Abstract
Objectives
To evaluate the self-perceived knowledge and confidence of inpatient and outpatient pharmacists in applying pharmacogenomics information to clinical practice.
Methods
A 19-question multiple-choice, electronic needs-assessment survey instrument was distributed to 480 inpatient and outpatient pharmacists in a large, academic, multi-campus healthcare system.
Results
The survey response rate was 64% (303). Most respondents (85%) agreed that pharmacists should be required to be knowledgeable about pharmacogenomics, and 65% agreed that pharmacists should be capable of providing information on the appropriate use of pharmacogenomics testing. Sixty-three percent felt they could not accurately apply the results of pharmacogenomics tests to drug-therapy selection, dosing, or monitoring.
Conclusion
Pharmacists believe pharmacogenomics knowledge is important to the profession, but they lack the knowledge and self-confidence to act on the results of pharmacogenomics testing and may benefit from pharmacogenomics education.
Keywords: pharmacogenomics, needs assessment, genetic testing, continuing education, survey
INTRODUCTION
The science of pharmacogenomics explores the contribution of genetic variations to drug response among individuals given the same drug and dose on the same schedule.1,2 While this concept has been discussed in academic and research circles, the completion of the Human Genome Project (HGP) in 2003 made the concept of personalized medicine more tangible to clinicians.3,4 Four years after the HGP was completed, the Food and Drug Administration (FDA) announced labeling changes to warfarin to include the potential usefulness of genetic testing and, in the past year, also mandated a black box warning regarding pharmacogenomics concerns associated with clopidogrel metabolism.5,6 The FDA has mandated pharmacogenomics testing for trastuzumab, cetuximab, maraviroc, and dasatinib, and highly recommended testing for abacavir, mercaptopurine, carbamazepine, and irinotecan, among others.7 The FDA's guiding statements for manufacturers marked a new era in which patient-specific dosing strategies are being developed for commonly prescribed medications.8
As point-of-care providers and drug experts, pharmacists are uniquely positioned in the healthcare system to educate providers and patients about interpreting and applying the results of pharmacogenomics testing.9 Pharmacists’ education and background also enable them to participate in pharmacogenomics conceptual development and practice integration.10 Through these activities, pharmacists have the potential to be an integral part of the new age of personalized medicine.
Despite the natural fit of pharmacists with pharmacogenomics, the concept remains in its infancy.11 Educational gaps in pharmacogenomics have been documented in academia.12 Accrediting institutions such as the American Association of Colleges of Pharmacy (AACP), the American Society of Health-System Pharmacists (ASHP), and the American College of Clinical Pharmacy (ACCP) have recommended the implementation of coordinated pharmacogenomics educational requirements and supported efforts that assess patient outcomes, improve drug dosing, and predict therapeutic response.13-16
Little is known about pharmacists’ opinions regarding pharmacogenomics and their impact on the profession or pharmacists’ self-perceived confidence in the practical application of pharmacogenomics information in the scientific literature. The objective of this study was to assess the pharmacogenomics educational needs of pharmacists within a large, academic, multi-campus healthcare system.
METHODS
A 19-question electronic, needs-assessment survey instrument was developed in collaboration with the institutional survey research support center.17-20 The survey instrument was composed of 2 sections, the first of which evaluated educational exposure, significance to clinical practice, perceived roles of the individual pharmacist and pharmacist profession, anticipated monetary implications, and self-assessed confidence regarding pharmacogenomics concepts. Responses were based on a 5-point Likert scale: strongly disagree, disagree, neutral, agree, and strongly agree. For ease of interpretation and analysis, the final results were collapsed into 3 categories: agree, neutral, and disagree.
The second section of the survey instrument contained multiple-choice questions to assess demographic information, including the pharmacist's practice setting, amount of time spent at the bedside, geographic location, years in practice, and preferred learning format for future education. The survey instrument was assembled and critiqued by the internal survey research center and field-tested by 6 pharmacists to refine survey items and enhance content validity.
The survey method used was a census-sample process, in that all known members of the population were surveyed. The target population included inpatient, outpatient, and administrative pharmacists in a large, academic, multi-campus healthcare system. The health system consisted of 3 regional centers with 4 hospitals located throughout the country that housed 214 to 1,296 beds, depending on location. One of these regions also served rural areas with a network of 70 community facilities. As a whole, the health system served more than 500,000 patients.
A distribution list of e-mail addresses for all pharmacists in the system (N = 543) was compiled. The status of each individual was verified using the internal employee directory. Three pharmacists with invalid e-mail addresses and 60 individuals found to be nonpharmacists were excluded from the distribution list. The survey instrument was administered electronically by the internal survey research center to maintain participant confidentiality and investigator blinding. Pharmacists were notified about the survey via an e-mail that included a Web link to the survey page, which also was operated and maintained by the center. A reminder e-mail was sent to nonrespondents at 7 and 12 days after initial contact. The survey was closed after 13 days. To ensure nonbiased results, the survey instrument did not include a definition of pharmacogenomics. Respondents who contacted the primary investigator regarding content questions were asked to complete the survey instrument to the best of their ability with no further detail provided.
To investigate relevant differences among respondents, the percentage who responded with “agree” to a given survey question was compared across practice setting groups using chi-square tests. Cochran-Armitage tests for trend were performed to determine if the percentage of agreement increased or decreased by years of experience (ordinal). Prior to study initiation, the institutional review board granted the study exempt status.
RESULTS
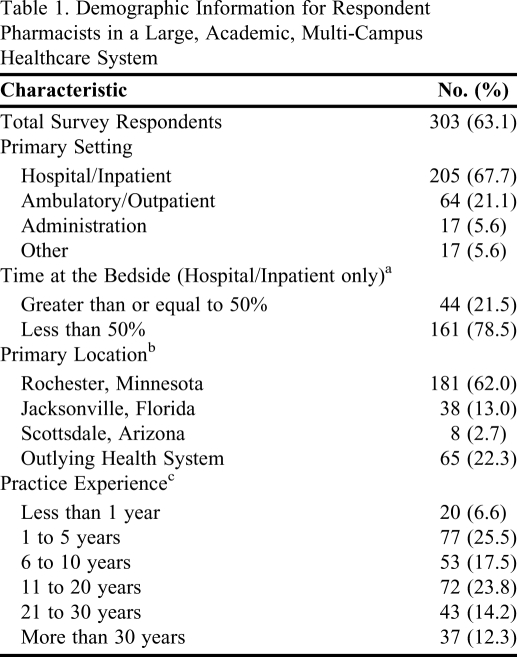
Four hundred eighty survey instruments were distributed. Three (0.6%) pharmacists asked to be removed from the mailing list, 174 (36.3%) did not respond, and 303 (63.1%) completed the electronic survey instrument. Pharmacist practice years among respondents varied: 97 (32%) had been in practice for 5 or fewer years, 53 (17.5%) for 6 to 10 years, and 152 (50.2%) for more than 10 years. A majority (68%) of respondents was hospital pharmacists and the greatest portion (60%) was based in Rochester, Minnesota. Baseline characteristics of respondents are listed in Table 1.
Table 1.
Demographic Information for Respondent Pharmacists in a Large, Academic, Multi-Campus Healthcare System
Not all participants responded to this item (N = 205)
Not all participants responded to this item (N = 292)
Not all participants responded to this item (N = 302)
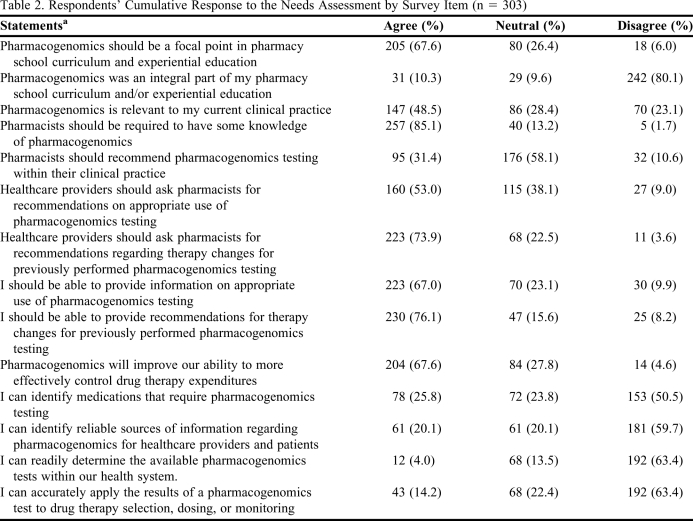
The first domain of the survey assessed educational exposure to pharmacogenomics. When evaluating their own experience, 80.1% disagreed that pharmacogenomics was an integral part of their education. In contrast, when asked if pharmacogenomics should be a focal point in pharmacy school education, 67.6% agreed. Survey items and quantified responses are listed in Table 2.
Table 2.
Respondents’ Cumulative Response to the Needs Assessment by Survey Item (n = 303)
Actual statements utilized a 5-point Likert scale. ‘Strongly agree’ was collapsed into ‘agree’ and ‘strongly disagree’ was collapsed into ‘disagree’
The second domain assessed pharmacists’ perception of the significance of pharmacogenomics in clinical practice. Nearly half (48.5%) of the respondents agreed that pharmacogenomics is relevant to their clinical practice, and a majority (85.1%) agreed that pharmacists should be required to have some knowledge of pharmacogenomics.
The third domain assessed respondents’ perceptions of their role in pharmacogenomics as a profession as well as individuals. With respect to the profession of pharmacy as a whole, 58.1% of respondents were neutral about whether pharmacists should recommend pharmacogenomics testing within their clinical practice, while 31.4% agreed that they should. More than half (53%) agreed that healthcare providers should be asking pharmacists for recommendations on appropriate use of pharmacogenomics testing. Most (73.9%) agreed that, when pharmacogenomics testing has already been completed, pharmacists should make therapy recommendations based on the results; only 3.6% disagreed. Regarding their personal role in pharmacogenomics, 67% agreed that they should be capable of providing information on pharmacogenomics testing and, if presented with test results, 76.1% agreed that they should be capable of providing therapy recommendations.
The fourth domain assessed perceived monetary implications of pharmacogenomics testing. In response to the statement that pharmacogenomics would improve pharmacists’ ability to control drug therapy expenditures more effectively, 67.6% agreed and 4.6% disagreed.
The last domain assessed the pharmacists’ personal confidence in their abilities. Only 25.8% of pharmacists agreed that they are capable of identifying medications that require pharmacogenomics testing, while 50.5% disagreed. With respect to their ability to identify reliable sources of pharmacogenomics information for healthcare providers and patients, 59.7% of respondents disagreed, and only 4% agreed that they are readily able to determine what pharmacogenomics tests are available within their hospital system. In response to the final survey item, only 14.2% agreed that they are confident in their ability to accurately apply the results of a pharmacogenomics test to drug therapy selection, dosing, or monitoring.
In order to facilitate future education in pharmacogenomics, respondents were asked about their educational format preferences. A majority of respondents (64%) preferred lecture format, either live or Web-archived, while 22.8% chose printed reading material. Other responses included CD/DVD format (8.3%) and audio podcast (4.3%).
Subgroup analyses revealed that, in general, survey responses were similar across practice settings with one exception. Pharmacists working in the hospital/inpatient setting were most likely to agree that they should be capable of recommending therapeutic changes based on the results of previously performed pharmacogenomics testing (80.9%). Comparatively, 70.3% of those working in the ambulatory/outpatient and 58.8% of those in administrative/other settings agreed (p = 0.009).
With a few exceptions, most survey responses were not associated with years of pharmacist experience. Respondents with 5 or fewer years of pharmacist experience were much more likely to agree that pharmacogenomics was an integral part of their pharmacy school curriculum and/or experiential education (25% for those with less than 1 year and 23.4% for those with 1 to 5 years) while less than 5% of respondents in practice for more than 5 years agreed (p < 0.0001). There was an inverse association between respondents’ years of experience and agreement with statements about being able to recommend therapy changes based on previous test results and having confidence in identifying medications that require pharmacogenomics testing: agreement with these survey items decreased steadily as years of respondent experience increased (p = 0.001).
DISCUSSION
Over the past 40 years, there has been great progress toward integrating pharmacists as members of the healthcare delivery team as well as in shifting daily activities from dispensing medications to delivering personalized pharmacotherapy services.21-25 While the pharmacy profession has fostered development of the clinical pharmacist, laboratory-based researchers have been discovering underlying pharmacogenomics principles and relationships, bringing the 2 concepts closer to a complementary relationship rather than a mutually exclusive one.11
In 2007, the National Coalition for Health Professional Education in Genetics (NCHPEG) published core competencies for all healthcare professionals designed to serve as a framework for continuing healthcare education. Hopefully, these competencies, which involve knowledge, skills, and attitudes, will result in healthcare practitioners integrating genomics into their daily practice.26
While it would seem intuitive for the practice of clinical pharmacy to encompass pharmacogenomics, the results of the current study clearly demonstrate that pharmacists lack self-confidence in their ability to base therapeutic decisions on pharmacogenomics. These results are corroborated by an evaluation in the community pharmacy setting in which pharmacists rated themselves as less than 40% (scale of 0% to 100%) confident in their own knowledge of the human genome project, pharmacogenomics, and genetic testing.19
Practitioners also have demonstrated a lack of confidence in clinical collaborative practice, another rapidly expanding area of pharmacy. One survey found that less than 50% of community pharmacists had adequate confidence in their clinical knowledge or felt sufficiently trained to provide clinical services to general practitioners.27 Despite these findings, the longitudinal portion of the survey found a 16% increase in general practitioners’ confidence that their local pharmacists are capable of providing medication management services in 2002, compared with results from 1998 (p < 0.001).27
Pharmacists’ lack of confidence in their knowledge of pharmacogenomics may stem from a deficiency of pharmacogenomics education in their pharmacy curricula. A 2010 survey regarding the state of pharmacogenomics education within doctor of pharmacy or postgraduate programs was distributed to 90 colleges and schools of pharmacy.28 Of the 75 responding schools, 69 were currently providing some form of pharmacogenomics education, although investigators identified high variability in the number of hours taught, faculty members involved, and plans for growth.28
This study demonstrates improvement from a similar survey in 2005, which was sent to 85 colleges and schools of pharmacy, producing 41 responses (48%).12 While 32 schools (78%) had established pharmacogenomics education programs, none had met all of the NCHPEG education goals.12 Ensuring that the educational content presented in these programs is both concise and clinically applicable to bedside medicine is a considerable obstacle to the effective initiation of education.29
While efforts underway to improve the pharmacogenomics knowledge base of new PharmD graduates represent progress, it is equally essential that practicing pharmacists receive continuing education in pharmacogenomics. As the results of the current survey indicate, continuing education emphasizing the importance of pharmacogenomics in the profession of pharmacy may be necessary to ensure that seasoned practitioners recognize the significance of this science.
In 2001, an assessment of pharmacogenomics continuing education at 3 national pharmacy meetings found over 60 hours of educational offerings devoted to the topic.29 Pharmacists attending these meetings could easily spend almost half (45%) of their time on pharmacogenomics sessions alone.29 Despite the available continuing education, this study and another conducted in the community pharmacy setting indicate a lack of association between the availability of continuing education and its impact on pharmacists.
There are limitations to this study that should be considered in interpreting the results. A Web-based survey may select for individuals who are more comfortable with technology-based communication. Because the health system that conducted this study relied heavily on electronic communication, respondents were likely to be familiar with Internet-based interactions. Selection bias may occur if individuals in the sample have strong positive or negative opinions on the survey subject matter because they may be more inclined to open the e-mail or share their opinions. Conversely, those who have not formed opinions or are indifferent about pharmacogenomics may provide fewer survey responses. A 64% response rate in this survey suggested that a majority of the surveyed population were interested enough to share their opinions. The 36% who did not participate in the survey may not be interested in pharmacogenomics, suggesting that organized education efforts should focus on increasing awareness about the significance of pharmacogenomics in pharmacy practice.
Survey fatigue associated with the length of the instrument or time required for completion may be a contributing factor in nonresponses. An attempt was made to minimize this effect by limiting the survey instrument to 19 items and an average completion time of 6 minutes. The amount of survey requests practitioners receive from outside sources may be another potential factor in nonresponses.
Survey research that uses a Likert scale is subject to a central tendency bias. If given a response option of “neutral” or “unsure,” participants who are unfamiliar with the topic may select the middle ground. Because a definition of pharmacogenomics was not provided, investigators elected to include neutral responses to represent the positions of individuals who are frankly uncertain about or unfamiliar with the topic. Despite this option, more than 50% of respondents either agreed or disagreed with most survey items, decreasing the impact of potential central tendency bias. Another source of potential bias, survey item interpretation, was addressed by having 6 pharmacists validate the survey instrument. Although this content validity methodology is not infallible, it is the one most commonly used in survey research.30
Finally, this survey could have exposure bias because it was conducted in a large, academic, multi-campus healthcare system. Compared with pharmacists in other settings, pharmacists in this type of environment may discuss or order pharmacogenomics tests more often, thereby incorporating pharmacogenomics into their daily medical and pharmacy practice. While this factor did not translate into substantial pharmacist confidence in our study, the confidence of pharmacists in other settings may be different. However, because the current survey included pharmacists at a large teaching hospital as well as those in outlying community medical centers, the results may be generalizable to a variety of institutions and pharmacists.
CONCLUSION
The surveyed pharmacists perceived themselves as lacking confidence and educational background in pharmacogenomics. If pharmacists are to become leaders in this age of personalized medicine, they must be prepared to make recommendations regarding pharmacogenomics testing, successfully interpret and apply results for pharmacogenomics-driven therapeutic decisions, and teach pharmacogenomics to other healthcare providers.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Mayo Clinic Eisenberg Genomics Educational Program for providing an educational grant to support this research (C.M.F. and W.T.N). Additionally, special thanks to Sarah Jenkins for her outstanding statistical support. Lastly, we thank the pharmacists for participating in this project.
Dr. Formea was supported by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
REFERENCES
- 1.Evans WE, McLeod HL. Pharmacogenomics–drug disposition, drug targets, and side effects. N Eng J Med. 2003;348(6):538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 2.Weinshilboum R. Inheritance and drug response. N Engl J Med. 2003;348(6):529–537. doi: 10.1056/NEJMra020021. [DOI] [PubMed] [Google Scholar]
- 3.Gurwitz D, Weizman A, Rehavi M. Education: Teaching pharmacogenomics to prepare future physicians and researchers for personalized medicine. Trends Pharmacol Sci. 2003;24(3):122–125. doi: 10.1016/S0165-6147(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 4.Regalado A. Inventing the pharmacogenomics business. Am J Health-Syst Pharm. 1999;56(1):40–50. doi: 10.1093/ajhp/56.1.40. [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Lesko LJ. Pharmacogenetics of warfarin: regulatory, scientific, and clinical issues. J Thromb Thrombolysis. 2008;25(1):45–51. doi: 10.1007/s11239-007-0104-y. [DOI] [PubMed] [Google Scholar]
- 6.Holmes DR, Dehmer GJ, Kaul S, Leifer D, O'Gara PT, Stein CM. ACCF/AHA Clopidogrel Clinical Alert: Approaches to the FDA “Boxed Warning”: A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56(4):321–341. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Shin J, Kayser SR, Langaee TY. Pharmacogenetics: from discovery to patient care. Am J Health-Syst Pharm. 2009;66(7):625–637. doi: 10.2146/ajhp080170. [DOI] [PubMed] [Google Scholar]
- 8. United States Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Guidance for industry pharmacogenomic data submissions. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079849.pdf. Accessed January 18, 2011.
- 9.Lee KC, Ma JD, Kuo GM. Pharmacogenomics: bridging the gap between science and practice. J Am Pharm Assoc. 2010;50(1):e1–14. doi: 10.1331/JAPhA.2010.09124. [DOI] [PubMed] [Google Scholar]
- 10.Ellingrod VL, Moline J. Incorporating Pharmacogenomics into Practice. J Pharm Pract. 2007;20(3):277–282. [Google Scholar]
- 11.Streetman DS. Emergence and evolution of pharmacogenetics and pharmacogenomics in clinical pharmacy over the past 40 years. Ann Pharmacother. 2007;41(12):2038–2041. doi: 10.1345/aph.1K273. [DOI] [PubMed] [Google Scholar]
- 12.Latif DA, McKay AB. Pharmacogenetics and pharmacogenomics instruction in colleges and schools of pharmacy in the United States. Am J Pharm Educ. 2005;66(2) Article 23. [Google Scholar]
- 13.American College of Clinical Pharmacy. The research agenda of the American College of Clinical Pharmacy. Pharmacotherapy. 2007;27(2):312–324. doi: 10.1592/phco.27.2.312. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Bootman JL, Evans WE, et al. Pharmacogenomics: A scientific revolution of pharmaceutical sciences and pharmacy practice. Report of the 2001-2002 Academic Affairs Committee. Am J Pharm Educ. 2002;66(4):12S–15S. [Google Scholar]
- 15. American Society of Health-system Pharmacy. ASHP Formulary Management Policy Position: Pharmacogenomics. http://www.ashp.org/DocLibrary/BestPractices/FormularyPositions.aspx. Accessed January 18, 2011.
- 16.Cavallari LH, Overholser BR, Anderson D, et al. Recommended basic sciences foundation necessary to prepare pharmacists to manage personalized pharmacotherapy. Pharmacotherapy. 2010;30(6):228e–235e. [Google Scholar]
- 17.Cerulli J, Malone M. Using CAPE outcome-based goals and objectives to evaluate community pharmacy advanced practice experiences. Am J Pharm Educ. 2003;(2):67. Article 34. [Google Scholar]
- 18.Hamilton WR, Monaghan MS, Turner PD. Comparison of pharmacy practitioner and pharmacy student attitudes toward complementary and alternative therapies in a rural setting. Am J Pharm Educ. 2002;66(2):55–58. [Google Scholar]
- 19.Sansgiry SS, Kulkarni AS. The Human Genome Project: assessing confidence in knowledge and training requirements for community pharmacists. Am J Pharm Educ. 2003;(2):67. Article 39. [Google Scholar]
- 20.Melnyk BM, Fineout-Overholt E, Fischbeck Feinstein N, et al. Nurses' perceived knowledge, beliefs, skills, and needs regarding evidence-based practice: implications for accelerating the paradigm shift. Worldviews Evid Based Nurs. 2004;1(3):185–193. doi: 10.1111/j.1524-475X.2004.04024.x. [DOI] [PubMed] [Google Scholar]
- 21.Law AV, Okamoto MP, Brock K. Perceptions of Medicare Part D enrollees about pharmacists and their role as providers of medication therapy management. J Am Pharm Assoc. 2008;48(5):648–653. doi: 10.1331/JAPhA.2008.07084. [DOI] [PubMed] [Google Scholar]
- 22.Smith WE, Ray MD, Shannon DM. Physicians' expectations of pharmacists. Am J Health-Syst Pharm. 2002;59(1):50–57. doi: 10.1093/ajhp/59.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Spencer JA, Edwards C. Pharmacy beyond the dispensary: general practitioners' views. Br Med J. 1992;304(6843):1670–1672. doi: 10.1136/bmj.304.6843.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapskin K, Johnson C, Cory P, Sorum S, Decker C. Forging a novel provider and payer partnership in Wisconsin to compensate pharmacists for quality-driven pharmacy and medication therapy management services. J Am Pharm Assoc. 2009;49(5):642–651. doi: 10.1331/JAPhA.2009.08158. [DOI] [PubMed] [Google Scholar]
- 25.Whitney HA, Jr., Nahata MC, Thordsen DJ. Francke's legacy–40 years of clinical pharmacy. Ann Pharmacother. 2008;42(1):121–126. doi: 10.1345/aph.1K660. [DOI] [PubMed] [Google Scholar]
- 26. National Coalition for Health Professional Education in Genetics. Core Competencies in Genetics for Health Professionals. http://www.nchpeg.org/index.php?option=com_content&view=article&id=94&Itemid=84. Accessed January 18, 2011.
- 27.Bryant LJ, Coster G, Gamble GD, McCormick RN. General practitioners' and pharmacists' perceptions of the role of community pharmacists in delivering clinical services. Res Social Admin Pharm. 2009;5(4):347–362. doi: 10.1016/j.sapharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JE, Green JS, Adams LA, Squire RB, Kuo GM, McKay A. Pharmacogenomics in the curricula of colleges and schools of pharmacy in the United States. Am J Pharm Educ. 2010;74(1):7. doi: 10.5688/aj740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brock TP, Faulkner CM, Williams DM, Smith SR. Continuing-education programs in pharmacogenomics for pharmacists. Am J Health-Syst Pharm. 2002;59(8):722–725. doi: 10.1093/ajhp/59.8.722. [DOI] [PubMed] [Google Scholar]
- 30.Hayes BE. Measuring Customer Satisfaction: Survey Design, Use, and Statistical Analysis Methods. 2nd ed. Milwaukee, WI: ASQ Quality Press; 1998. [Google Scholar]