Abstract
The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is an immediate-early gene that has been widely implicated in synaptic plasticity and in the consolidation of a variety of hippocampal- and amygdala-dependent memory tasks. The functional role of Arc/Arg3.1 in memory reconsolidation processes, however, has not been systematically studied. In the present study, we examined the role of Arc/Arg3.1 in the reconsolidation of an amygdala-dependent auditory pavlovian fear memory. We show that Arc/Arg3.1 protein is regulated in the lateral nucleus of the amygdala (LA) by retrieval of an auditory fear memory. Next, we show that antisense knockdown of Arc/Arg3.1 in the LA impairs fear memory reconsolidation of both a recent (1-d-old) as well as a well-consolidated (2-week-old) fear memory; that is, post-retrieval short-term memory, tested at 3 h after retrieval, is intact, whereas post-retrieval long-term memory, tested ∼24 h after retrieval, is significantly impaired. The effect of Arc/Arg3.1 knockdown was observed to be time limited and specific to an actively reactivated fear memory. Moreover, the reconsolidation deficit induced by Arc/Arg3.1 knockdown was not found to be sensitive to spontaneous recovery, reinstatement, or a shift in the testing context, suggesting that our behavioral effects are not attributable to facilitated extinction. Collectively, our findings provide the first comprehensive look at the functional role of Arc/Arg3.1 in memory reconsolidation processes in the mammalian brain.
Introduction
Considerable progress has been made at defining the cellular and molecular mechanisms underlying memory “reconsolidation” in the mammalian brain (Dudai and Eisenberg, 2004; Tronson and Taylor, 2007). With notable exceptions (Alberini, 2005), findings suggest that reconsolidation shares many of the core molecular features with that of initial memory consolidation, including NMDA-receptor (NMDAR)-driven activation of protein kinase signaling cascades (Duvarci et al., 2005; Ben Mamou et al., 2006; Tronson et al., 2006), the involvement of transcription factors (Kida et al., 2002), and de novo mRNA and protein synthesis (Nader et al., 2000; Da Silva et al., 2008; Duvarci et al., 2008).
Although the importance of transcription and translation in memory reconsolidation has been well established (Nader et al., 2000; Kida et al., 2002; Da Silva et al., 2008; Duvarci et al., 2008) (but see Parsons et al., 2006), relatively little is known about the downstream genes that are critical for the reconsolidation process. The activity-regulated cytoskeletal associated protein (Arc/Arg3.1) is an effector immediate-early gene (IEG) that has been widely implicated in experience-dependent synaptic plasticity and memory formation (Lyford et al., 1995; Steward et al., 1998; Guzowski et al., 2000; Plath et al., 2006; Ploski et al., 2008). Global deletion of Arc/Arg3.1 has been shown to impair the consolidation of a variety of hippocampal- and amygdala-dependent memory tasks, including spatial learning, object recognition, contextual and auditory fear conditioning, and conditioned taste aversion (Plath et al., 2006). Furthermore, local knockdown of Arc/Arg3.1 protein within the hippocampus or amygdala using antisense oligodeoxynucleotides (ODNs) selectively impairs the consolidation of spatial learning and auditory fear conditioning, respectively (Guzowski et al., 2000; Ploski et al., 2008).
Although the role of Arc/Arg3.1 has been extensively studied in the acquisition and consolidation phases of a variety of memory tasks, little is known about the role of Arc/Arg3.1 in memory reconsolidation processes. Although several studies have used Arc/Arg3.1 expression as a neuronal marker to examine the brain regions necessary for memory retrieval (Guzowski et al., 2001; Gusev et al., 2005; Zhang et al., 2005), none of these has asked whether Arc/Arg3.1 is functionally involved in memory reconsolidation.
In the present study, we have examined the role of Arc/Arg3.1 in the reconsolidation of a Pavlovian fear memory. We first examine the regulation of Arc/Arg3.1 protein within the lateral nucleus of the amygdala (LA) after auditory fear memory retrieval. Next, we use local infusions of an Arc/Arg3.1 ODN to examine the functional role of Arc/Arg3.1 in auditory fear memory reconsolidation in the LA.
Materials and Methods
Subjects.
Adult male Sprague Dawley rats (Harlan), weighing 300–350 g and aged 2–3 months, were housed individually in plastic cages and maintained on a 12 h light/dark cycle with food and water provided ad libitum.
Surgery.
Rats were anesthetized with intraperitoneal administration of ketamine (100 mg/kg) and xylazine (6.0 mg/kg) and implanted with 26-gauge stainless-steel guide cannulas (Plastics One) in the LA (−3.2 mm, ±5.2 mm, −8.0 mm relative to bregma). Guide cannulas were secured to screws in the skull using a mixture of dental acrylic and cement, and 31-gauge dummy cannulas were inserted into the guide to prevent obstruction. Buprenex (0.2 mg/kg) was administered as an analgesic, and rats were provided with at least 5 d postoperative recovery time. All surgical procedures were conducted under the guidelines provided in the National Institutes of Health Guide for the Care and Use of Experimental Rats and were approved by the Yale University Institutional Animal Care and Use Committee.
Western blotting experiments.
For Western blotting experiments examining Arc/Arg3.1 expression after auditory fear memory retrieval, rats were habituated to handling and to both conditioning and testing chambers (30 min/d per chamber) for 4 d before training to limit the ability of handling stress or exposure to the testing chamber alone to drive Arc/Arg3.1 expression in the LA (Ploski et al., 2010). On the conditioning day, rats received either two tone–shock pairings (30 s, 5 kHz, 75 dB; 1.0 mA) or two presentations of a tone without shock. The conditioning chamber (chamber A) was a lit chamber with a grid floor, whereas the testing chamber (chamber B) was dark and contained a black plastic floor that had been washed with a distinctive peppermint soap. Twenty-four hours after conditioning, rats in the “reactivation” and “tone-alone” groups were placed in chamber B and presented with a single tone conditioned stimulus (CS) (30 s, 5 kHz, 75 dB). Rats in the “no-reactivation” group were placed in chamber B for the same amount of time as those in the “reactivation” group but were not presented with a reactivation trial. Two hours after the reactivation (or no-reactivation session), rats in both experiments were rapidly and deeply anesthetized with chloral hydrate (600 mg/kg, i.p.), and brains were removed and frozen at −80°C until processed. “Naive” rats were handled but not exposed to either the conditioning or testing chambers before being killed.
Punches containing the LA were obtained with a 1 mm punch tool (Fine Science Tools) from 400-μm-thick sections taken on a sliding freezing microtome. Punched slices were examined using low-power light microscopy to verify the accuracy of the LA punch. Only those rats with punches confined to the borders of the LA were included in the analysis. Punches were manually dounced in 100 μl of ice-cold hypotonic lysis buffer [10 mm Tris-HCl, pH 7.5, 1 mm EDTA, 2.5 mm sodium pyrophosphate, 1 mm phenylmethylsulfonyl fluoride, 1 mm β-glycerophosphate, 1% Igepal CA-630, 1% protease inhibitor cocktail (Sigma), and 1 mm sodium orthovanadate]. Sample buffer was immediately added to the homogenates, and the samples were boiled for 4 min. Homogenates were electrophoresed on 10% Tris-HCl gels and blotted to Immobilon-P (Millipore). Western blots were then blocked in TTBS buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 0.05% Tween 20) with 5% dry milk and then incubated with anti-Arc antibody (1:1000; catalog #SC17839; Santa Cruz Biotechnology). Blots were then incubated with anti-mouse antibody conjugated to horseradish peroxidase (Cell Signaling Technology) and developed using West Dura chemiluminescent substrate (Pierce). Western blots were developed in the linear range used for densitometry. Densitometry was conducted using NIH Image J software. To control for inconsistencies in loading, optical densities were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein (1:20,000; catalog #ab9484; Abcam). Data were normalized to the average value of naive controls and analyzed using ANOVA.
Immunohistochemical experiments.
Immunohistochemistry experiments examining Arc/Arg3.1 expression after auditory fear memory retrieval used procedures identical to those used in the Western blotting experiments; however, 2 h after the reactivation (or no-reactivation session), rats were rapidly and deeply anesthetized with chloral hydrate (600 mg/kg, i.p.) and transcardially perfused with PBS, followed by ice-cold 4% paraformaldehyde in 0.1 m phosphate buffer (PB). Naive rats were handled but not exposed to either the conditioning or testing chambers before being killed.
Brains were removed and postfixed in 4% paraformaldehyde–PB for 12 h and then cryoprotected in 20% glycerol–0.1 m PB for 48–72 h. Free-floating sections (40 μm) containing the LA were cut using a sliding microtome and collected in PBS containing 0.1% sodium azide for storage. Every sixth section was processed for Arc/Arg3.1 immunoreactivity. After extensive washing, sections were blocked in PBS consisting of 1% bovine serum albumin (BSA) (Fraction V; catalog #A-3059; Sigma) and 0.1% Triton X-100. Slices were then incubated overnight at room temperature in anti-Arc antibody (1:500; mouse monoclonal; catalog #SC17839; Santa Cruz Biotechnology) in PBS, 1% BSA, and 0.1% Triton X-100. After three washes in PBS, tissue sections were visualized using VectaStain ABC kit (Vector Laboratories) and developed in DAB peroxidase substrate (Sigma) for 5 min. Sections were mounted on Fisherbrand electrostatic slides and coverslipped.
Sections from comparable anteroposterior levels were selected for scoring, ∼3.2–3.4 mm posterior to bregma. At this level, the LA, the central nucleus (CE), and basal (B) nuclei are all well represented. Cell counts were taken from at least five sections per rat and scored using NIH Image J. For analysis, cell counts for each region were averaged into a single score for each rat, and data were analyzed using ANOVA.
Oligodeoxynucleotide design and preparation.
Arc/Arg3.1 antisense and scrambled ODN (Midland Certified Reagent Company) design followed the guidelines used in a previous study (Guzowski et al., 2000). The Arc ODN encoded an antisense sequence for the Arc/Arg3.1 mRNA sequence near the translation start site (Lyford et al., 1995). The scrambled ODN served as a control and did not show significant homology to sequences in the GenBank database. Both ODNs contained phosphorothioate linkages on the bases of both the 5′ and 3′ ends and phosphodiester internal bonds, because this nucleotide design is reportedly more stable than unmodified phosphodiester ODNs in vivo and less toxic than fully phosphorotioate ODNs (Guzowski et al., 2000; Guzowski, 2002). The following sequences were used (∼ denotes a phosphorothioate linkage): 5′-G∼T∼C∼CAGCTCCATCTGCT∼C∼G∼C-3′ (antisense) and 5′- C∼G∼T∼GCACCTCTCGCAGC∼T∼T∼C-3′ (scrambled). This antisense sequence has been shown previously to effectively knock down Arc/Arg3.1 protein expression in the amygdala (Ploski et al., 2008).
Behavioral procedures.
Rats were handled for 2 d before conditioning. On the second handling day, dummy cannulas were removed and infusion cannulas were briefly inserted. Rats were then habituated to the conditioning chamber (chamber A) for 15 min (day 1). The following day (day 2), rats were placed in chamber A and exposed to two tone–shock pairings consisting of a 30 s, 5 kHz, 75 dB tone that coterminated with a 1 s, 2.0 mA footshock. The next day (day 3), rats received intra-LA infusion of either Arc/Arg3.1 antisense or scrambled ODNs (200 pmol; 1 μl/side). Infusions were made over 4 min, and the infusion cannulas were left in place for at least 2 min after infusion to facilitate diffusion of the ODN throughout the LA. Ninety minutes after infusion, rats were placed in the testing chamber (chamber B) and received a single presentation of a tone CS (30 s, 5 kHz, 75 dB) to serve as a memory reactivation trial. Three hours after reactivation, rats were returned to chamber B and tested for post-reactivation short-term memory (PR-STM) consisting of presentation of three tones (30 s, 5 kHz, 75 dB). Twenty-four hours later (day 4), all rats were retuned to chamber B and received a post-reactivation long-term memory (PR-LTM) test that consisted of 10 tone presentations (30 s, 5 kHz, 75 dB). Rats used to examine the effect of Arc/Arg3.1 knockdown on the reconsolidation of a “well-consolidated” fear memory were trained and tested under identical parameters, with the exception that they were returned to their home cage for 2 weeks after conditioning before ODN infusions, reactivation, and the subsequent PR-STM and PR-LTM tests.
An additional behavioral experiment examined whether the reconsolidation deficit induced by Arc/Arg3.1 knockdown in the LA was sensitive to spontaneous recovery, reinstatement, or a shift in the testing context. Rats in this experiment were trained in chamber A and reactivated 24 h later in chamber B as described above. Three and 24 h after reactivation, rats were returned to chamber B and tested for PR-STM and PR-LTM, respectively, as described above. One week after the initial PR-LTM test, rats were returned to chamber B and tested for spontaneous recovery with five tone presentations. The next day, they were placed in a novel context (chamber C), scented with cedar and brightly illuminated, and given a reinstatement session consisting of two unsignaled footshocks (1 s, 2.0 mA). Twenty-four hours later, all rats were returned to chamber B and tested for reinstatement with five tone presentations. The next day, rats were introduced to a final novel context (chamber D), consisting of a lit behavior box with a scented cotton-padded floor and tested with three tone presentations to examine the context generality of the reconsolidation deficit.
All behavioral testing was videotaped for subsequent scoring. Freezing was defined as a lack of movement, excluding that necessary for respiration, and was quantified as a percentage of the amount of time the rat spent engaged in freezing behavior during the CS presentations. All data were analyzed using ANOVA and Duncan's post hoc t tests. Repeated-measures ANOVAs were used for multiple trial comparisons. Differences were considered significant if p < 0.05. Only data from those rats with bilateral LA placed cannulas were included in the subsequent analyses.
Results
Arc/Arg3.1 is significantly regulated in the LA after auditory fear memory retrieval
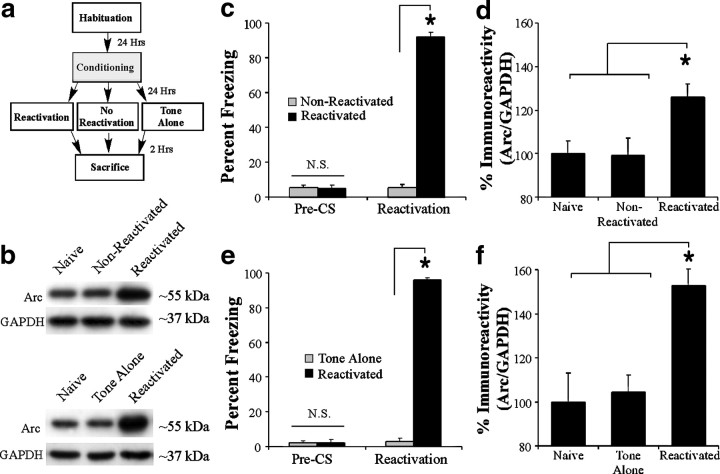
In our first series of experiments, we used a combination of Western blotting and immunohistochemistry to ask whether Arc/Arg3.1 is regulated by retrieval of an auditory fear memory. In the first experiment, rats underwent auditory fear conditioning in chamber A, followed 24 h later by exposure to either a memory reactivation trial (reactivated) or to a no-reactivation session in which they were placed in the a distinct chamber (chamber B) but not presented with a tone (non-reactivated) (Fig. 1a). A third group of rats was handled and habituated but did not undergo conditioning or memory reactivation (naive) (Fig. 1a). Analysis of the behavioral data revealed no difference in pre-CS freezing between the non-reactivated and reactivated groups (t(11) = 0.01). During the reactivation trial, only the reactivated group displayed increased freezing during the CS presentation, suggesting significant memory retrieval (t(11) = 20.59, p < 0.01) (Fig. 1c). Western blotting revealed a significant regulation of Arc/Arg3.1 protein within the LA (F(2,16) = 5.11, p < 0.05) (Fig. 1d). Duncan's post hoc tests revealed a significant difference between the reactivated group and the non-reactivated and naive groups (p < 0.05), whereas no significant difference between naive and non-reactivated groups was observed (p > 0.05). No difference was observed between the levels of the loading control GAPDH (F(2,16) = 0.13) (data not shown). Representative blots can be viewed in Figure 1b (top).
Figure 1.
Western blot analysis of Arc/Arg3.1 expression in the LA after fear memory retrieval. a, Schematic of the behavioral protocol. Rats underwent auditory fear conditioning, followed 24 h later by either exposure to a memory reactivation trial (Reactivation) or to no reactivation in which they were placed in a distinct chamber but not presented with a tone (No Reactivation). In a second experiment, rats received either two tone–shock pairings (Reactivated) or two presentations of the tone alone (Tone Alone) during training, followed by a memory reactivation trial 24 h later. b, Representative Western blots for each experiment. c, Memory reactivation scores for the non-reactivated and reactivated groups. d, Western blot analysis of Arc/Arg3.1 protein in the LA of reactivated (n = 6), non-reactivated (n = 6), and naive (n = 7) groups after fear memory retrieval. e, Memory reactivation scores for the tone-alone and reactivated groups. f, Western blot analysis of Arc/Arg3.1 protein in the LA of reactivated (n = 7), tone-alone (n = 7), and naive (n = 6) groups after fear memory retrieval. *p < 0.05 relative to naive and non-reactivated groups.
In a second experiment, we asked whether the regulation of Arc/Arg3.1 in the LA is specific to fear memory retrieval rather than to exposure to tone alone. On the conditioning day, rats received either two tone-shock pairings (reactivated) or two presentations of the tone alone in chamber A. The next day, rats in both groups were placed into chamber B and given a single tone presentation (Fig. 1a). As before, a third group of rats was handled and habituated but did not undergo conditioning or memory reactivation (naive). Analysis of the behavioral data revealed no difference in pre-CS freezing between the tone alone and reactivated groups (t(12) = 0.53). During the reactivation trial, only the reactivated group displayed increased freezing during the CS presentation (t(12) = 49.23, p < 0.01) (Fig. 1e). Western blotting revealed a significant regulation of Arc/Arg3.1 protein within the LA (F(2,17) = 9.87, p < 0.05) (Fig. 1f). Duncan's post hoc tests revealed a significant difference between the reactivated group and the tone-alone and naive groups (p < 0.05), whereas no significant difference between naive and tone-alone groups was observed (p > 0.05). No difference was observed between the levels of the loading control GAPDH (F(2,17) = 0.29) (data not shown). Representative blots can be viewed in Figure 1b (bottom).
Next, we used immunohistochemistry to examine the anatomical localization of Arc/Arg3.1 regulation in the amygdala after auditory fear memory retrieval. As in our previous experiments, rats underwent auditory fear conditioning in chamber A, followed 24 h later by exposure to either a memory reactivation trial (reactivated) or to a no-reactivation session in which they were placed in chamber B but not presented with a tone (non-reactivated) (Fig. 2a). Analysis of the behavioral data revealed no difference in pre-CS freezing between the non-reactivated and reactivated groups (t(10) = 0.48). During the reactivation trial, only the reactivated group displayed increased freezing during the CS presentation (t(10) = 37.59, p < 0.01) (Fig. 2b). Analysis of the immunohistochemistry revealed a high level of Arc/Arg3.1-labeled cells in the reactivated group relative to both the naive and non-reactivated groups (Fig. 2h). Specifically, a main effect of group was observed in the dorsolateral LA (LAd) (F(2,11) = 43.14, p < 0.01) and the ventrolateral LA (LAv) (F(2,11) = 32.86, p < 0.01) but not in the basal nucleus (F(2,11) = 3.48) or the CE (F(2,11) = 0.53) of the amygdala. Duncan's post hoc tests revealed a significant increase in Arc/Arg3.1-labeled cells in the reactivated group within the LAd and LAv (p < 0.05), whereas no significant differences were observed between naive and non-reactivated groups (p > 0.05). Representative photomicrographs for reactivated, non-reactivated, and naive rats are displayed in Figure 2c–e, whereas higher-magnification photomicrographs of a reactivated rat are displayed in Figure 2, f and g.
Figure 2.
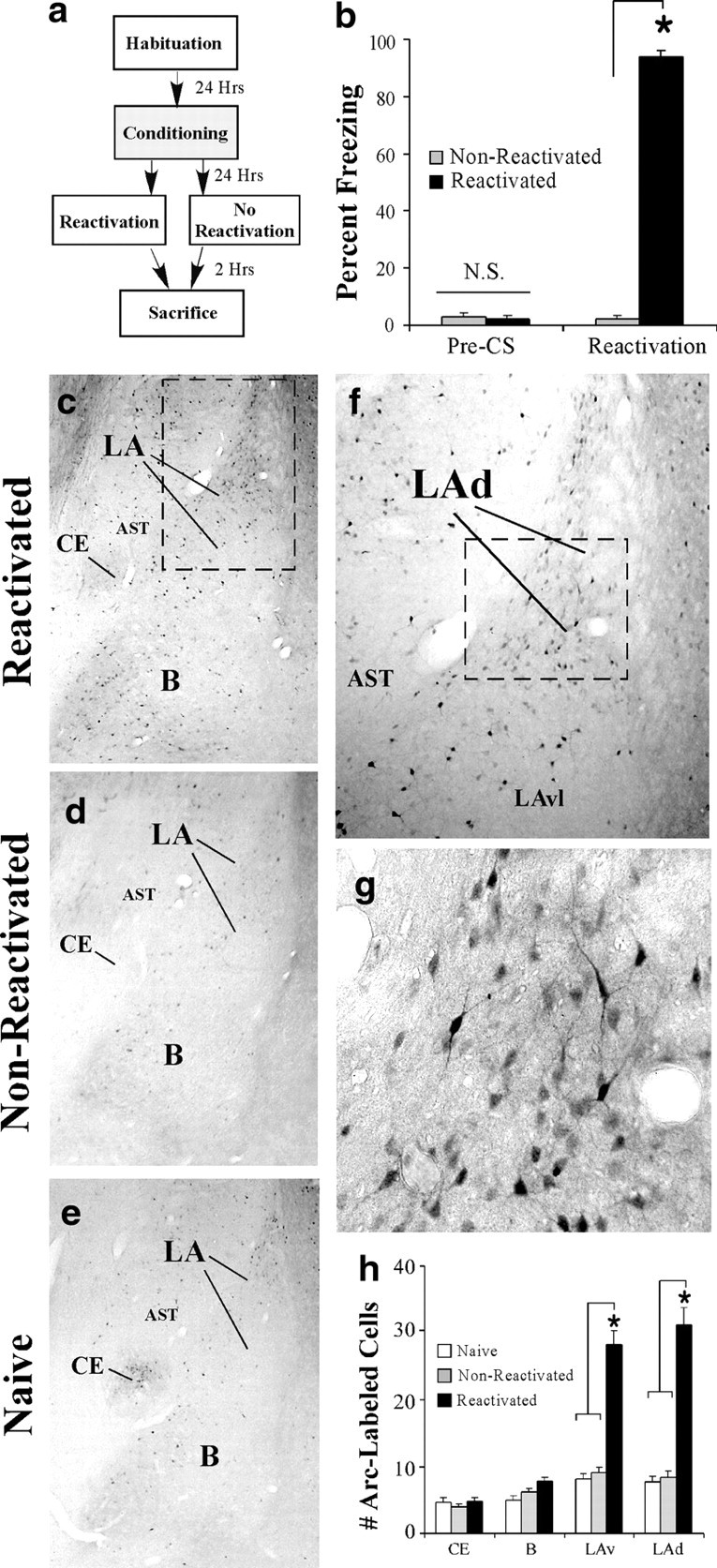
Immunohistochemical analysis of Arc/Arg3.1 expression in the amygdala after fear memory retrieval. a, Schematic of the behavioral protocol. Rats underwent auditory fear conditioning, followed 24 h later by exposure to either a memory reactivation trial (Reactivation) or to no reactivation in which they were placed in a distinct chamber but not presented with a tone (No Reactivation). b, Memory reactivation scores for the non-reactivated and reactivated groups. c–e, Representative 10× photomicrographs of immunolabeled Arc/Arg3.1 cells in a reactivated, non-reactivated, and naive rat, respectively. f, g, Higher-level (20× and 40×, respectively) magnifications of Arc/Arg3.1-labeled cells from the reactivated rat. The box in f represents the area outlined in c, whereas g represents the area outlined in f. h, Quantification of Arc/Arg3.1-labeled cells in the CE, B, LAv, and LAd of naive (n = 4), non-reactivated (n = 5), and reactivated (n = 5) groups after fear memory retrieval. *p < 0.05 relative to naive and non-reactivated groups. AST, Amygdala–striatal transition zone.
Arc/Arg3.1 knockdown in the LA impairs fear memory reconsolidation
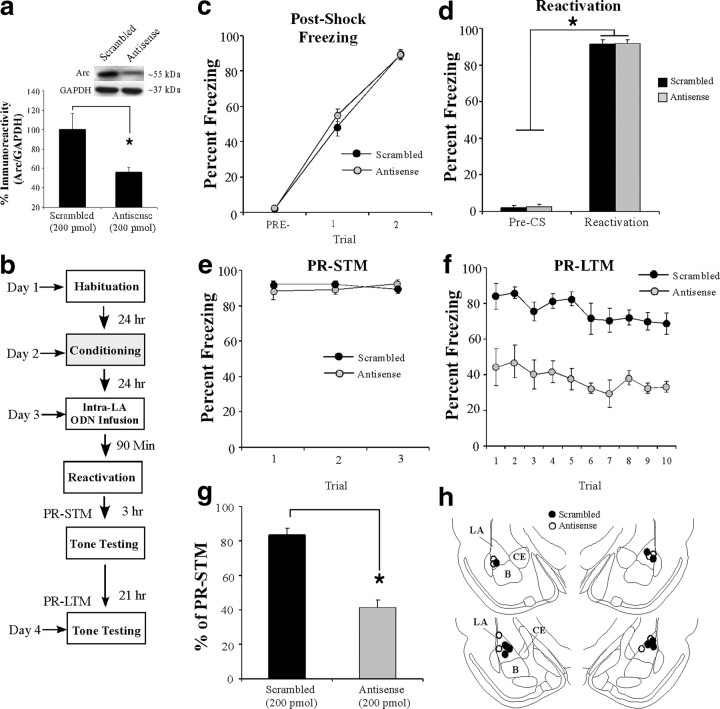
Our initial series of experiments showed that Arc/Arg3.1 protein is regulated in the LA by auditory fear memory retrieval. In this next series of experiments, we asked whether Arc/Arg3.1 is obligatory for fear memory reconsolidation using localized antisense ODN knockdown of Arc/Arg3.1 protein in the LA. In our first experiment, we verified the efficacy of the Arc/Arg3.1 antisense ODN in reducing expression of Arc/Arg3.1 protein in the LA after auditory fear memory reactivation. Rats received two tone–shock pairings in chamber A consisting of a 30 s, 5 kHz, 75 dB tone that coterminated with a 1 s, 2.0 mA footshock. The next day, rats received intra-LA infusion of an Arc/Arg3.1 antisense ODN (200 pmol; 1 μl) on one side of the brain and a scrambled ODN (200 pmol; 1 μl) on the contralateral side. Ninety minutes later, rats were exposed to a fear memory reactivation trial in chamber B consisting of presentation of a single tone CS (30 s, 5 kHz, 75 dB) and were killed 2 h later. Western blot analysis revealed a significant knockdown of Arc/Arg3.1 protein on the antisense-infused side of the brain compared with the scrambled-infused side (t(6) = 3.21, p < 0.05) (Fig. 3a). No significant difference was observed between the levels of the loading control GAPDH (t(6) = 1.65) (data not shown).
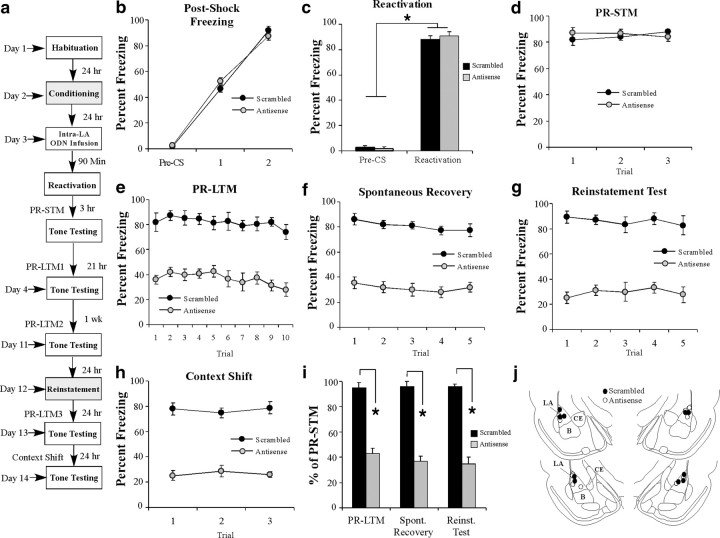
Figure 3.
Antisense knockdown of Arc/Arg3.1 protein in the LA impairs auditory fear memory reconsolidation. a, Western blot analysis of Arc/Arg3.1 protein from LA homogenates from rats given intra-LA infusion of antisense and scrambled ODNs (200 pmol; 1 μl) on opposite sides of the brain, reactivated, and killed 2 h later (n = 7). *p < 0.05 relative to the scrambled ODN-infused side. b, Schematic of the behavioral protocol. c, Postshock freezing scores in Arc/Arg3.1 ODN (n = 4) and scrambled ODN (n = 5) groups immediately after the conditioning trials. d, Freezing scores in each group during the memory reactivation trial. e, PR-STM assessed at 3 h after memory reactivation in each group. f, PR-LTM assessed 24 h after reactivation in each group. g, PR-LTM depicted as a percentage of PR-STM for each rat in each group. *p < 0.01 relative to the scrambled group. h, Histological verification of cannula placements for rats infused with Arc/Arg3.1 antisense (white circles) or scrambled (black circles) ODNs. Panels adapted from Paxinos and Watson (1998).
To examine the functional role of Arc/Arg3.1 in auditory fear memory reconsolidation, rats were trained with two tone–shock pairings in chamber A, followed 24 h later by intra-LA infusion of either Arc/Arg3.1 antisense or scrambled ODNs (200 pmol; 1 μl). Ninety minutes later, rats received a reactivation trial in chamber B (Fig. 3b). There was no difference in levels of postshock freezing between the scrambled and antisense groups (Fig. 3c). The ANOVA revealed only a significant main effect of trial (F(2,14) = 485.60, p < 0.01); there was no significant main effect of group (F(1,7) = 0.40) or group × trial interaction (F(2,14) = 0.91). Furthermore, both groups showed equivalent levels of freezing during the pre-CS period and the tone–CS presentation during the reactivation trial (Fig. 3d). An ANOVA (group × trial) revealed no significant effect of group (F(1,7) = 0.058) or group × trial interaction (F(1,7) = 0.01); however, there was a significant main effect of trial (F(1,7) = 1619.13, p < 0.01), indicating that there was an increase in freezing to the tone CS relative to the pre-CS period in both groups. Three hours after tone memory reactivation, rats were given a PR-STM test (Fig. 3e). The ANOVA (group × trial) revealed no significant effect of group (F(1,7) = 0.11), trial (F(2,14) = 0.08), or group × trial interaction (F(2,14) = 2.19).
On the following day, rats were given a PR-LTM test in chamber B, and the group infused with the Arc/Arg3.1 antisense ODN exhibited impaired PR-LTM (Fig. 3f). The ANOVA (group × trial) revealed significant main effects of group (F(1,7) = 42.22, p < 0.01) and trial (F(9,63) = 3.26, p < 0.01) but no significant group × trial interaction (F(9,63) = 0.25). As another measure of the reconsolidation deficit observed in the Arc/Arg3.1 antisense ODN group, each rat's freezing score during the PR-LTM test was expressed as a percentage of that exhibited during the PR-STM test (Fig. 3g). The Arc/Arg3.1 antisense ODN group exhibited significantly less retention during the PR-LTM test than the scrambled group (t(7) = 55.3, p < 0.01). Cannula placements are shown in Figure 3h.
The effect of Arc/Arg3.1 knockdown on reconsolidation of an auditory fear memory is specific to an actively reactivated memory
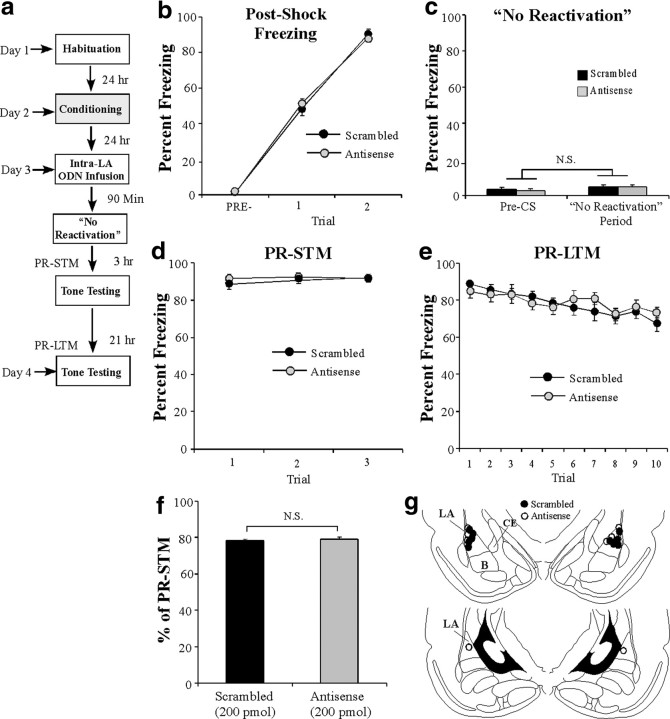
We next examined the effect of Arc/Arg3.1 knockdown on memory reconsolidation in the absence of fear memory reactivation. Rats were trained with two tone–shock pairings in chamber A, followed 24 h later by intra-LA infusion of either Arc/Arg3.1 antisense or scrambled ODNs (200 pmol; 1 μl). Ninety minutes later, rats were placed in chamber B for the same amount of time as those that received tone reactivation in the previous experiment but were not presented with a tone (Fig. 4a). Both groups showed similar levels of postshock freezing on the training day (Fig. 4b). The ANOVA revealed a significant main effect of trial (F(2,20) = 833.76, p < 0.01), a nonsignificant main effect of group (F(1,10) = 0.19), and a nonsignificant group × trial interaction (F(2,20) = 1.22). On the next day, both groups showed equivalent levels of freezing during the pre-CS period and during the 30 s when the tone would have been presented during the reactivation trial (Fig. 4c). An ANOVA (group × trial) revealed no significant effect of group (F(1,10) = 0.46), trial (F(1,10) = 0.62), or group × trial interaction (F(1,10) = 0.07). In addition, both groups exhibited intact memory during the PR-STM and PR-LTM tests (Fig. 4d,e). The ANOVA (group × trial) for PR-STM revealed nonsignificant effects of group (F(1,10) = 0.03), trial (F(2,20) = 1.97), and the group × trial interaction (F(2,20) = 3.18). The ANOVA (group × trial) for PR-LTM revealed a nonsignificant main effect of group (F(1,10) = 0.01); however, there was a significant main effect of trial (F(9,90) = 12.99, p < 0.01) and group × trial interaction (F(9,90) = 2.74, p < 0.05). Furthermore, no significant difference in retention was observed between the two groups when PR-LTM was expressed as a percentage of PR-STM (t(10) = 0.06) (Fig. 4f). Cannula placements can be viewed in Figure 4g.
Figure 4.
The effect of Arc/Arg3.1 knockdown on auditory fear memory reconsolidation is specific to reactivated memories. a, Schematic of the behavioral protocol. b, Postshock freezing scores in Arc/Arg3.1 ODN (n = 6) and scrambled ODN (n = 6) groups immediately after the conditioning trials. c, Freezing scores in each group during the no-reactivation trial. d, PR-STM assessed at 3 h after the no-reactivation trial in each group. e, PR-LTM assessed 24 h after the no-reactivation trial in each group. f, PR-LTM depicted as a percentage of PR-STM for each rat in each group. g, Histological verification of cannula placements for rats infused with Arc/Arg3.1 antisense (white circles) or scrambled (black circles) ODNs. Panels adapted from Paxinos and Watson (1998).
The effect of Arc knockdown in the LA on auditory fear memory reconsolidation is time limited
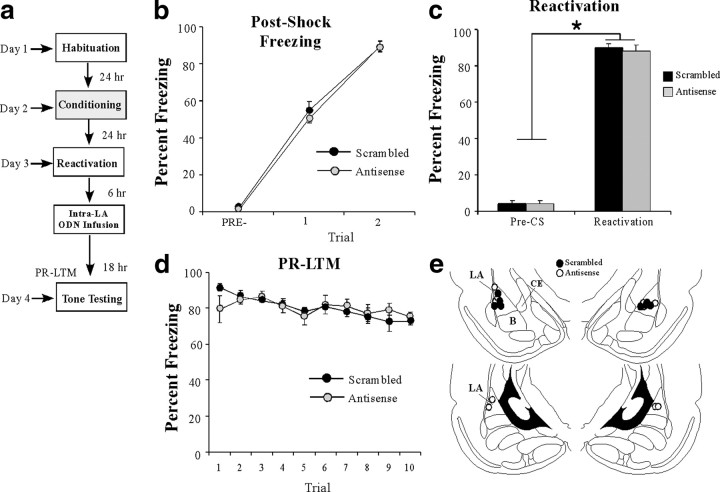
In our next experiment, we asked whether the effect of Arc/Arg3.1 knockdown on memory reconsolidation has temporal constraints. Rats were trained with two tone–shock pairings in chamber A, followed 24 h later by a tone-reactivation trial in chamber B. Six hours after reactivation, rats received intra-LA infusion of either Arc/Arg3.1 antisense or scrambled ODNs (200 pmol; 1 μl), followed by a PR-LTM test 18 h later (Fig. 5a). There was no significant difference between the scrambled and antisense groups in the level of postshock freezing after training (Fig. 5b). The ANOVA revealed a main effect of trial (F(2,14) = 539.93, p < 0.01) but no significant main effect of group (F(1,7) = 0.51) or the group × trial interaction (F(2,14) = 0.376). On the next day, both groups exhibited equivalent levels of memory reactivation (Fig. 5c). An ANOVA (group × trial) revealed no significant effect of group (F(1,7) = 0.329) or group × trial interaction (F(1,7) = 0.14); however, there was a significant main effect of trial (F(1,7) = 1203.6, p < 0.01), indicating that there was an increase in freezing to the tone CS relative to the pre-CS period in both groups. Furthermore, no significant group difference in freezing levels was observed during the PR-LTM test (Fig. 5d). The ANOVA revealed a nonsignificant effect for group (F(1,7) = 0.01); however, there were significant effects of trial (F(9,63) = 6.65, p < 0.05) and group × trial interaction (F(9,63) = 2.32, p < 0.05). Duncan's post hoc tests for the group × trial interaction revealed only a significant difference in freezing between scrambled and antisense groups on the first trial. Cannula placements can be viewed in Figure 5e.
Figure 5.
The effect of Arc/Arg3.1 knockdown on auditory fear memory reconsolidation is temporally graded. a, Schematic of the behavioral protocol. b, Postshock freezing scores in Arc/Arg3.1 ODN (n = 5) and scrambled ODN (n = 4) groups immediately after the conditioning trials. c, Freezing scores in each group during the memory reactivation trial. d, PR-LTM assessed 18 h after infusions for both antisense and scrambled groups. e, Histological verification of cannula placements for rats infused with Arc/Arg3.1 antisense (white circles) or scrambled (black circles) ODNs. Panels adapted from Paxinos and Watson (1998).
The reconsolidation deficit induced by Arc/Arg3.1 knockdown is not sensitive to spontaneous recovery, reinstatement, or a shift in testing context
Our experiments thus far collectively suggest that knockdown of Arc/Arg3.1 in the LA impairs reconsolidation of an auditory fear memory. An alternative interpretation, however, is that knockdown of Arc/Arg3.1 has facilitated fear memory extinction. To distinguish among these possibilities, we examined whether the reconsolidation deficit induced by Arc/Arg3.1 knockdown in the LA is sensitive to spontaneous recovery, reinstatement, or a shift in the testing context, all features that are characteristic of extinguished fear memories (Pavlov, 1927; Bouton and Bolles, 1979a,b). Rats were trained with two tone–shock pairings in chamber A, followed 24 h later by intra-LA infusion of either Arc/Arg3.1 antisense or scrambled ODNs (200 pmol; 1 μl). Ninety minutes later, rats in each group received a reactivation trial in chamber B, followed by tests of PR-STM and PR-LTM either 3 or 24 h later, respectively (Fig. 6a). One week later, rats were retested for PR-LTM in chamber B to test for spontaneous recovery of the fear memory. The next day, rats underwent a fear reinstatement session in a novel context (chamber C) consisting of exposure to two unsignaled footshocks (Duvarci and Nader, 2004), followed 24 h later by a third test of PR-LTM in chamber B (reinstatement test). Finally, rats were placed in a third novel context (chamber D) and retested for PR-LTM to examine the generality of the memory reconsolidation deficit (context shift) (Fig. 6a).
Figure 6.
The effect of Arc/Arg3.1 knockdown on auditory fear memory reconsolidation is not sensitive to spontaneous recovery, reinstatement, or a shift in testing context. a, Schematic of the behavioral protocol (for details, see Results). b, Postshock freezing scores in Arc/Arg3.1 ODN (n = 5) and scrambled ODN (n = 5) groups immediately after the conditioning trials. c, Freezing scores in each group during the memory reactivation trial. d, PR-STM assessed at 3 h after the reactivation trial in each group. e, PR-LTM assessed 24 h after the reactivation trial in each group. f, Spontaneous recovery assessed 1 week after the PR-LTM test. g, Reinstatement test assessed 24 h after the reinstatement session in each group. h, Context shift test assessed 24 h after the reinstatement test in each group. i, PR-LTM depicted as a percentage of PR-STM for each rat in each group. *p < 0.01 relative to the scrambled group. j, Histological verification of cannula placements for rats infused with Arc/Arg3.1 antisense (white circles) or scrambled (black circles) ODNs. Panels adapted from Paxinos and Watson (1998).
There was no difference in levels of postshock freezing during the training session between the scrambled and antisense ODN groups (Fig. 6b). The ANOVA revealed only a significant main effect of trial (F(2,16) = 661.59, p < 0.01); there was no significant main effect of group (F(1,8) = 0.10) or group × trial interaction (F(2,16) = 2.46). Furthermore, both groups showed equivalent levels of freezing during the pre-CS period and the tone–CS presentation during the reactivation trial (Fig. 6c). An ANOVA (group × trial) revealed no significant effect of group (F(1,8) = 0.17) or group × trial interaction (F(1,8) = 0.58); however, there was a significant main effect of trial (F(1,8) = 1584.21, p < 0.01), indicating that there was an increase in freezing to the tone CS relative to the pre-CS period in both groups.
Analysis of the PR-STM data revealed no differences between the groups (Fig. 6d). The ANOVA (group × trial) revealed a nonsignificant effect of group (F(1,8) = 0.16), trial (F(2,16) = 0.12), and group × trial interaction (F(2,16) = 1.62). In contrast to the PR-STM test, rats infused with the Arc/Arg3.1 antisense ODN exhibited impaired PR-LTM (Fig. 6e). The ANOVA (group × trial) revealed significant main effects of group (F(1,8) = 62.05, p < 0.01) and trial (F(9,72) = 2.57, p < 0.05) but no significant group × trial interaction (F(9,72) = 0.36). During the test of spontaneous recovery 1 week later, rats infused with the Arc/Arg3.1 antisense ODN exhibited sustained memory impairment, whereas the scrambled ODN control group retained high levels of freezing (Fig. 6f). An ANOVA (group × trial) revealed a significant main effect of group (F(1,8) = 121.47, p < 0.01) but no significant effect of trial (F(4,32) = 1.90) or group × trial interaction (F(4,32) = 0.29).
During the reinstatement session, both scrambled and antisense ODN groups exhibited significant postshock freezing in chamber C (data not shown). An ANOVA (group × trial) revealed no main effect of group (F(1,8) = 0.01) or group × trial interaction (F(2,16) = 0.04) but did reveal a significant main effect of trial (F(2,16) = 348.18), suggesting an increase in freezing relative to the preshock period in both groups. When tested for memory reinstatement in chamber B 24 h later, however, rats infused with the Arc/Arg3.1 antisense ODN continued to exhibit sustained memory impairment, whereas the scrambled ODN control group exhibited high levels of freezing (Fig. 6g). An ANOVA (group × trial) revealed a significant main effect of group (F(1,8) = 130.04, p < 0.01) but no significant effect of trial (F(4,32) = 0.40) or group × trial interaction (F(4,32) = 0.40).
During the context shift test in chamber D, rats infused with the Arc/Arg3.1 antisense ODN continued to exhibit sustained memory impairment, whereas the group infused with scrambled ODN sustained high levels of freezing, suggesting that the observed reconsolidation deficit is not context specific (Fig. 6h). An ANOVA (group × trial) revealed a significant main effect of group (F(1,8) = 107.58, p < 0.01), but no effect of trial (F(2,16) = 0.03) or the group × trial interaction (F(2,16) = 0.97).
As another measure of the reconsolidation deficit observed in the Arc/Arg3.1 antisense ODN group, each rat's freezing score during each of the first three PR-LTM tests was expressed as a percentage of that exhibited during the PR-STM test (Fig. 6i). The Arc/Arg3.1 antisense ODN group exhibited significantly less retention across all PR-LTM tests relative to the scrambled group, which exhibited sustained fear memory retention. An ANOVA group × test revealed significant main effect of group (F(1,8) = 233.21, p < 0.01) but no significant main effect of test (F(2,16) = 0.56) or group × test interaction (F(2,16) = 0.79). Cannula placements are shown in Figure 6j.
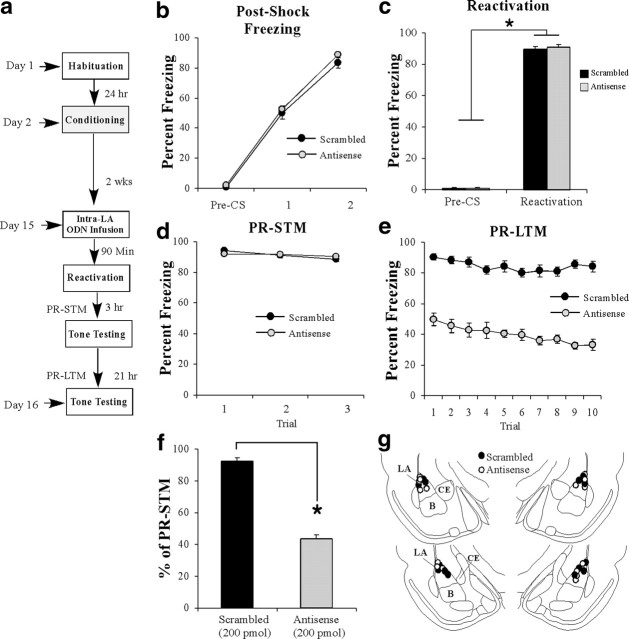
Arc/Arg3.1 knockdown impairs reconsolidation of a well-consolidated auditory fear memory
In each of our previous experiments, we reactivated the fear memory under the influence of an Arc/Arg3.1 ODN within 24 h after fear conditioning. Thus, it might be argued that the observed memory impairments that we have attributed to impaired reconsolidation processes are in fact attributable to interference with a later wave of Arc/Arg3.1 expression that is critical for initial memory consolidation. Our delayed infusion experiments (Fig. 5) suggest that this is an unlikely possibility. As a more definitive test of this hypothesis, however, we asked whether Arc/Arg3.1 is necessary for the reconsolidation of a well-consolidated (e.g., 2-week-old) auditory fear memory.
Rats were trained with two tone–shock pairings in chamber A. Two weeks later (Nader et al., 2000), rats were given intra-LA infusion of either Arc/Arg3.1 antisense or scrambled ODNs (200 pmol; 1 μl), followed by a memory reactivation trial 90 min later in chamber B (Fig. 7a). There was no difference in levels of postshock freezing during training between the scrambled and antisense groups (Fig. 7b). The ANOVA revealed only a significant main effect of trial (F(2,26) = 924.64, p < 0.01); there was no significant main effect of group (F(1,13) = 1.91) or group × trial interaction (F(2,26) = 0.61). Furthermore, both groups showed equivalent levels of freezing during the pre-CS period and the tone–CS presentation during the reactivation trial (Fig. 7c). An ANOVA (group × trial) revealed no significant effect of group (F(1,13) = 0.18) or group × trial interaction (F(1,13) = 0.32); however, there was a significant main effect of trial (F(1,13) = 5025.8, p < 0.01), indicating that there was an increase in freezing to the tone CS relative to the pre-CS period in both groups. Three hours after tone memory reactivation, rats were given a PR-STM test (Fig. 7d). The ANOVA (group × trial) revealed no significant effect of group (F(1,13) = 0.01) or group × trial interaction (F(2,26) = 1.33) yet revealed a significant effect of trial (F(2,26) = 4.38).
Figure 7.
Arc/Arg3.1 is required for the reconsolidation of a well-consolidated auditory fear memory. a, Schematic of the behavioral protocol. b, Postshock freezing scores in Arc/Arg3.1 ODN (n = 8) and scrambled ODN (n = 7) groups immediately after the conditioning trials. c, Freezing scores in each group during the memory reactivation trial administered 2 weeks after training. d, PR-STM assessed at 3 h after the reactivation trial in each group. e, PR-LTM assessed 24 h after the reactivation trial in each group. f, PR-LTM depicted as a percentage of PR-STM for each rat in each group. *p < 0.01 relative to the scrambled group. g, Histological verification of cannula placements for rats infused with Arc/Arg3.1 antisense (white circles) or scrambled (black circles) ODNs. Panels adapted from Paxinos and Watson (1998).
On the following day, rats were given a PR-LTM test, and the group infused with the Arc/Arg3.1 antisense ODN exhibited impaired PR-LTM (Fig. 7e). The ANOVA (group × trial) revealed significant main effects of group (F(1,13) = 181.93, p < 0.01) and trial (F(9,117) = 4.32, p < 0.01) but no significant group × trial interaction (F(9,117) = 1.35). As another measure of the reconsolidation deficit observed in the Arc/Arg3.1 antisense ODN group, each rat's freezing score during the PR-LTM test was expressed as a percentage of that exhibited during the PR-STM test (Fig. 7f). The Arc/Arg3.1 antisense ODN group exhibited significantly less retention during the PR-LTM test than the scrambled group (t(13) = 219.6, p < 0.01). Cannula placements are shown in Figure 7g.
Discussion
Although considerable progress has been made in defining the role of NMDAR-driven protein kinase signaling cascades and transcription factors in memory reconsolidation processes, very little is known about the downstream transcriptional targets of these pathways and how they contribute to memory reconsolidation (Tronson et al., 2007). In the present study, we have examined the functional role of Arc/Arg3.1 in fear memory reconsolidation. We show that Arc/Arg3.1 protein is upregulated in the LA after retrieval of a recently acquired auditory fear memory and that antisense knockdown of Arc/Arg3.1 in the LA impairs fear memory reconsolidation.
The transcriptional characteristics and rapid subcellular trafficking of Arc/Arg3.1 have made it an attractive tool to map regions of the brain that are involved in memory retrieval (Guzowski et al., 2001). Arc/Arg3.1 has been shown to be regulated within the hippocampus after reexposure to a familiar environment or retrieval of a spatial memory (Guzowski et al., 1999; Gusev et al., 2005), within the hippocampus and amygdala after retrieval of a contextual fear memory (Zhang et al., 2005; Mamiya et al., 2009), and within the amygdala after retrieval of an appetitive memory (Petrovich et al., 2005). In our experiments, we show using both Western blotting and immunohistochemistry that Arc/Arg3.1 protein is regulated in an anatomically restricted manner in the LA after retrieval of a recently acquired auditory fear memory. Furthermore, our non-reactivated and tone-alone groups show that retrieval-induced Arc/Arg3.1 expression in the LA is specific to memory retrieval and is not attributable to exposure to the testing context alone, tone alone, or recent (e.g., within 24 h) exposure to footshock stress. Interestingly, previous work in our laboratory that has examined IEG expression after auditory fear conditioning has observed significant regulation of both Arc/Arg3.1 and EGR-1 in the LA, including the LAd and LAv, as well as in the basal nucleus of the amygdala (Ploski et al., 2008; Maddox et al., 2011). In contrast to those findings, our immunohistochemical data suggest that retrieval-induced expression of Arc/Arg3.1 is primarily restricted to the LA, particularly the LAd and LAv; very little Arc/Arg3.1 was observed in the basal nucleus. These findings are consistent with those of a recent study from our laboratory that examined retrieval-induced expression of EGR-1 in the amygdala (Maddox et al., 2011) and might suggest that, although plasticity within several different amygdala nuclei play a role in fear memory acquisition and/or consolidation, the LA may play a selective role in auditory fear memory reconsolidation.
Despite the numerous studies that have shown that Arc/Arg3.1 is regulated by memory retrieval (Guzowski et al., 1999; Zhang et al., 2005), ours is the first study, of which we are aware, to systematically examine the functional role of Arc/Arg3.1 in memory reconsolidation processes. Our behavioral experiments show that retrieval-induced expression of Arc/Arg3.1 in the LA is critical for fear memory reconsolidation; intra-LA infusion of Arc/Arg3.1 antisense before retrieval leaves PR-STM (tested at 3 h) intact, whereas PR-LTM (tested at 24 h) is impaired. Furthermore, this effect on fear memory reconsolidation was observed to be time limited and specific to an actively reactivated memory; Arc/Arg3.1 knockdown in the absence of fear memory retrieval failed to result in a reconsolidation impairment. This pattern of findings attests to the specificity of our memory reactivation parameters; rats in the non-reactivated group exhibit very little freezing to the testing context, suggesting that there is little to no generalization of fear between our training and testing contexts. Moreover, we showed that knockdown of Arc/Arg3.1 in the LA was equally effective at impairing both new and well-consolidated auditory fear memories. This latter finding is consistent with those of previous reports in the literature that have examined the role of protein synthesis (Nader et al., 2000) and noradrenergic signaling (Debiec and LeDoux, 2004) in fear memory reconsolidation and suggests that memory impairments induced by an Arc/Arg3.1 ODN after retrieval are not attributable to interference with a later wave of Arc/Arg3.1 expression that may be critical for initial memory consolidation. Rather, our findings collectively support the conclusion that Arc/Arg3.1 is required for fear memory reconsolidation in the LA.
In our experiments, rats that received a memory reactivation trial under the influence of an Arc/Arg3.1 ODN exhibited impaired memory recall on subsequent tests. Given that in each of our experiments PR-STM (at 3 h) was intact whereas PR-LTM (at 24 h) was impaired, we interpreted these findings to indicate that fear memory reconsolidation was impaired. As with any reconsolidation study, however, it is critical to rule out the possibility that we have instead interfered with fear memory expression by facilitating memory extinction. Previous studies that have examined the role of protein synthesis (Duvarci and Nader, 2004) and noradrenergic signaling (Debiec and LeDoux, 2004) in fear memory reconsolidation processes have suggested that this is an unlikely possibility. Nonetheless, to distinguish among these possibilities, we showed that the reconsolidation deficit induced by Arc/Arg3.1 knockdown in the LA is not sensitive to spontaneous recovery, reinstatement after a series of reminder footshocks, or a shift in the testing context, features that are each hallmarks of extinguished fear memories (Pavlov, 1927; Bouton and Bolles, 1979a,b). Our findings support and extend those of previous studies (Debiec and LeDoux, 2004; Duvarci and Nader, 2004) and add additional support to the notion that amygdala-dependent fear memories that are lost as a result of interference with reconsolidation processes are not readily amenable to recovery (Duvarci and Nader, 2004; Tronson and Taylor, 2007; Nader and Einarsson, 2010).
The mechanisms by which Arc/Arg3.1 contributes to memory reconsolidation are presently unknown. Arc/Arg3.1 is known to interact with endophilin and dynamin to modulate AMPAR endocytosis and reduce AMPAR surface expression, thus allowing Arc/Arg3.1 to influence synaptic strength and excitability and synaptic homeostasis (Chowdhury et al., 2006; Shepherd et al., 2006). Although it is well established that AMPAR regulation is critical for memory formation and for fear memory consolidation (Rumpel et al., 2005), few studies have examined whether similar trafficking of AMPARs underlies the reconsolidation process. A recent study, however, suggested that fear memories that are lost as a result of interference with reconsolidation are accompanied by a removal of calcium-permeable AMPARs at LA synapses (Clem and Huganir, 2010). Furthermore, Arc/Arg3.1 has been found to be necessary for cofilin phosphorylation and stable expansion of the filamentous actin (F-actin) cytoskeleton in the dentate gyrus during the consolidation of long-term potentiation (LTP) (Messaoudi et al., 2007). Thus, it remains possible that Arc/Arg3.1 may participate in the restabilization of the synapse after memory-retrieval-induced destabilization, a hypothesis that has yet to be examined.
In summary, the results of the present study provide strong evidence that Arc/Arg3.1 is critical for memory reconsolidation processes. These findings expand nicely on previous work that has used Arc/Arg3.1 as a tool to map out brain areas necessary for memory retrieval in hippocampal- and amygdala-dependent memory tasks (Gusev et al., 2005; Zhang et al., 2005) and further contribute to our understanding of the cellular and molecular mechanisms of fear memory reconsolidation within the LA.
Footnotes
This research was supported by National Institutes of Health Grant MH 073949 (G.E.S.) and Yale University. This research was made with government support under and awarded by the Department of Defense, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate Fellowship 32 CRF 168a (S.A.M.).
References
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979a;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979b;10:445–466. [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva WC, Bonini JS, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. Inhibition of mRNA synthesis in the hippocampus impairs consolidation and reconsolidation of spatial memory. Hippocampus. 2008;18:29–39. doi: 10.1002/hipo.20362. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learn Mem. 2008;15:747–755. doi: 10.1101/lm.1027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusev PA, Cui C, Alkon DL, Gubin AN. Topography of Arc/Arg3.1 mRNA expression in the dorsal and ventral hippocampus induced by recent and remote spatial memory recall: dissociation of CA3 and CA1 activation. J Neurosci. 2005;25:9384–9397. doi: 10.1523/JNEUROSCI.0832-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Peña de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Maddox SA, Monsey MS, Schafe GE. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learn Mem. 2011;18:24–38. doi: 10.1101/lm.1980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Einarsson EO. Memory reconsolidation: an update. Ann N Y Acad Sci. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci. 2006;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. New York: Dover; 1927. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bösl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploski JE, Park KW, Ping J, Monsey MS, Schafe GE. Identification of plasticity-associated genes regulated by Pavlovian fear conditioning in the lateral amygdala. J Neurochem. 2010;112:636–650. doi: 10.1111/j.1471-4159.2009.06491.x. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Zhang WP, Guzowski JF, Thomas SA. Mapping neuronal activation and the influence of adrenergic signaling during contextual memory retrieval. Learn Mem. 2005;12:239–247. doi: 10.1101/lm.90005. [DOI] [PMC free article] [PubMed] [Google Scholar]