of BM-MSCs to be cancerous under certain conditions.29 In contrast, Bernardo et al reported that human BM-derived MSCs do not exhibit spontaneous transformation after long term in vitro expansion, making this issue controversial.30 However, it was not reported whether non-manipulated short-term cultured MSCs can acquire severe chromosomal abnormality and induce tumors in vivo.
In this study, using short-term cultured unmodified mouse BM-MSCs, we aimed to treat experimental diabetic neuropathy and MI. During 4–8 weeks of follow-up, rapidly growing tumors were observed in both models. Histopathologic studies revealed that these tumors were primitive sarcoma, and the BM-MSCs had chromosomal abnormalities even at early passages. This is the first study showing malignant tumor formation in the heart following implantation of any adult stem cells.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournlas.org.
Mouse bone marrow mononuclear cells were isolated from the femur and tibia of 8 week-old, male C57/BL mice by density gradient centrifugation. Adherent cells were cultured on 10 cm plastic dishes in complete media (CM) consisting of Dulbecco’s modified Eagle’s Media -glutamine. Diabetes was induced in 8 week-old, male C57/BL mice by injection of streptozotocin (150 mg/kg in 0.9% saline). One million (1×106) MSCs were transplanted into limb of the mice via bilateral intramuscular injection. Acute myocardial infarction was induced by ligation of the left anterior descending coronary artery of mice. One hundred thousand (1 × 105) cultured MSCs were injected into the peri-infarct areas and apex. The sciatic nerves and hearts were stained with either hematoxylin and eosin (H & E) or antibodies against desmin and α-smooth muscle actin (αSMA). Flow cytometry was performed by staining with phycoerythrin (PE)-conjugated antibodies against CD44, CD29, c-kit (CD117), CD31, and CD34. Chromosome analysis was performed at the Cytogenetics Core of the Dana Farber Harvard Cancer Center. Fifty cells were scored by determining the number of acrocentric chromosomes, fused chromosomes, chromosome fragments, and ring chromosomes.
Results
We locally injected cultured MSCs into the hindlimb muscles for treating diabetic neuropathy. In a first series of the study (n = 10), we used MSCs at passages P3 to P4. In this experiment, seven mice were sacrificed at 2 and 4 weeks for histologic analysis as scheduled. Another three mice, due to be sacrificed at 8 weeks, died at 5 weeks. We believe that high blood glucose might have been responsible for the death of these mice, as diabetic control mice which did not receive MSCs sometimes died as well. In a second series of experiments, we injected MSCs at P4–P6 in the same animal model (n = 10). According to the study design, seven mice were sacrificed at 2 and 4 weeks for histologic examination. The remaining three mice were scheduled to be sacrificed at 8 weeks; however, one mouse died at 5 weeks and two mice developed rapidly growing tumors in both hindlimbs between 5 and 8 weeks. These mice were sacrificed at 8 weeks and the tumors were harvested. In both series of experiments, we could not observe tumors by gross examination by 4 weeks.
Grossly, the tumors protruded visibly and were huge enough to cripple the hindlimbs (Figure 1A). The tumor covered the entire hindlimb which received the MSCs. Gross examination of the removed tumors demonstrated soft-flesh consistency, slippery surface and multiple sites of necrosis (Figure 1A and 1B). Microscopic examination following H & E staining revealed fascicular arrangement of pleomorphic spindle cells, which is typical for myoma or sarcoma, and some areas of geographic necrosis due to rapid growth (Figure 1C). The tumor cells showed hypercelluarity, nuclear hyperchromatism, pleomorphic nucleoli, cytologic atypia (Figure 1D), and increased mitotic figures, indicative of malignant sarcoma (Figure 1E and 1F). To more precisely determine the nature of the tumors, we performed immunostaining with anti-desmin and anti-αSMA antibodies. The tumors were not stained for desmin but stained for αSMA, indicating that the tumors originated from smooth muscle cells (Figure 1G).
Figure 1. Transplanted MSCs generated malignant tumors in a mouse model of diabetic neuropathy.
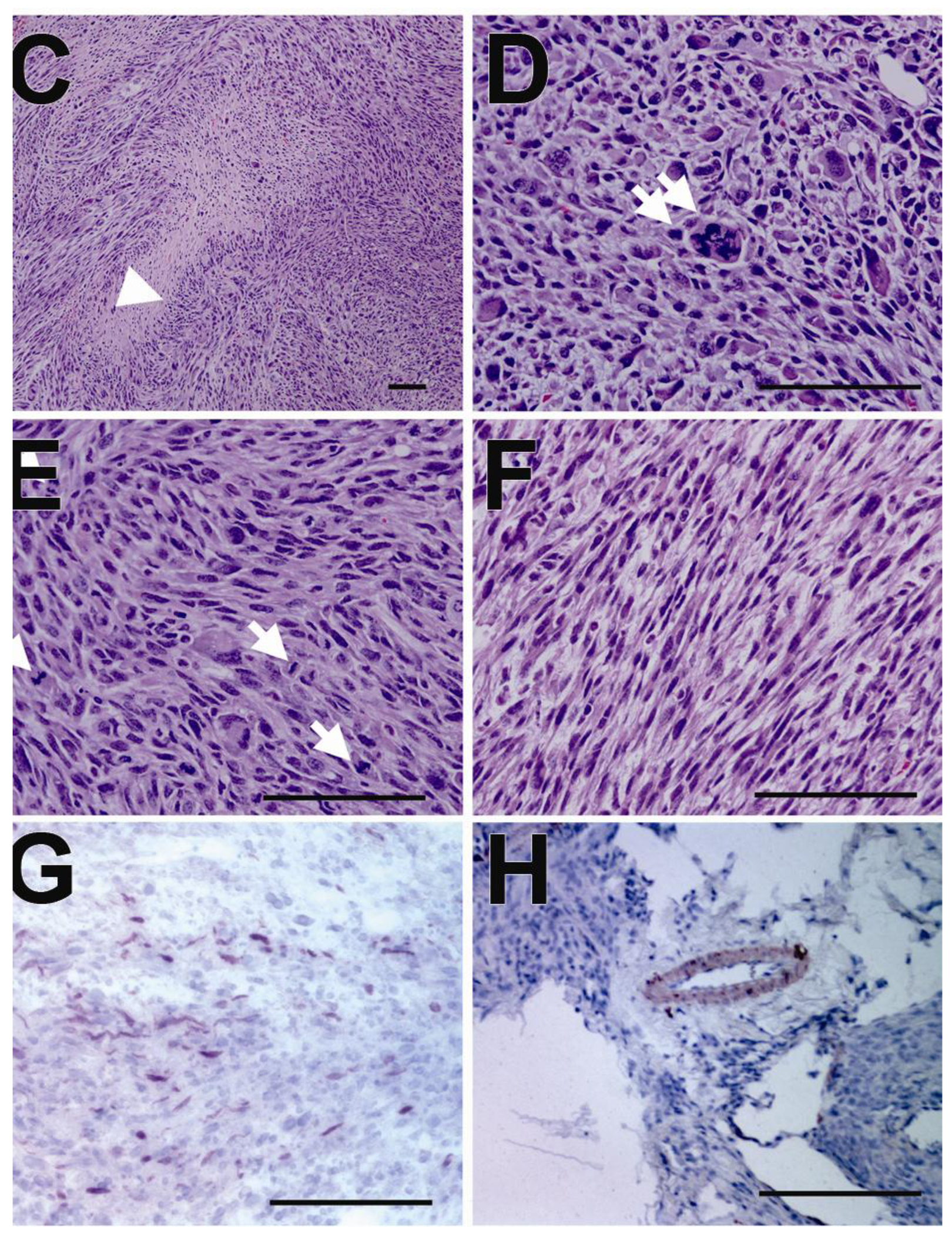
A, Representative tumors (arrows) in the hindlimbs following BM-MSC transplantation into hindlimb muscles. B, Serial cross sectional images of the tumors showed multiple sites of necrosis (arrowheads). C–F, H & E staining showed fascicular arrangement of pleomorphic spindle cells and area of geographic necrosis (C, an arrowhead). The tumor cells showed nuclear hyperchromatism, pleomorphism, atypical mitosis (D, double arrows), and increased mitotic figures (E, arrows) suggesting pleomorphic sarcoma. The tumor cells were elongated and had abundant pinkish cytoplasms (F). The nuclei were centrally located and blunt ended. Original magnification ×400. Scale bar, 100 µm. G, Immunohistochemistry with α-smooth muscle actin showed that tumors were focally positive for α-smooth muscle actin, which is compatible with the pleomorphic sarcoma with myogenic differentiation. H, Positive control of α-smooth muscle actin staining (scale bar, 100 µm).
To further confirm the causal relationship of tumor and MSCs, we injected the same line of MSCs at P6 to P8 into the diabetic neuropathy model (n = 15). In this series, 14 mice developed tumors at earlier time points (3–4 weeks) than in the second series of experiments. Only one mouse was free from gross tumor until 4 weeks. Together, tumors developed in 16 out of 35 diabetic mice (46%) in three independent series of cell transplantation experiments for diabetic neuropathy (Table 1). There was a trend that later passage MSCs induced tumors earlier and more frequently compared to earlier passage MSCs.
Table 1.
The incidence of sarcoma formation.
| Disease Animal Model | Tumor formation |
|---|---|
| DM neuropathy | 16/35 (46%) |
| Non-DM MI model | 3/10 (30%) |
Non-DM: non-diabetes
In another experiment, we sought to treat experimental MI with these MSCs. We used non-diabetic mice as an MI model to circumvent the effects of diabetes on tumor formation. We injected the same line of MSCs (1 × 105) at P6 to P10 into the peri-infarct area immediately after creation of MI (n = 10). To monitor the development of tumors, we performed echocardiography every week. Until 6 weeks, we were not able to find any distinct masses; however, one mouse died at seven weeks. During necropsy, infiltrating tumor was observed which was extended into the posterior part of the heart (Figure 2). As this finding suggests that our echocardiographic resolution may not clearly detect the occurrence of tumors, we sacrificed all the mice and performed necropsy. Gross and microscopic examinations of these mice revealed that three out of 10 mice (30%) had tumors at 7 weeks (Table 1).
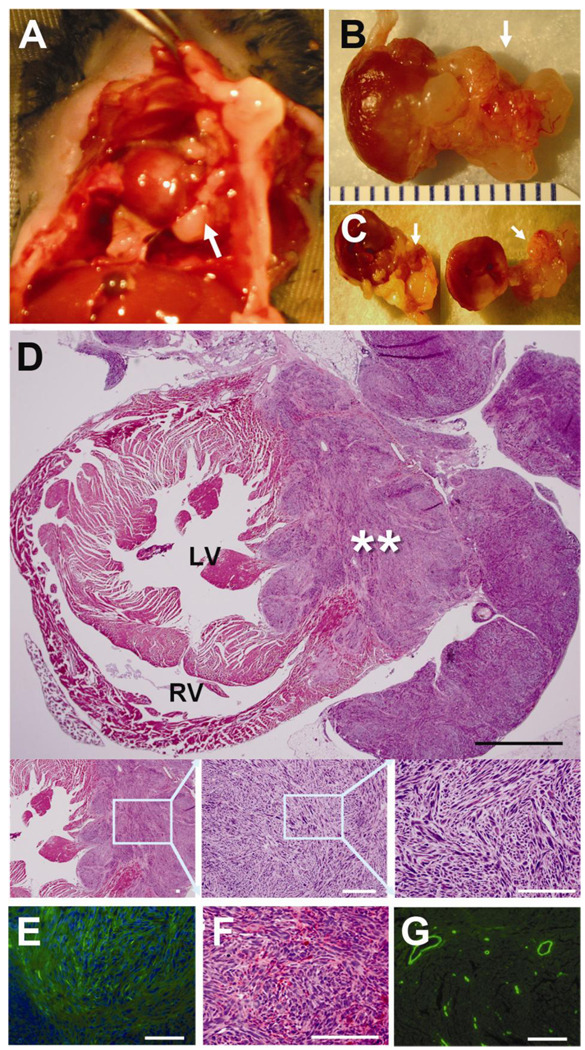
Figure 2. Transplanted MSCs generated malignant tumors in a mouse model of acute myocardial infarction.
A, Gross necropsy examination after opening the chest wall showed that the tumor mass (arrow) is extended to pericardial sac and invaded into the lung and chest wall. B and C, The explanted tumor showed infiltrative growth into the myocardium, pericardium and chest wall. D, Light microscopic examination after H & E staining revealed infiltrative growth of hypercellular tumor surrounding almost one third of the heart and protruding out of heart. The tumor showed fascicular arrangement of atypical spindle cells having pleomorphic, hyperchromatic nuclei and eosinophilic cytoplasm. Black scale bar 1000 µm. White scale bar 100 µm. E and F, IHC with α-smooth muscle actin showed the tumor was focally positive for α-smooth muscle actin staining. Green fluorescence, α-smooth muscle actin; Blue fluorescence, DAPI for nuclei. G, Positive control of α-smooth muscle actin staining (scale bar 100 µm).
By gross examination, tumors showed an invasive nature: tumors invaded internally into myocardium and externally into the pericardium and further into the lung and the chest wall (Figure 2A, 2B, and 2C). Microscopic examination of H & E stained cardiac tissues clearly demonstrated infiltrative growth of hypercellular tumors occupying one third of the myocardial wall and invading externally into the pericardium and the chest wall. The tumors showed fascicular arrangement of atypical spindle cells having pleomorphic, hyperchromatic nuclei and eosinophilic cytoplasm (Figure 2D). Immunohistochemistry showed that the tumors were positive for αSMA (Figure 2E and 2F). These tumors were identified as the same type of tumors that were observed in the diabetic neuropathy model. Based on these gross and microscopic features, the tumors were diagnosed as spindle cell sarcoma with myogenic differentiation, which is a primitive and malignant type of sarcoma.
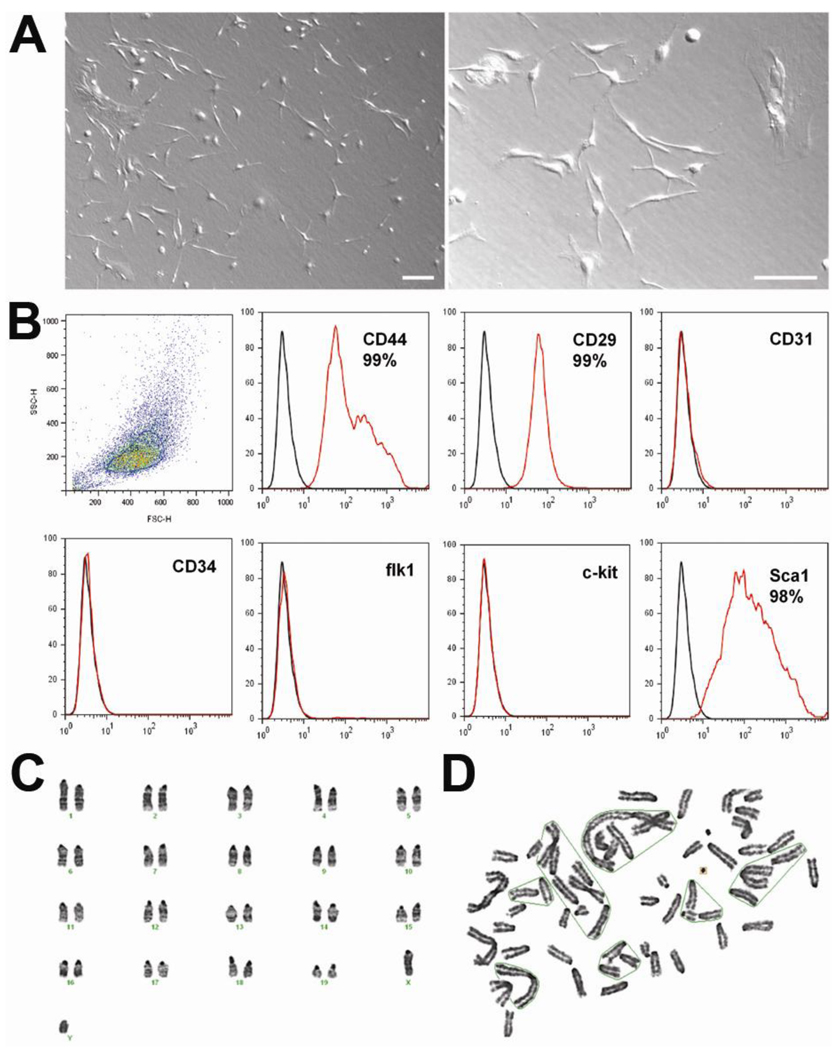
We wondered whether these MSCs had any phenotypic distinction from normal MSCs. We therefore investigated the cell biologic characteristics of these tumor-forming MSCs using FACS and chromosome analyses. The MSCs at P4 showed typical morphology of spindle cells (Figure 3A). FACS analysis demonstrated that 99% of these cells expressed typical MSC markers such as CD44 and CD29, and did not exhibit hematopoietic lineage markers such as c-kit, flk1, or CD34 (Figure 3B). We used BM mononuclear cells for culturing MSCs at the start, and there are usually contaminating hematopoietic lineage cells up to passage 2. Thus passage 3 cells are regarded as the first clone of MSCs in our culture method. Chromosome analysis of these tumor-forming MSCs showed multiple chromosomal abnormalities including fusion, fragmentation, and ring formation (Figure 3D, Table 2). Such chromosome abnormalities were found in both P4 and P8 MSCs. These findings suggest that tumor-forming MSCs have chromosomal abnormalities without demonstrating any abnormal morphology or unusual surface epitopes of MSCs.
Figure 3. Characterization of tumor-forming MSCs.
A, MSCs showed typical spindle-shaped morphology. Scale bar 100 µm. B, FACS analysis showed that 99% of these MSCs expressed CD44 and CD29, but not c-kit, CD31, and CD34. C, Chromosome analyses of normal MSCs showed normal karyotype, 40 XY. D Chromosome analyses of tumor-forming MSCs demonstrated multiple chromosomal abnormalities including fusion, fragmentation, and ring formation.
Table 2.
Chromosome analysis of MSCs.
| Scope | Slide | Vernier Reading |
Acrocentrics | Fused | Fragments | Rings | Total Count |
|
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 149.5×8.2 | 40 | 12 | 5 | 0 | 57 |
| 2 | 2 | 3 | 131.2×17.1 | 44 | 13 | 1 | 0 | 58 |
| 3 | 2 | 1 | 140.7×15.8 | 41 | 10 | 5 | 0 | 56 |
| 4 | 2 | 1 | 144.4×1.8 | 43 | 11 | 3 | 0 | 57 |
| 5 | 2 | 1 | 144.6×23.3 | 38 | 14 | 3 | 0 | 55 |
| 6 | 2 | 3 | 145.1×7.1 | 42 | 12 | 5 | 0 | 59 |
| 7 | 2 | 3 | 164.5×3.3 | 43 | 8 | 5 | 1 | 57 |
| 8 | 2 | 3 | 166.0×4.2 | 49 | 11 | 2 | 0 | 62 |
| 9 | 1 | 2 | 168.0×14.0 | 46 | 11 | 2 | 0 | 59 |
| 10 | 2 | 1 | 168.4×7.4 | 44 | 11 | 2 | 0 | 57 |
| 11 | 1 | 1 | 170.7×6.8 | 47 | 9 | 2 | 0 | 58 |
| 12 | 2 | 2 | 171.3×11.5 | 42 | 13 | 2 | 0 | 57 |
| 13 | 2 | 3 | 164.9×3.6 | 27 | 7 | 3 | 0 | 37 |
| 14 | 2 | 3 | 135.2×7.6 | 26 | 7 | 0 | 0 | 33 |
| 15 | 2 | 3 | 161.9×23.8 | 33 | 4 | 1 | 0 | 38 |
| 16 | 2 | 3 | 149.2×23.2 | 41 | 7 | 7 | 1 | 56 |
| 17 | 2 | 3 | 147.0×23.4 | 46 | 10 | 3 | 0 | 59 |
| 18 | 2 | 1 | 143.0×2.2 | 40 | 10 | 3 | 0 | 53 |
| 19 | 2 | 1 | 153.0×1.8 | 42 | 9 | 0 | 1 | 52 |
| 20 | 2 | 1 | 167.7×7.6 | 43 | 12 | 2 | 0 | 57 |
| 21 | 2 | 2 | 173.5×14.7 | 47 | 10 | 2 | 0 | 59 |
| 22 | 2 | 2 | 173.4×18.7 | 42 | 12 | 6 | 0 | 60 |
| 23 | 2 | 4 | 130.9×1.7 | 39 | 12 | 4 | 0 | 55 |
| 24 | 2 | 4 | 131.5×0.9 | 45 | 13 | 3 | 0 | 61 |
| 25 | 2 | 4 | 145.9×1.3 | 36 | 9 | 2 | 0 | 47 |
| 26 | 2 | 4 | 146.0×0.4 | 42 | 9 | 4 | 0 | 55 |
| 27 | 2 | 4 | 147.8×0.2 | 39 | 12 | 5 | 0 | 56 |
| 28 | 2 | 4 | 149.1×0.2 | 32 | 6 | 1 | 0 | 39 |
| 29 | 2 | 4 | 152.1×0.6 | 43 | 9 | 3 | 0 | 55 |
| 30 | 2 | 4 | 156.3×0.4 | 45 | 12 | 1 | 0 | 58 |
| 31 | 2 | 4 | 167.7×4.0 | 41 | 11 | 0 | 0 | 52 |
| 32 | 2 | 4 | 161.6×3.9 | 45 | 11 | 2 | 0 | 58 |
| 33 | 2 | 4 | 160.2×3.5 | 46 | 10 | 2 | 0 | 58 |
| 34 | 2 | 4 | 154.3×4.0 | 43 | 10 | 2 | 0 | 55 |
| 35 | 2 | 4 | 135.7×4.0 | 48 | 12 | 6 | 0 | 66 |
| 36 | 2 | 4 | 134.1×7.0 | 44 | 11 | 3 | 0 | 58 |
| 37 | 2 | 4 | 134.0×6.4 | 37 | 9 | 5 | 0 | 51 |
| 38 | 2 | 4 | 137.8×7.8 | 44 | 9 | 5 | 1 | 59 |
| 39 | 2 | 4 | 156.9×7.9 | 29 | 11 | 0 | 0 | 40 |
| 40 | 2 | 4 | 166.8×9.8 | 46 | 8 | 6 | 0 | 60 |
| 41 | 2 | 4 | 165.0×10.0 | 30 | 5 | 4 | 0 | 39 |
| 42 | 2 | 4 | 163.9×10.1 | 41 | 8 | 5 | 0 | 54 |
| 43 | 2 | 4 | 162.5×10.9 | 41 | 11 | 4 | 0 | 56 |
| 44 | 2 | 4 | 156.4×11.1 | 44 | 12 | 3 | 0 | 59 |
| 45 | 2 | 4 | 142.2×11.7 | 43 | 11 | 5 | 0 | 59 |
| 46 | 2 | 4 | 133.9×10.3 | 47 | 9 | 4 | 0 | 60 |
| 47 | 2 | 4 | 124.1×13.1 | 44 | 11 | 2 | 0 | 57 |
| 48 | 2 | 4 | 146.2×14.1 | 46 | 6 | 6 | 0 | 58 |
| 49 | 2 | 4 | 155.5×15.6 | 46 | 11 | 1 | 0 | 58 |
| 50 | 2 | 4 | 168.1×15.7 | 42 | 10 | 6 | 0 | 58 |
Fifty cells were scored for the number of acrocentric chromosome, fused chromosomes, chromosome fragments, and ring chromosomes.
Discussion
We demonstrate that culture-expanded BM-MSCs possess chromosomal aberrations even at passage 4 of culture, and transplantation of these cells induces malignant tumors in mouse diabetic neuropathy and MI models. P4 is equivalent to passage 2 as a MSC line and can be usually obtained within 4 weeks of culture. This is often the minimum number of passages required to obtain enough cells to be used in pre-clinical or clinical studies. Clinical trials are underway using human MSCs for treating ischemic heart diseases.31 Since the heart is an organ which only rarely develops malignant tumors,32 the present study suggests the aggressiveness of anomalous MSCs and prompts more careful attention to the potential side effects of cell therapy using MSCs in humans.
MSCs have been widely used for regenerating bone, cartilage, skeletal muscle and even neural tissues in patients as well as in animal models due to their multipotency.33, 34 However, tumor formation has not been reported in animal or human studies to date with adult stem cells including MSCs that were passaged for short-term periods without genetic modification. Recently, spontaneous transformation of BM-derived rat MSCs, with abnormalities in cell morphology, cell proliferation rate, surface marker expression, and chromosome number, was found at passages as early as passage 3, whereas no tumors were observed in any organs in nude mice after intravenous injection of these transformed MSCs.35 There was a report of sarcoma formation by cultured mouse MSCs that had been genetically modified using transposons.26 On the other hand, there have been two reports that late-passaged mouse and human MSCs (P16 to P20) developed chromosomal abnormalities and underwent spontaneous cancerous transformation in vitro and in vivo.25, 27 The mechanism by which MSCs are transformed into malignant cells is known to be related to chromosomal abnormalities, including structural and numerical aberrations, and increases with higher passage numbers.27 Miura et al intentionally induced spontaneous immortalization after numerous passages (P29 – P54) and demonstrated a contribution of these transformed cells to fibrosarcoma formation in vivo. Rubio et al showed that although MSCs can be managed safely during the standard ex vivo expansion period (6–8 weeks), human MSCs can undergo spontaneous transformation following long-term in vitro culture (4–5 months), and the transformed cells lead to formation of tumors in mice.25 We used ‘unmodified’ MSCs at early passages to avoid potential chromosomal abnormalities that result from long-term culture. However, these MSCs developed chromosomal abnormalities and induced malignant tumors when injected in vivo, although the cells display conventional morphology and surface marker characteristics. The accelerated rate of tumor formation by P6–P10 MSCs compared to P4–P6 MSCs, suggest that cumulative abnormalities through ex-vivo culture expansion is likely to promote tumorigenesis in vivo. Studies including ours suggested that sarcoma could originate from implanted MSCs with altered chromosomal stability. It is also possible that tumors could be formed by fusion between the MSCs and resident smooth muscle cells, or that they originate from resident smooth muscle cells stimulated by the MSCs.27 The possibility of MSC-released cytokines transforming unidentified host cells into tumor cells is unlikely. As our experimental results indicate tumors were formed regardless of the host organs (here skeletal muscle or hearts) that were treated with the same MSC transplantation, albeit not excluded, this possibility appears to be minimal. Interestingly, opposing effects of MSCs have been reported on surrounding tumor growth. In general, due to their immunosuppressive actions, MSCs are known to favor tumor growth.36 However, studies have reported that MSCs may exert antitumorigenic effects in vitro and in a model of Kaposi’s sarcoma.37, 38
Over the last decade clinical trials have been underway for treating cardiovascular diseases with various types of adult cells such as endothelial progenitor cells,39 MSCs,40 skeletal myoblasts,41 and BM mononuclear cells.42 Considering the choice of cells for regenerative therapy, safety should be considered as a priority in human use. From the standpoint of tumorigenic potential, more differentiated and uncultured cells may be beneficial; however high inflammatory and tissue-calcifying activities of uncultured BM cells were reported to have side effects.43, 44 Multipotency, on the other hand can be associated with tumorigenic or unwanted cellular differentiation. Therefore, using selected uncultured cells such as CD34, CD133 or CD31 cells45 or short-term cultured cells may be a safer option for achieving neovascularization in ischemic cardiovascular diseases.
This is the first study to report that BM-derived MSCs could possess chromosomal abnormalities at very early passages of culture and that locally transplanted unmodified BMderived MSCs could generate malignant tumors in cardiovascular animal models. Although this study did not use human MSCs, and the chromosomal aberrations are usually more common in mouse cells than human cells, careful monitoring of chromosome status is warranted for using culture expanded MSCs for cell therapy.
Novelty and Significance.
What Is Known?
Bone marrow (BM)-derived mesenchymal stem cells (MSCs) hold a great promise for cell therapy owing to their multipotency and culture-expandability.
Experimental studies and pilot clinical trials have shown therapeutic benefits of BM-derived MSCs for ischemic heart disease.
What New Information Does This Article Contribute?
Unmodified mouse BM-derived MSCs underwent chromosomal abnormalities even at early passages and induced malignant sarcoma when transplanted into hearts and hindlimbs.
We sought to apply BM-derived MSCs for treating diabetic neuropathy and acute myocardial infarction based on their multipotent differentiation potential and paracrine effects. We isolated mononuclear cells from mouse BM and cultured MSCs using a conventional manner. During the follow-up of 4–8 weeks after cell transplantation, growing tumors developed 30~50% of animals. Histopathologic examination revealed the identity of this tumor as malignant sarcoma and chromosomal analysis showed multiple chromosomal aberrations of the injected MSCs. This study for the first time demonstrated that genetically unaltered MSCs even at early passages can undergo chromosomal abnormalities and generate malignant sarcoma in vivo. This study clearly highlights the importance of chromosomal status of MSCs and the requirement for close monitoring thereof when cultured MSCs are used for cell therapy.
Acknowledgements
We would like to thank Andrea Wecker and Julie J. Kim for critical reading of the manuscript.
Sources of Funding
This work was supported in part by NIH grants, RO1HL084471, R21HL097353, RC1 GM092035, P01GM85354, and HHSN268201000043C (Program of Excellence in Nanotechnology Award); and Stem Cell Research Center of the 21st Century Frontier Research Program grant SC4300, funded by the Ministry of Science and Technology, Republic of Korea.
Non-standard Abbreviations and Acronyms
- BM
bone marrow
- MI
myocardial infarction
- MSC
mesenchymal stem cells
Footnotes
Disclosure
None
References
- 1.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 2.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 3.Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 5.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase i clinical trial of the treatment of crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 9.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min JY, Sullivan MF, Yang Y, Zhang JP, Converso KL, Morgan JP, Xiao YF. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg. 2002;74:1568–1575. doi: 10.1016/s0003-4975(02)03952-8. [DOI] [PubMed] [Google Scholar]
- 11.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 12.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji H, Miyoshi S, Ikegami Y, Hida N, Asada H, Togashi I, Suzuki J, Satake M, Nakamizo H, Tanaka M, Mori T, Segawa K, Nishiyama N, Inoue J, Makino H, Miyado K, Ogawa S, Yoshimura Y, Umezawa A. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res. 2010;106:1613–1623. doi: 10.1161/CIRCRESAHA.109.205260. [DOI] [PubMed] [Google Scholar]
- 14.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 16.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi C, Yamagishi M, Yamahara K, Hagino I, Mori H, Sawa Y, Yagihara T, Kitamura S, Nagaya N. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;374:11–16. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 19.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Haider H, Jiang S, Idris NM, Ashraf M. Igf-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of sdf-1alpha/cxcr4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 21.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 22.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 23.Obrosova IG. Diabetic painful and insensate neuropathy: Pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647. doi: 10.1016/j.nurt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong JO, Kim MO, Kim H, Lee MY, Kim SW, Ii M, Lee JU, Lee J, Choi YJ, Cho HJ, Lee N, Silver M, Wecker A, Kim DW, Yoon YS. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation. 2009;119:699–708. doi: 10.1161/CIRCULATIONAHA.108.789297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 26.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 27.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 28.Zhou YF, Bosch-Marce M, Okuyama H, Krishnamachary B, Kimura H, Zhang L, Huso DL, Semenza GL. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66:10849–10854. doi: 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- 29.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 30.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 31.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Gomez I, Elvira G, Zapata AG, Lamana ML, Ramirez M, Castro JG, Arranz MG, Vicente A, Bueren J, Garcia-Olmo D. Mesenchymal stem cells: Biological properties and clinical applications. Expert Opin Biol Ther. 2010;10:1453–1468. doi: 10.1517/14712598.2010.519333. [DOI] [PubMed] [Google Scholar]
- 34.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furlani D, Li W, Pittermann E, Klopsch C, Wang L, Knopp A, Jungebluth P, Thedinga E, Havenstein C, Westien I, Ugurlucan M, Li RK, Ma N, Steinhoff G. A transformed cell population derived from cultured mesenchymal stem cells has no functional effect after transplantation into the injured heart. Cell Transplant. 2009;18:319–331. doi: 10.3727/096368909788534906. [DOI] [PubMed] [Google Scholar]
- 36.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 37.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: Impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 38.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of kaposi's sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (topcare-ami) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 40.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The myoblast autologous grafting in ischemic cardiomyopathy (magic) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 42.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with st-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto K, Nishigami K, Nagaya N, Akutsu K, Chiku M, Kamei M, Soma T, Miyata S, Higashi M, Tanaka R, Nakatani T, Nonogi H, Takeshita S. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114:2679–2684. doi: 10.1161/CIRCULATIONAHA.106.644203. [DOI] [PubMed] [Google Scholar]
- 44.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. Cd31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: Novel role of nonendothelial cd31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]