Cellular sensing of DNA damage, along with concomitant cell cycle arrest, is mediated by a great many proteins and enzymes. One focus of pharmaceutical development has been the inhibition of DNA damage signaling, and checkpoint kinases (Chks) in particular, as a means to sensitize proliferating tumor cells to chemotherapies that damage DNA. Although the clinical development of Chk inhibitors has overcome many initial obstacles, such drugs have nevertheless failed to show a high level of clinical activity when combined with DNA-damaging chemotherapeutic agents. One very likely reason is the induction of compensatory activities in response to the Chk inhibitor itself. A variety of experimental approaches indicate that the Chk inhibitors of interest influence basic signaling events that could not have been predicted. In this way, the clinical development of such inhibitors is tied to attempts to understand very broad connections among cell signaling pathways.
Abstract
Cellular sensing of DNA damage, along with concomitant cell cycle arrest, is mediated by a great many proteins and enzymes. One focus of pharmaceutical development has been the inhibition of DNA damage signaling, and checkpoint kinases (Chks) in particular, as a means to sensitize proliferating tumor cells to chemotherapies that damage DNA. 7-Hydroxystaurosporine, or UCN-01, is a clinically relevant and well-studied kinase activity inhibitor that exerts chemosensitizing effects by inhibition of Chk1, and a multitude of Chk1 inhibitors have entered development. Clinical development of UCN-01 has overcome many initial obstacles, but the drug has nevertheless failed to show a high level of clinical activity when combined with chemotherapeutic agents. One very likely reason for the lack of clinical efficacy of Chk1 inhibitors may be that the inhibition of Chk1 causes the compensatory activation of ATM and ERK1/2 pathways. Indeed, inhibition of many enzyme activities, not necessarily components of cell cycle regulation, may block Chk1 inhibitor–induced ERK1/2 activation and enhance the toxicity of Chk1 inhibitors. This review examines the rationally hypothesized actions of Chk1 inhibitors as cell cycle modulatory drugs as well as the impact of Chk1 inhibition upon other cell survival signaling pathways. An understanding of Chk1 inhibition in multiple signaling contexts will be essential to the therapeutic development of Chk1 inhibitors.
Introduction
DNA damage is a ubiquitous process, occurring in all cells and organelles that contain DNA. Damage to DNA can result from errors made during DNA replication as well as from agents that chemically modify or intercalate within the DNA structure. In tumor cells, elevated levels of endogenous reactive oxygen species constantly cause DNA damage, which is one probable factor for the genomic instability of such cells (1, 2). Cells have multiple mechanisms for sensing DNA damage and for initiating processes to permit DNA repair, with varying levels of fidelity. If levels of damage sustained cannot be repaired, the cell may undergo rapid or delayed forms of reproductive cell death (3, 4).
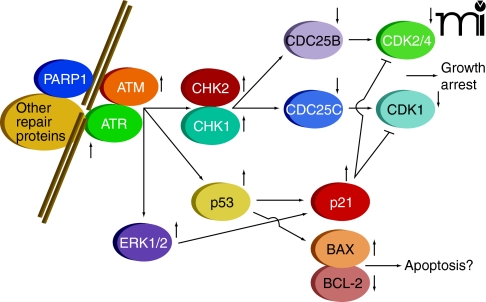
Cellular sensing of DNA damage is mediated through formation of several distinct complexes of proteins, determined by the nature of the DNA lesions, that act to catalyze repair (5, 6). Simultaneously, with the formation of repair complexes, signaling processes are initiated that lead to cell cycle arrest, thereby permitting DNA repair without the occurrence of additional DNA replication or cell division. The sensing of DNA damage with concomitant cell cycle arrest is mediated by pathways involving multiple enzyme activities (Figure 1), including: 1) poly(ADP–ribose) polymerase 1 (PARP1); 2) distinct members of the phosphatidylinositol 3-kinase protein family, known as ataxia-telangiectasia mutated (ATM) and ataxia-telangiectasia and Rad3-related (ATR); 3) checkpoint kinases 1 and 2 (CHK1 and CHK2); 4) the dual-specificity protein phosphatases CDC25A–C; and 5) cyclin-dependent kinases (CDKs, specifically, CDK1 and CDK2/4) (7–16). Additional regulators of these pathways include p53 and the cyclin kinase inhibitor p21. Inhibition of cyclin-dependent kinases pursuant to DNA damage is of central importance for reducing the rate of progression through the cell cycle so that DNA repair can be effected.
Figure 1. Cell signaling processes caused by DNA damage.
Damage is sensed and repaired in multi-protein complexes. Signaling caused by this damage results in cell cycle arrest and a choice between repair or progression to apoptosis.
A variety of clinical and laboratory observations have led to our understanding of the signaling pathways related to DNA repair. It was recognized that DNA isolated from ataxia-telangiectasia (AT) patients exhibited inherently more evidence of damage and that AT patients were more likely to develop malignancies; the expression of ATM was eventually linked to the disease (17, 18). A multitude of studies in which DNA repair was inhibited elucidated the regulatory pathways downstream of ATM and the related ATR protein; in turn, pharmaceutical companies and ultimately the National Cancer Institute began to explore the development and characterization of novel compounds to inhibit the kinase activities of ATM, ATR, Chk1, and Chk2 [see (19, 20)]. Based on this work, abrogation of DNA damage–induced cell cycle arrest became a major focus of anticancer chemotherapeutic research. Specifically, it was reasoned that chemotherapies that cause DNA damage might be made more effective in the presence of agents that interfere with cell cycle control. By causing “inappropriate” cell cycle progression in tumor cells, frequently characterized by damaged DNA, it was hypothesized, patient survival might be improved. Inhibitors of cell cycle control were thus envisaged as chemosensitizers, exploiting the proliferative nature of transformed cells, and invoking various forms of short-term and long-term reproductive cell death. In this review, the development of Chk1 inhibitors and the cellular responses to such inhibitors are discussed.
The Chk1 Inhibitor 7-Hydroxystaurosporine (UNC-01)
There are almost 400 studies referenced in the National Library of Medicine that cover the use of UCN-01, originally isolated from Streptomyces (21), to explore tumor cell signaling and cell death responses. Although UCN-01 became widely recognized as a broad-spectrum inhibitor of the protein kinase C (PKC) family of enzymes, it proved unique among PKC inhibitors for promoting the activation of Cdk1and Cdk2 and thereby driving cell cycle progression and killing tumor cells (22). More specifically, UCN-01 was demonstrated to abrogate the DNA damage–dependent G2 checkpoint that can be induced by cisplatin treatment; the activity of UCN-01 as a G2-checkpoint inhibitor was found to enhance cisplatin toxicity by as much as sixtyfold (23). Subsequently, several interesting activities associated with UCN-01 were determined, including: 1) radiosensitization associated with the abrogation of ionizing radiation–induced G2/M arrest; 2) enhancement of the toxicity of 1-[beta-D-arabinofuranosyl] cytosine (Ara-C); and 3) the potentiation of lethality of topoisomerase inhibitors, thymidylate synthase inhibitors, and temozolomide (24–28). The cell cycle regulatory effects of UCN-01 were clearly linked to its inhibition of Chk1 and to dysregulation of the dual-specificity phosphatase Cdc25C (29). Although UCN-01 has more recently been shown to inhibit PDK-1 (i.e., the kinase upstream of AKT within the “classic” PI3K pathway), checkpoint abrogation appears to play the major role in the anticancer properties of UCN-01 (30).
In light of its inherent toxicity, as well as its chemosensitizing effects in vitro and in a wide variety of in vivo systems, UCN-01 was chosen by the National Cancer Institute for evaluation in patients. Its pharmacokinetic properties in animal studies had predicted that UCN-01 would lead to sustainable free drug levels in patients and single-agent antitumor effects; however, several phase 1 trials established that the drug had a very long half-life, owing to its binding to alpha-1 acidic glycoprotein, and the levels of free drug in patient plasma were only about 100 nM (31, 32). Although a UCN-01 administration schedule has recently been developed that results in more favorable pharmacokinetics (33), the drug has not shown significant clinical activity, alone or in combination with other agents, and it presently remains unclear whether UCN-01 will become an FDA-approved anticancer drug.
Other Chk1 Inhibitors
Based on the realization that Chk1 was a druggable target and that its inhibition could enhance tumor cell killing by DNA-damaging agents, Chk1 inhibitors distinct from UCN-01 have been investigated. AZD7762 (Astra Zeneca) is a potent Chk1 inhibitor that enhances the toxicity of a variety of DNA-damaging agents against cancer in preclinical models (34). AZD7762 is presently undergoing phase 1 evaluation in a variety of tumor types in combination with DNA-damaging agents. Similarly, PF-477736 (Pfizer), SCH900776 (Schering Plough), and LY2606368 (Eli Lily) have been reported to inhibit Chk1 in tumor cells and enhance chemotherapy sensitivity; they, too, are undergoing phase 1 evaluation in patients (35–37).
Newton’s Third Law and the Development of Combinatorial Cancer Therapeutics
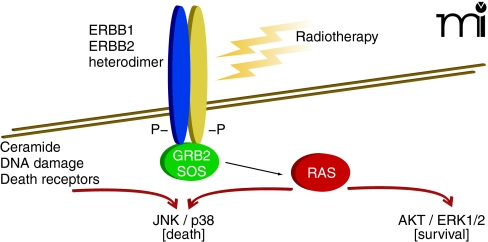
For every action there is an equal and opposite reaction. Cells respond proportionally to stress by adjusting their signaling pathway activities. This observation extends to tumor cells that are exposed, within limits, to a chemotherapeutic agent (e.g., at concentrations that result in a loss of ~30% or less of reproductive survival). Some stress-induced signals are toxic to the cell, depending on the mode of action of the therapeutic agent; other signals, however, may be induced to modulate these stress-induced toxic signals (38). For example, when a carcinoma cell is exposed to ionizing radiation, the epidermal growth factor receptor (EGFR) is activated (39). EGFR activation can, alongside ceramide and reactive oxygen and nitrogen species, play a supporting role in propagating a toxic signal (e.g., activation of the JNK pathway or CD95 death receptor) and coincidentally play a direct role in activation of protective pathways (e.g., activation of AKT and ERK) (40). Inhibitors of EGFR can (like inhibitors of PI3K or MEK1/2) thus radiosensitize tumor cells by shifting the balance of toxic and protective signals toward toxicity (Figure 2) (41). Moreover, toxic signals in this context can offer a chemo-therapeutic advantage because signaling pathway activity levels are usually elevated and more unstable in cancer cells, relative to nontransformed cells. However, the reaction of a tumor cell to a given kinase inhibitor at a concentration that alone causes little cell killing will also be a function of any other administered agent that acts (e.g., in a compensatory fashion) within the nexus of signaling pathways (42).
Figure 2. Radiation exposure and the activation of multiple signaling pathways.
Activated signaling pathways act in balance and can promote both cell survival as well as cell death. The combination of inhibitors that block AKT/ERK1/2 activation with radiotherapy enhances activation of the toxic JNK/p38 pathways to cause tumor cell death.
Chk1 Inhibitors and ERK1/2 Signaling
Almost a decade ago, it was noted that treatment of tumor cells at modest but clinically relevant concentrations of UCN-01 caused a rapid and sustained activation of the ERK1/2 pathway (43, 44). More recently, ERK1/2 activation has been observed in response to the chemically unrelated Chk1 inhibitor AZD7762; similar activation is promoted upon transient expression of a dominant–negative form of Chk1 (45, 46). Chk1 inhibitor–mediated activation of ERK1/2, however, becomes limited upon expression of dominant–negative Chk1. In addition, although knockdown of Chk1 reduces basal ERK1/2 activation, treatment of knockdown cells with Chk1 inhibitors raises ERK1/2 activity (45, 46). In developing Drosophila pupae, loss of Chk1 function has been shown to promote MEK1/2 activation, which provides independent genetic confirmation of studies in cancer cells (47).
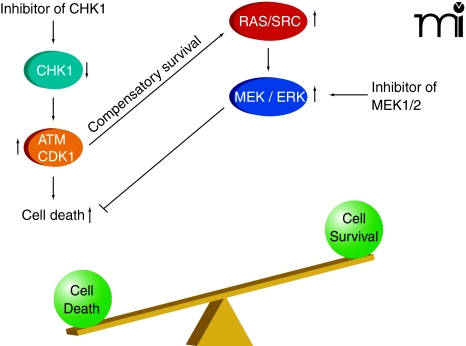
In agreement with the concept that ERK1/2 activation is a compensatory protective signal in response to the loss of cell cycle control that occurs upon Chk1 inhibition, tumor cell death rates significantly increase when Chk1 inhibitor–treated cells are subjected to inhibition of MEK1/2 (43–46, 48–61). In addition, the prevention of Ras activity at the cell membrane (i.e., by preventing Ras prenylation; see Figure 3) enhances Chk1 inhibitor–induced toxicity (54, 56, 58, 60). Suppression of mTOR signaling, NFκ B signaling, and HSP90 function has also been shown to enhance, to some degree, Chk1 inhibitor–induced lethality (51, 52, 55). More recently, inhibition of SRC kinase signaling was shown to block Chk1 inhibitor–induced ERK1/2 activation and to strongly enhance Chk1 inhibitor–induced lethality in vitro and in vivo (48, 62).
Figure 3. Inhibition of CHK1 and activation of ATM and CDK1.
Activated ATM and CDK1 can promote cell killing which is suppressed by a compensatory survival signal through ERK1/2. Inhibition of this ERK1/2 survival signal shifts the balance of signaling causing high levels of tumor cell killing.
Mechanisms of Chk1 Inhibitor–induced ERK1/2 Activation and Tumor Cell Death
Data from several groups have shown that inhibition of checkpoint kinases causes a compensatory activation of ATM [e.g., (45)]. Hypothetically, the compensatory activation of ATM could reflect the enhancement of DNA damage by checkpoint inhibition and concomitant G2/M cell cycle progression, but this hypothesis is challenged by in vitro data using primary human multiple myeloma blasts, which are to all intents and purposes suspended in the G0 phase of the cell cycle. Specifically, even in these “G0-phase cells,” Chk1 inhibitors still activate ERK1/2, and MEK1/2 inhibitors still promote Chk1 inhibitor–mediated toxicity (46, 48, 49, 61). Thus, Chk1 inhibitors may invoke the activation of Cdk1 or other signaling events, rather than acceleration through the cell cycle per se, to promote the activation of ATM. Indeed, the activation of ERK1/2 in response to Cdk1 activation has been established in Xonopus oocytes [see (63)]. Nevertheless, in time course studies, Chk1 inhibitor–induced MEK1/2 phosphorylation was found to precede Cdk1 activation (i.e., dephosphorylation of tyrosine Y15), whereas ectopic expression of Cdk1 or siRNA-mediated Cdk1 knockdown did not alter Chk1 inhibitor–induced ERK1/2 activation (46, 61). Thus, inappropriate activation of Cdk1 cannot be presumed to be the direct outcome of the Chk1 inhibitor–induced activation of ERK1/2. The possibility that Chk1 has alternative targets, which influence ATM and ERK1/2 activity, has not been adequately explored. In contrast, several studies have causally linked DNA damage–induced activation of ATM to DNA damage–induced activation of ERK1/2 (64, 65). In addition, ERK1/2 activation has also been shown to reinforce further ATM activation, suggesting that there is a regulatory feed-forward loop between ATM and the ERK1/2 pathway (65).
Given that Chk1 inhibitors activate ERK1/2 rapidly and do so in a p53-independent fashion, signal transduction between ATM and ERK1/2 is unlikely to require transcription (43, 44). Otherwise, several hypothetical mechanisms by which ATM might promote the SRC-RAS-ERK1/2 pathway might be considered. For example, because Chk1 inhibitors such as UCN-01 and AZD7762 do not inhibit Chk2, Chk1 inhibitor–mediated activation of ATR could allow for activation of Chk2 (66). Chk2–dependent phosphorylation of Cdc25A, in turn, which is a protein phosphatase that is rapidly degraded upon its own phosphorylation, could hinder dephosphorylation of activated Raf-1, thereby enhancing, through RAS and SRC, the ERK1/2 pathway (67–70).
The complex regulation of Cdc25C, which is essential to cyclin B/Cdk1 activity, may also play a role in drug toxicity. Although Cdk1 activation is not a direct result of the ERK1/2 activation that arises from Chk1 and MEK1/2 inhibition, it should be recalled that a dominant–negative form of Cdk1 was found to suppress the toxicity associated with combined Chk1 and MEK1/2 inhibition (46). Inappropriate activation of cyclin-dependent kinases has been noted in multiple systems to kill transformed cells [see (71, 72)]. ERK1/2 pathway signaling promotes Cdc25C phosphorylation at multiple residues so as to increase the phosphatase activity that activates Cdk1 (73). Perhaps more importantly, Chk1-dependent phosphorylation of Cdc25C (at S216) promotes 14-3-3 protein binding and inactivates phosphatase function, thereby increasing phosphorylation of Cdk1 tyrosine residues and reducing Cdk1 activity (74). However, ERK1/2-dependent phosphorylation also causes proteolytic degradation of Cdc25A and Cdc25C, which would thereby facilitate Cdk1 phosphorylation (75). MCL-1, a substrate of Cdk1, is destabilized upon phosphorylation (76); the protective functions of BCL-2 and BCL-XL are similarly inactivated by Cdk1 (77). Accordingly, sustained activation of Cdk1 has been shown to promote apoptosis (78, 79).
In addition to altering ERK1/2 pathway activity, other MAP kinase pathways play a role in regulating Chk1 inhibitor toxicity. For example, the toxicity associated with combining Chk1 inhibitors and ERK1/2 pathway inhibitors has been linked to activation of the JNK and p38 MAPK pathways, and as JNK and p38 are also both downstream targets of ATM, it is probable that ATM signaling could be responsible for the survival and cell death signals caused by drug combination treatment (43–61).
Enhancement of Chk1 inhibitors Lethality by PARP1 Inhibitors
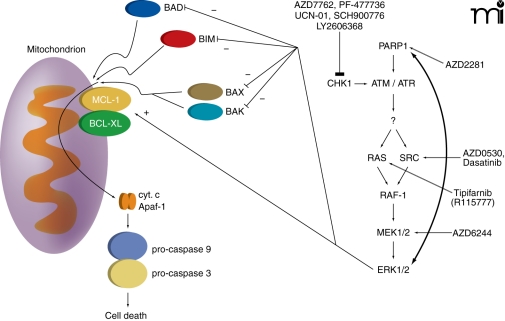
As stated earlier, DNA repair processes involve the formation of multi-protein complexes that associate with damaged DNA. The mechanisms by which DNA damage induces activation of ATM are still not fully understood, but PARP1 is clearly involved [see (7, 80)]. Upon its activation by DNA damage, PARP1 catalyzes the ADP ribosylation of multiple repair proteins (including PARP1 itself), which likely facilitates complex formation. It is of interest, based on prior statements with respect to ionizing radiation–induced ERK1/2 activation and the interconnected nature of ATM and ERK1/2 signaling, that some studies have shown radiation-induced activation of ERK1/2 is dependent on PARP1 function (81). As Chk1 inhibitor–induced activation of ERK1/2 is mediated through an ATM-dependent pathway, it could be hypothesized that inhibition of PARP1 signaling would block any form of ATM activation, and in the case of cells being treated with a Chk1 inhibitor, thereby suppressing the compensatory survival signal of ERK1/2. And indeed, multiple PARP1 inhibitors, as well as knockdown of PARP1 expression, block Chk1 inhibitor–induced activation of both ATM and ERK1/2, and PARP1 inhibitors significantly enhance Chk1 inhibitor lethality in breast cancer cells (Figure 4) (45). However, unlike studies combining Chk1 inhibitors and MEK1/2 inhibitors, where expression of an activated form of MEK1 almost abolishes killing, expression of activated AKT to a greater extent than expression of activated MEK1 is required to reduce PARP1 inhibitor and Chk1 inhibitor lethality. The subtle differences in survival signaling owing to use of MEK1/2 inhibitors versus PARP1 inhibitors in combination with Chk1 inhibitors have yet to be fully explored.
Figure 4. Multiple CHK1 inhibitors and compensatory activation pathways.
Multiple CHK1 inhibitors promote compensatory activation of the RAS-SRC / MEK1/2 / ERK1/2 pathway. The survival pathway can be inhibited at various points along its course that reveals the true toxicity of CHK1 inhibitor exposure. Killing is mediated via the intrinsic / mitochondrial cell death pathway.
Conclusions
The development of new anticancer drugs, through the clinic to ultimate FDA approval, involves not only the initial assessment of dose-limiting drug toxicity but also the identification of patient populations in which the drug, as a single agent, elicits antitumor responses. As a single agent, it would be predicted that any Chk1 inhibitor would have little to no antitumor effect; instead, Chk1 inhibitors would be expected to enhance the toxicity of drugs that cause DNA damage. Thus, “modulator” drugs such as Chk1 inhibitors are placed at a relative disadvantage in the drug development pipeline. For example, and in a similar manner to Chk1 inhibitors, Cdk inhibitory drugs, such as flavopiridol (Alvocidib), which act to suppress Cdk7/Cdk9 activity, have not shown significant anticancer effects as single agents but are showing some ability at reversing bortezomib (Velcade) resistance in blood tumor cells. In our own studies, in breast cancer tumor xenografts, a two-day exposure to either UCN-01 or a MEK1/2 inhibitor did not have any impact on tumor growth, whereas combined exposure to both drugs could be shown nearly to eliminate tumor growth (57). Collectively, these findings suggest that the “standard” model of drug development needs to be slightly modified, taking into account new advances in our understanding of how specific combinations of kinase inhibitors, although individually ineffective, can lead to high levels of tumor cell killing.
Nevertheless, despite the lack of single agent antitumor effects, the NCI has invested considerable resources into the clinical development of UCN-01 as a modulator of cell cycle checkpoints with the possible goal of enhancing the toxicity of marketed DNA-damaging anticancer drugs. Unfortunately, in the phase 1 and phase 2 studies that have been and are being performed in patients wherein Chk1 inhibitors are combined with “traditional” cytotoxic chemotherapeutic drugs, there have as yet been no widespread reports in the literature of profound enhancements in tumor control or patient survival.
One relatively unexplored component of Chk1 biology in the development and application of Chk1 inhibitors has been their effect on activation of ATM and of the ERK1/2 pathway, as discussed above. As activation of ERK1/2 is frequently associated with tumor cell survival, it may well be responsible for reducing the overall chemosensitization effect caused by checkpoint abrogation. For example, elevated levels of ERK1/2 signaling have often been associated with protection of tumor cells from the toxic actions of therapeutic drugs and ionizing radiation (82–85). Thus the rational therapeutic application of Chk1 inhibitors may require, in addition to combination with the “traditional” cytotoxic agent, the concurrent inhibition of compensatory ATM or ERK1/2 activation (e.g., through the use of PARP1 or MEK1/2 inhibitors, respectively). Such combined inhibitor therapy is under investigation, for example, in the case of EGFR and VEGFR inhibitors concurrent with gemcitabine treatment (86).
Acknowledgements
This work was funded by the Public Health Service (Grants R01-DK52825, P01-CA104177, R01-CA108325, R01-CA141703, R01-CA150214, R01-CA100866, and R01-CA93738) and by the U.S. Department of Defense (Grants DAMD17-03-1-0262 and W81XWH-10-1-0009). The work was also funded by The Jim Valvano “Jimmy V” Foundation. PD is the holder of the Universal Inc. Professorship in Signal Transduction Research; S.G. is the holder of the Olsen Distinguished Professorship; PBF holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center and is an SWCRF Investigator.
Biography
Paul Dent, PhD, is Professor and Vice Chair of Research in the Department of Neurosurgery at Virginia Commonwealth University. Email
pdent@vcu.edu; fax 804-827-1014.

Footnotes
Authorship contribution.
Wrote or contributed to the writing of the manuscript: Dent, Grant, Fisher, and Dai.
Other: Tang and Yacoub performed literature searches; Grant, Fisher, and Dai proofread the manuscript and contributed additional concepts.
References
- 1.Lonkar P, Dedon PC. (2010) Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int J Cancer, 10.1002/ijc.25815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boesch P, Weber-Lotfi F, Ibrahim N, Tarasenko V, Cosset A, Paulus F, Lightowlers RN, Dietrich A. (2011) DNA repair in organelles: Pathways, organization, regulation, relevance in disease and aging. Biochim Biophys Acta 1813:186–200 [DOI] [PubMed] [Google Scholar]
- 3.Al-Ejeh F, Kumar R, Wiegmans A, Lakhani SR, Brown MP, Khanna KK. (2010) Harnessing the complexity of DNA-damage response pathways to improve cancer treatment outcomes. Oncogene 29:6085–98 [DOI] [PubMed] [Google Scholar]
- 4.Chen F, Wang W, El-Deiry WS. (2010) Current strategies to target p53 in cancer. Biochem Pharmacol 80:724–730 [DOI] [PubMed] [Google Scholar]
- 5.Ciccia A, Elledge SJ. (2010) The DNA damage response: making it safe to play with knives. Mol Cell 40:179–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai Y, Grant S. (2010) New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res 16:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J, Tho LM, Xu N, Gillespie DA. (2010) The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res 108:73–112 [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Poon RY. (2008) The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability. Front Biosci 13:5016–5029 [DOI] [PubMed] [Google Scholar]
- 9.Bartek J, Bartkova J, Lukas J. (2007) DNA damage signaling guards against activated oncogenes and tumour progression. Oncogene 26:7773–7779 [DOI] [PubMed] [Google Scholar]
- 10.Choudhury A, Cuddihy A, Bristow RG. (2006) Radiation and new molecular agents part I: targeting ATM-ATR checkpoints, DNA repair, and the proteasome. Semin Radiat Oncol 16:51–58 [DOI] [PubMed] [Google Scholar]
- 11.Stracker TH, Usui T, Petrini JH. (2009) Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair (Amst) 8:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavecchia A, Di Giovanni C, Novellino E. (2009) CDC25A and B dual-specificity phosphatase inhibitors: potential agents for cancer therapy. Curr Med Chem 16:1831–1849 [DOI] [PubMed] [Google Scholar]
- 13.Kiyokawa H, Ray D. (2008) In vivo roles of CDC25 phosphatases: biological insight into the anti-cancer therapeutic targets. Anticancer Agents Med Chem 8:832–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aressy B, Ducommun B. (2008) Cell cycle control by the CDC25 phosphatases. Anticancer Agents Med Chem 8:818–824 [DOI] [PubMed] [Google Scholar]
- 15.Satyanarayana A, Kaldis P. (2009) Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28:2925–2939 [DOI] [PubMed] [Google Scholar]
- 16.Stark GR, Taylor WR. (2006) Control of the G2/M transition. Mol Biotechnol 32:227–248 [DOI] [PubMed] [Google Scholar]
- 17.Derheimer FA, Kastan MB. (2010) Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett 584:3675–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavin MF. (2008) Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 9:759–769 [DOI] [PubMed] [Google Scholar]
- 19.Reinhardt HC, Yaffe MB. (2009) Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol 21:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Poon RY. (2008) The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability. Front Biosci 13:5016–5029 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi I, Kobayashi E, Asano K, Yoshida M, Nakano H. (1987) UCN-01, a selective inhibitor of protein kinase C from Streptomyces. J Antibiot (Tokyo) 40:1782–1784 [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Worland PJ, Clark JL, Carlson BA, Sausville EA. (1995) Apoptosis in 7-hydroxy-staurosporine-treated T lymphoblasts correlates with activation of cyclin-dependent kinases 1 and 2. Cell Growth Differ 6:927–936 [PubMed] [Google Scholar]
- 23.Bunch RT, Eastman A. (1996) Enhancement of cisplatin-induced cytotoxicity by 7-hydroxy-staurosporine (UCN-01), a new G2-checkpoint inhibitor. Clin Cancer Res 2:791–797 [PubMed] [Google Scholar]
- 24.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O’Connor PM. (1996) UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst 88:956–965 [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Vrana JA, Bartimole TM, Freemerman AJ, Jarvis WD, Kramer LB, Krystal G, Dent P, Grant S. (1997) Agents that down-regulate or inhibit protein kinase C circumvent resistance to 1-beta-D-arabinofuranosylcytosine-induced apoptosis in human leukemia cells that overexpress Bcl-2. Mol Pharmacol 52:1000–1009 [DOI] [PubMed] [Google Scholar]
- 26.Tse AN, Schwartz GK. (2004) Potentiation of cytotoxicity of topoisomerase i poison by concurrent and sequential treatment with the checkpoint inhibitor UCN-01 involves disparate mechanisms resulting in either p53-independent clonogenic suppression or p53-dependent mitotic catastrophe. Cancer Res 64:6635–6644 [DOI] [PubMed] [Google Scholar]
- 27.Kortmansky J, Shah MA, Kaubisch A, Weyerbacher A, Yi S, Tong W, Sowers R, Gonen M, O’reilly E, Kemeny N, et al. (2005) Phase I trial of the cyclin-dependent kinase inhibitor and protein kinase C inhibitor 7-hydroxy-staurosporine in combination with Fluorouracil in patients with advanced solid tumors. J Clin Oncol 23:1875–1884 [DOI] [PubMed] [Google Scholar]
- 28.Hirose Y, Berger MS, Pieper RO. (2001) Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res 61:5843–5849 [PubMed] [Google Scholar]
- 29.Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O’Connor PM, Piwnica-Worms H. (2000) The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem 275:5600–5605 [DOI] [PubMed] [Google Scholar]
- 30.Komander D, Kular GS, Bain J, Elliott M, Alessi DR, Van Aalten DM. (2003) Structural basis for UCN-01 (7-hydroxystaurosporine) specificity and PDK1 (3-phosphoinositide-dependent protein kinase-1) inhibition. Biochem J 375:255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuse E, Hashimoto A, Sato N, Tanii H, Kuwabara T, Kobayashi S, Sugiyama Y. (2000) Physiological modeling of altered pharmacokinetics of a novel anticancer drug, UCN-01 (7-hydroxystaurosporine), caused by slow dissociation of UCN-01 from human alpha1-acid glycoprotein. Pharm Res 17:553–564 [DOI] [PubMed] [Google Scholar]
- 32.Kurata N, Imabeppu S, Nitoh M, Kobayashi H, Kuwabara T, Kobayashi S. (2000) The effect of different dosing schedules of UCN-01 on its pharmacokinetics and cardiohaemodynamics in dogs. J Pharm Pharmacol 52:1327–1335 [DOI] [PubMed] [Google Scholar]
- 33.Dees EC, Baker SD, O’Reilly S, Rudek MA, Davidson SB, Aylesworth C, Elza-Brown K, Carducci MA, Donehower RC. (2005) A phase I and pharmacokinetic study of short infusions of UCN-01 in patients with refractory solid tumors. Clin Cancer Res 11:664–671 [PubMed] [Google Scholar]
- 34.Zabludoff SD, Deng C, Grondine MR, Sheehy AM, Ashwell S, Caleb BL, Green S, Haye HR, Horn CL, Janetka JW, et al. (2008) AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol Cancer Ther 7:2955–2966 [DOI] [PubMed] [Google Scholar]
- 35.Ashwell S, Janetka JW, Zabludoff S. (2008) Keeping checkpoint kinases in line: new selective inhibitors in clinical trials. Expert Opin Investig Drugs 17:1331–1340 [DOI] [PubMed] [Google Scholar]
- 36.Daud A, Springett GM, Mendelson DS, Munster PN, Goldman JW, Strosberg JR, Kato G, Nesheiwat T, Isaacs R, Rosen LS. (2010) A phase I dose-escalation study of SCH 900776, a selective inhibitor of checkpoint kinase 1 (CHK1), in combination with gemcitabine (Gem) in subjects with advanced solid tumors. J Clin Oncol 28 (15), supp: 3064 [Google Scholar]
- 37.http://clinicaltrials.gov/ct2/show/NCT01115790.
- 38.Dent P, Curiel DT, Fisher PB, Grant S. (2009) Synergistic combinations of signaling pathway inhibitors: mechanisms for improved cancer therapy. Drug Resist Updat 12:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt-Ullrich R. (1999) Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell 10:2493–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reardon DB, Contessa JN, Mikkelsen RB, Valerie K, Amir C, Dent P, Schmidt-Ullrich RK. (1999) Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene 18:4756–4766 [DOI] [PubMed] [Google Scholar]
- 41.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. (2007) Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther 6:789–801 [DOI] [PubMed] [Google Scholar]
- 42.Grant S, Dent P. (2007) Simultaneous interruption of signal transduction and cell cycle regulatory pathways: implications for new approaches to the treatment of childhood leukemias. Curr Drug Targets 8:751–759 [DOI] [PubMed] [Google Scholar]
- 43.Dai Y, Yu C, Singh V, Tang L, Wang Z, McInistry R, Dent P, Grant S. (2001) Pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) kinase/MAPK cascade interact synergistically with UCN-01 to induce mitochondrial dysfunction and apoptosis in human leukemia cells. Cancer Res 61:5106–5115 [PubMed] [Google Scholar]
- 44.McKinstry R, Qiao L, Yacoub A, Dai Y, Decker R, Holt S, Hagan MP, Grant S, Dent P. (2002) Inhibitors of MEK1/2 interact with UCN-01 to induce apoptosis and reduce colony formation in mammary and prostate carcinoma cells. Cancer Biol Ther 1:243–253 [DOI] [PubMed] [Google Scholar]
- 45.Mitchell C, Park M, Eulitt P, Yang C, Yacoub A, Dent P. (2010) Poly (ADP-ribose) polymerase 1 modulates the lethality of CHK1 inhibitors in carcinoma cells. Mol Pharmacol 78:909–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pei XY, Li W, Dai Y, Dent P, Grant S. (2006) Dissecting the roles of checkpoint kinase 1/CDK1 and mitogen-activated protein kinase kinase 1/2/extracellular signal-regulated kinase 1/2 in relation to 7-hydroxystaurosporine-induced apoptosis in human multiple myeloma cells. Mol Pharmacol 70:1965–1973 [DOI] [PubMed] [Google Scholar]
- 47.Mogila V, Xia F, Li WX. (2006) An intrinsic cell cycle checkpoint pathway mediated by MEK and ERK in Drosophila. Dev Cell 11:575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai Y, Chen S, Shah R, Pei XY, Wang L, Almenara JA, Kramer LB, Dent P, Grant S. (2011) Disruption of Src function potentiates Chk1 inhibitor-induced apoptosis in human multiple myeloma cells in vitro and in vivo. Blood 117:1947–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai Y, Landowski TH, Rosen ST, Dent P, Grant S. (2002) Combined treatment with the checkpoint abrogator UCN-01 and MEK1/2 inhibitors potently induces apoptosis in drug-sensitive and -resistant myeloma cells through an IL-6-independent mechanism. Blood 100:3333–3343 [DOI] [PubMed] [Google Scholar]
- 50.Yu C, Dai Y, Dent P, Grant S. (2002) Coadministration of UCN-01 with MEK1/2 inhibitors potently induces apoptosis in BCR/ABL+ leukemia cells sensitive and resistant to ST1571. Cancer Biol Ther 1:674–682 [DOI] [PubMed] [Google Scholar]
- 51.Jia W, Yu C, Rahmani M, Krystal G, Sausville EA, Dent P, Grant S. (2003) Synergistic antileukemic interactions between 17-AAG and UCN-01 involve interruption of RAF/MEK- and AKT-related pathways. Blood 102:1824–1832 [DOI] [PubMed] [Google Scholar]
- 52.Dai Y, Pei XY, Rahmani M, Conrad DH, Dent P, Grant S. (2004) Interruption of the NF-kappaB pathway by Bay 11-7082 promotes UCN-01-mediated mitochondrial dysfunction and apoptosis in human multiple myeloma cells. Blood 103:2761–2770 [DOI] [PubMed] [Google Scholar]
- 53.Dai Y, Dent P, Grant S. (2003) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) promotes mitochondrial dysfunction and apoptosis induced by 7-hydroxystaurosporine and mitogen-activated protein kinase kinase inhibitors in human leukemia cells that ectopically express Bcl-2 and Bcl-xL. Mol Pharmacol 64:1402–1409 [DOI] [PubMed] [Google Scholar]
- 54.Dai Y, Rahmani M, Pei XY, Khanna P, Han SI, Mitchell C, Dent P, Grant S. (2005) Farnesyltransferase inhibitors interact synergistically with the Chk1 inhibitor UCN-01 to induce apoptosis in human leukemia cells through interruption of both Akt and MEK/ERK pathways and activation of SEK1/JNK. Blood 105:1706–1716 [DOI] [PubMed] [Google Scholar]
- 55.Hahn M, Li W, Yu C, Rahmani M, Dent P, Grant S. (2005) Rapamycin and UCN-01 synergistically induce apoptosis in human leukemia cells through a process that is regulated by the Raf-1/MEK/ ERK, Akt, and JNK signal transduction pathways. Mol Cancer Ther 4:457–470 [DOI] [PubMed] [Google Scholar]
- 56.Pei XY, Dai Y, Rahmani M, Li W, Dent P, Grant S. (2005) The farnesyltransferase inhibitor L744832 potentiates UCN-01-induced apoptosis in human multiple myeloma cells. Clin Cancer Res 11:4589–4600 [DOI] [PubMed] [Google Scholar]
- 57.Hawkins W, Mitchell C, McKinstry R, Gilfor D, Starkey J, Dai Y, Dawson K, Ramakrishnan V, Roberts JD, Yacoub A, et al. (2005) Transient exposure of mammary tumors to PD184352 and UCN-01 causes tumor cell death in vivo and prolonged suppression of tumor regrowth. Cancer Biol Ther 4:1275–1284 [DOI] [PubMed] [Google Scholar]
- 58.Dai Y, Khanna P, Chen S, Pei XY, Dent P, Grant S. (2007) Statins synergistically potentiate 7-hydroxystaurosporine (UCN-01) lethality in human leukemia and myeloma cells by disrupting Ras farnesylation and activation. Blood 109:4415–4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pei XY, Dai Y, Tenorio S, Lu J, Harada H, Dent P, Grant S. (2007) MEK1/2 inhibitors potentiate UCN-01 lethality in human multiple myeloma cells through a Bim-dependent mechanism. Blood 110:2092–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamed H, Hawkins W, Mitchell C, Gilfor D, Zhang G, Pei XY, Dai Y, Hagan MP, Roberts JD, Yacoub A, et al. (2008) Transient exposure of carcinoma cells to RAS/MEK inhibitors and UCN-01 causes cell death in vitro and in vivo. Mol Cancer Ther 7:616–629 [DOI] [PubMed] [Google Scholar]
- 61.Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, Dent P, Grant S. (2008) Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood 112:2439–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell C, Hamed HA, Cruickshanks N, Tang Y, Bareford MD, Hubbard N, Tye G, Yacoub A, Dai Y, Grant S, et al. (2011) Simultaneous exposure of transformed cells to SRC family Inhibitors and CHK1 inhibitors causes cell death. Unpublished results [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borysov SI, Guadagno TM. (2008) A novel role for Cdk1/cyclin B in regulating B-raf activation at mitosis. Mol Biol Cell 19:2907–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. (2007) Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res 67:1046–1053 [DOI] [PubMed] [Google Scholar]
- 65.Golding SE, Rosenberg E, Valerie N, Hussaini I, Frigerio M, Cockcroft XF, Chong WY, Hummersone M, Rigoreau L, Menear KA, et al. (2009) Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol Cancer Ther 8:2894–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carlessi L, Buscemi G, Larson G, Hong Z, Wu JZ, Delia D. (2007) Biochemical and cellular characterization of VRX0466617, a novel and selective inhibitor for the checkpoint kinase Chk2. Mol Cancer Ther 6:935–944 [DOI] [PubMed] [Google Scholar]
- 67.Perona R, Moncho-Amor V, Machado-Pinilla R, Belda-Iniesta C, Sánchez Pérez I. (2008) Role of CHK2 in cancer development. Clin Transl Oncol 10:538–542 [DOI] [PubMed] [Google Scholar]
- 68.Gaul L, Mandl-Weber S, Baumann P, Emmerich B, Schmidmaier R. (2008) Bendamustine induces G2 cell cycle arrest and apoptosis in myeloma cells: the role of ATM-Chk2-Cdc25A and ATM-p53-p21-pathways. J Cancer Res Clin Oncol 134:245–253 [DOI] [PubMed] [Google Scholar]
- 69.Oguri T, Lazo JS. (2004) Activation of the Raf-1/MEK/Erk kinase pathway by a novel Cdc25 inhibitor in human prostate cancer cells. Prostate 58:95–102 [DOI] [PubMed] [Google Scholar]
- 70.Chow JP, Siu WY, Fung TK, Chan WM, Lau A, Arooz T, Ng CP, Yamashita K, Poon RY. (2003) DNA damage during the spindle-assembly checkpoint degrades CDC25A, inhibits cyclin-CDK1 complexes, and reverses cells to interphase. Mol Biol Cell 14:3989–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donaldson KL, Goolsby GL, Kiener PA, Wahl AF. (1994) Activation of p34cdk1 coincident with taxol-induced apoptosis. Cell Growth Differ 5:1041–1050 [PubMed] [Google Scholar]
- 72.Martin SJ, McGahon AJ, Nishioka WK, LaFace D, Guo X, Th’ng J, Bradbury EM, Green DR. (1995) p34cdk1 and apoptosis. Science 269:106–107 [DOI] [PubMed] [Google Scholar]
- 73.Wang R, Jung SY, Wu CF, Qin J, Kobayashi R, Gallick GE, Kuang J. (2010) Direct roles of the signaling kinase RSK2 in Cdc25C activation during Xenopus oocyte maturation. Proc Natl Acad Sci USA 107:19885–19890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma CX, Janetka JW, Piwnica-Worms H. (2011) Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med (2): 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Isoda M, Kanemori Y, Nakajo N, Uchida S, Yamashita K, Ueno H, Sagata N. (2009) The extracellular signal-regulated kinase-mitogen-activated protein kinase pathway phosphorylates and targets Cdc25A for SCF beta-TrCP-dependent degradation for cell cycle arrest. Mol Biol Cell 20:2186–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harley ME, Allan LA, Sanderson HS, Clarke PR. (2010) Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdk10-dependent destruction during mitotic arrest. EMBO J 29:2407–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terrano DT, Upreti M, Chambers TC. (2010) Cyclin-dependent kinase 1-mediated Bcl-xL/Bcl-2 phosphorylation acts as a functional link coupling mitotic arrest and apoptosis. Mol Cell Biol 30:640–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng P, Li Y, Yang L, Wen Y, Shi W, Mao Y, Chen P, Lv H, Tang Q, Wei Y. (2009) Hepatitis B virus X protein (HBx) induces G2/M arrest and apoptosis through sustained activation of cyclin B1-CDK1 kinase. Oncol Rep 22:1101–1107 [DOI] [PubMed] [Google Scholar]
- 79.Konishi Y, Bonni A. (2003) The E2F-Cdk1 cell-cycle pathway specifically mediates activity deprivation-induced apoptosis of postmitotic neurons. J Neurosci 23:1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huber A, Bai P, de Murcia JM, de Murcia G. (2004) PARP-1, PARP-2 and ATM in the DNA damage response: functional synergy in mouse development. DNA Repair (Amst) 3:1103–1108 [DOI] [PubMed] [Google Scholar]
- 81.Hagan MP, Yacoub A, Dent P. (2007) Radiation-induced PARP activation is enhanced through EGFR-ERK signaling. J Cell Biochem 101:1384–1393 [DOI] [PubMed] [Google Scholar]
- 82.Javvadi P, Hertan L, Kosoff R, Datta T, Kolev J, Mick R, Tuttle SW, Koumenis C. (2010) Thioredoxin reductase-1 mediates curcumin-induced radiosensitization of squamous carcinoma cells. Cancer Res 70:1941–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shukla A, Hillegass JM, Macpherson MB, Beuschel SL, Vacek PM, Pass HI, Carbone M, Testa JR, Mossman BT. (2010) Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol Cancer 9:314–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yacoub A, Gilfor D, Hawkins W, Park MA, Hanna D, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. (2006) MEK1/2 inhibition promotes Taxotere lethality in mammary tumors in vivo. Cancer Biol Ther 5:1332–1339 [DOI] [PubMed] [Google Scholar]
- 85.Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK. (2002) Gamma-synuclein promotes cancer cell survival and inhibits stress- and chemotherapy drug-induced apoptosis by modulating MAPK pathways. J Biol Chem 277:35050–35060 [DOI] [PubMed] [Google Scholar]
- 86.Burris H, 3rd, Rocha-Lima C. (2008) New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist 13:289–298 [DOI] [PubMed] [Google Scholar]