Abstract
Developmental exposure to polychlorinated biphenyls (PCBs) disrupts reproduction in animals. Human data are lacking. We measured PCBs in preserved mothers' serum samples collected during 1960-1963, 1-3 days after their daughters' birth. We recorded time to pregnancy (TTP) in 289 daughters 28-31 years later. PCB congeners 187, 156, and 99 in mother's serum were associated with longer TTP in their daughters while PCB congeners 105,138 and 183 were associated shorter TTP. Probability of pregnancy fell by 38% (95% CI 17%-53%) and infertility was higher (30% not pregnant after 13 cycles vs.11% not pregnant after 13 cycles) among women whose mothers had a higher proportion of PCB congeners associated with longer TTP (75th percentile versus 25th percentile).This study demonstrates, for the first time, that developmental exposure to PCBs may disrupt pregnancy in humans.
Keywords: polychlorinated biphenyls, PCBs, time to pregnancy, DDT, DDE, fecundability, time to pregnancy, TTP, Child Health and Development Studies, prospective study, cohort, prenatal, developmental origins of health and disease
1. Introduction
In 1986 Baird, Wilcox and Weinberg published a seminal paper proposing the use of fecundability (probability of pregnancy in each cycle) as a sensitive marker to identify reproductive hazards in humans. [1] The paper set forth a method for estimating fecundability by measuring time to pregnancy (TTP), defined as the number of non-contracepting cycles required to conceive a recognized pregnancy. The authors described the biological meaning of TTP, noting that longer TTP may indicate a problem at one or more of several stages of human reproduction including “gametogenesis, transport of gametes in both and male and female reproductive tracts, fertilization, migration of the zygote to the uterus, implantation and early survival of the conceptus”, p.471[1]
Hence the biological rationale for studying fecundability is to identify factors that increase TTP and therefore could be considered hazardous at any of these stages. The method is statistically powerful and also sensitive because it does not rely on identifying the specific mechanism of effects. In essence, analysis of fecundability, estimated by TTP, is a powerful screening tool to identify potential hazards to human reproduction, regardless of the mechanism. [1]
Following the logic of Baird, Wilcox and Weinberg, in this paper we test the hypothesis that in utero exposure to polychloriniated biphenyls (PCBs) increases TTP in humans. Prior studies assessed PCB exposure near the time of conception and reported mixed results as recently reviewed. [2-3] To our knowledge this is the first study to measure maternal exposures in blood samples collected near delivery in relation to her daughter's TTP decades later.
2. Material and Methods
2.1 Study Design and Participants
The study sample consists of women born between 1960 and 1963 in the Oakland, California area, whose mothers were enrolled in the Kaiser Permanente Health Plan. These women and their mothers were among participants in the Child Health and Development Studies (CHDS), an investigation of prenatal determinants of infant, child, adolescent and adult health. [4] Subjects were respondents to a follow-up study conducted in 1990-1991 [5-6] which investigated prenatal determinants of TTP. Women were ages 28-31 years (65% participation rate after 30 years since their birth) when they completed a questionnaire on the number of non-contracepting menstrual cycles that preceded their most recent pregnancy; or the number of non-contracepting cycles during which they were at risk for pregnancy even when pregnancy was not achieved. Women who did not plan their pregnancies as well as women who did plan their pregnancies were included in the study as long as they could report their TTP, reducing planning bias described in some previous studies of TTP. [7] This report is based on the same 289 women in our previous report on prenatal DDT exposure and TTP. [6] Details of the study sample are given below.
CHDS daughters born during 1960-1963 who resided in the San Francisco Bay area through ages 15-17 (N=1,003) were eligible for this study if their mothers had reported information on smoking during pregnancy (N=991). TTP data was originally collected for a study of prenatal tobacco exposure conducted by one of us (P.S) for her dissertation research. Of these 991 eligible women, 345 did not participate for the following reasons: no address, N=117, no response to mail, N=197, deceased or institutionalized, N=7, refused, N=24. There were 646 participants in the study (65% of 991 eligible), of which 357 were excluded from analysis of TTP for the following reasons: respondent was never at risk for pregnancy (N=163), respondent could not estimate TTP due to sporadic birth control use or birth control failure (N=119), incomplete questionnaire (N=47), insufficient prenatal serum for assays (N=18), younger sister (N=5), under age 16 at most recent pregnancy “attempt” (N=5).
Women in the following categories were included in the present study whether or not they had planned their pregnancy, as long as they could report TTP: women who omitted birth control just once, women who became pregnant during an interval between birth control methods or after stopping birth control, women who became pregnant when breast feeding, women who never used birth control, women who were attempting pregnancy after a prior pregnancy, and women who never became pregnant, but had been at risk for pregnancy for a known interval, whether or not they desired to become pregnant. Women were asked to report their TTP for their most recent pregnancy in order to minimize recall errors. Among the 289 women included in this report, 43% of women reported TTP for their first pregnancy, 26% for their second pregnancy, 15% for their third pregnancy, 8% for their fourth pregnancy, and 6% for their fifth or more, with 2% where gravidity before attempt was unknown. Women reporting on time to first pregnancy include women who were at risk for pregnancy, but failed to conceive.
In human observational studies where being at risk for pregnancy is not an experimental condition, there are expected differences between women who can report TTP and women who cannot. The two primary groups of women who are deleted from the TTP analysis are women never at risk for pregnancy (N=163) and women who became pregnant and could not answer about TTP (N=119). Compared to the analysis sample, women never at risk for pregnancy had higher personal incomes, were much more likely to have never been married, and were more highly educated and more likely to be Asian. Compared to the analysis sample, women who used birth control sporadically and could not report their TTP were more likely to be African American or Asian, slightly more likely to be never married, somewhat better educated, but were similar to the analysis sample for personal income. These differences result from personal, social and economic factors related to reproductive behavior, and are not surprising. Our results apply to those women who were at risk for pregnancy and could provide a TTP, including women who planned their pregnancies and women who did not plan their pregnancies. This research was approved by the Institutional Review Board of the Public Health Institute and study subjects gave informed consent.
2.2 Exposure Measurement
In utero exposure to PCBs among CHDS daughters was estimated by assays of serum samples drawn during the early postpartum period from their mothers, within one to three days of their delivery. PCBs have a long half-life and prior work has established stability of organochlorine levels assayed across all trimesters of pregnancy and the early postpartum. [8] Postpartum samples were used to conserve valuable archived serum samples drawn in each trimester for future studies where timed samples are essential.
PCBs were assayed in the laboratory of Dr. Mary Wolff [9-11] using modifications of methods developed previously by Brock and colleagues. [12] Briefly, a polar extraction of serum lipids is followed by a column chromatographic clean-up and enrichment step, with analysis by gas chromatography with electron capture detection. Limit of detection was approximately 0.07 ng/mL for individual compounds based on three times the standard deviation of the levels found in the lowest quality control plasma pool. [13] When the serum pool and blanks were considered together, [14] the limit of detection was 0.01-0.1 ng/mL; the instrumental limit of detection based on a peak-to-noise ratio of 3, was 0.01-0.03 ng/mL for tetra- through hepta-chlorobiphenyls, using 1-1.5 mL plasma.
2.3 Statistical Analysis
The fecundability ratio (ratio of probability of pregnancy in each cycle, for daughters exposed to higher versus lower PCB levels in utero) was estimated by a discrete analogue of the Cox proportional hazards model. [15] A fecundability ratio greater than 1 indicates a greater probability of pregnancy per cycle and therefore a shorter TTP; a fecundability ratio less than 1 indicates a lower probability of pregnancy per cycle and therefore a longer TTP.
Prior to any data analysis, PCB congeners were categorized by their functionality using a previously suggested classification by Wolff et.al. [16] Group 1 consisted of congeners detected in the CHDS serum samples that were considered to be potentially estrogenic and persistent (PCB 101,187, 201). Group 2 consisted of congeners detected in the CHDS serum samples that were considered to be potentially antiestrogenic, immunotoxic, dioxin-like: Group 2A which are non-ortho or mono-ortho in their structure (PCB 66, 74,105,118,156,167) and Group 2B which are di-ortho and have more limited dioxin-like activity (PCB138 and 170). Group 3 consisted of Phenobarbital, CYP1A and CYP2B inducers (PCB 99, 153,180,203,183).
We assumed, a priori, that it would not be possible to estimate coefficients for all PCB congeners entered into the model simultaneously due to limitations of sample size and covariance of the congeners. For this reason we chose, a priori, a two step method.
The first step entered congeners with potentially opposing effects (estrogenic and anti-estrogenic) in a single model in order to avoid overlooking any associations for estrogenic compounds that might be confounded by the presence of correlated anti-estrogenic compounds, and vice versa. The second step added all remaining congeners classified as PB, CYP1A and CYP2B inducers in a single group by Wolff et. al.[16]. Details of these two analysis steps are given below.
All models were adjusted for whether the daughter was breastfed (yes vs. no) to control for postnatal PCB exposure and race (African-American vs. all other) to control for a known source of confounding since we had previously observed both higher levels of organochlorine compounds in African Americans and longer TTP. We initially omitted lipids as adjustment variables based on an a priori decision to limit the number of independent variables in the model. We later adjusted the final model for lipids to determine whether they confounded PCB associations.
The rationale for entering individual congeners classified as estrogenic and anti-estrogenic together in a single first step was to account for the possibility that the estrogenic and anti-estrogenic congeners could mask each other's associations in models not mutually adjusted. We further hypothesized that differing potencies, multiple biological effects, and varying concentrations of individual congeners argue against creating summed variables within categories. At the first step, we removed congeners that were not significant (significance probability (p)>0.10) and checked whether removing these congeners from the model changed the coefficients of the remaining congeners by more than 10 percent. Since removing the non-significant terms did not affect the associations observed for the other terms, we proceeded to add the third group (phenobarbital, CYP1A and CYP2B inducers) to the model, entered as individual congeners. As for the prior step, we then removed non-significant terms (p>0.10) and checked to see whether removing the non-significant terms substantially changed the coefficients of the remaining congeners. In a prior report we found evidence for effects of in utero exposure to 1,1,1-Trichloro-2,2-bis(p-chlorophenyl)ethane (p,p′-DDT, the primary component of the pesticide, DDT) and 1,1′-Dichloro-2, 2′-bis(p-chlorophenyl)ethylene (p,p′-DDE, the primary metabolite of p,p′-DDT) on TTP in this same study population. [6] Therefore we also examined whether these variables altered observed PCB associations by adjusting the final model for them. We note that the practice of accounting for multiple compounds in the same analysis has been recommended since exposure to mixtures is usual in natural animal and human populations. [17-18] The model was further adjusted for the following maternal variables: age and body mass index (weight in kg/(height in cm)2) and maternal lipids (triglycerides, cholesterol) to determine whether these altered associations observed.
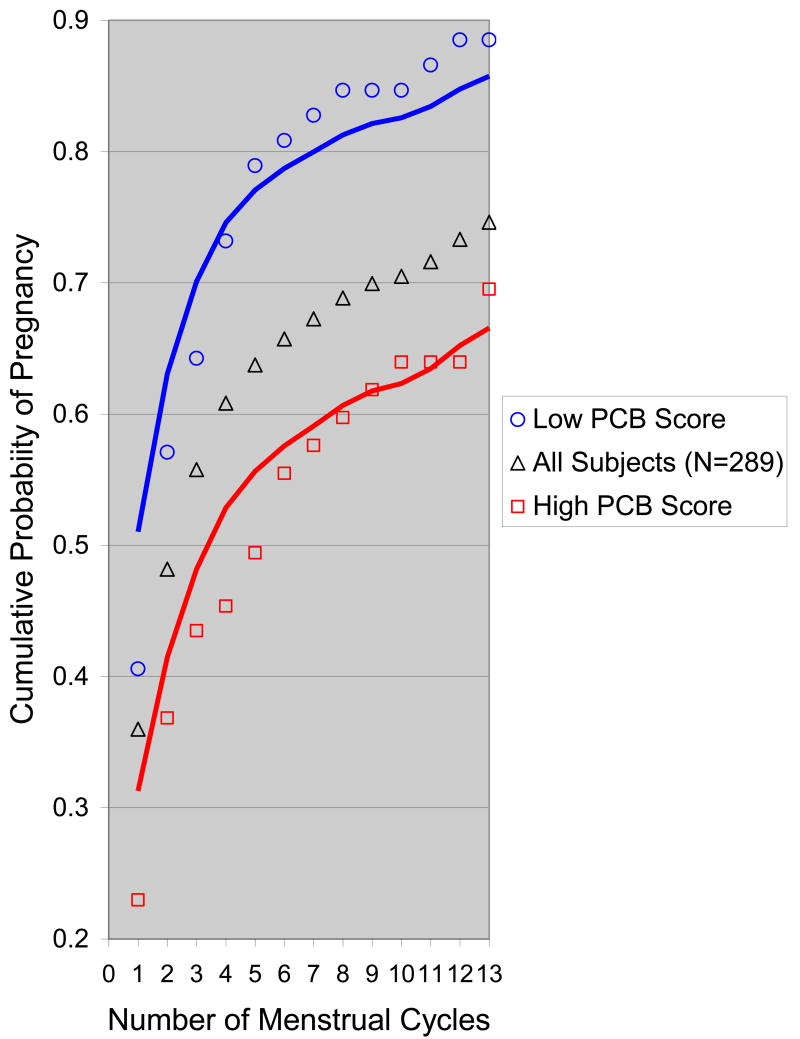
A PCB score was defined, post-hoc, after identifying the PCB congeners that predicted TTP and was composed of the ratio of the sum of congeners that were associated with longer TTP to the sum of congeners that were associated with shorter TTP (sum of PCBs 187,156,99/ sum of PCBs 105,138,183). We constructed the PCB score to be positively correlated to TTP based on the results of hypothesis testing for individual PCB congeners (results shown in Table 2). The purpose of the post-hoc score was to summarize the TTP observed in the study sample in association with various PCB mixtures. We focused on longer TTP, because it is interpreted as a screen for reproductive hazards. [1] Kaplan-Meier curves showing observed cumulative probability of pregnancy over thirteen menstrual cycles by PCB score percentile were constructed. Predicted curves were derived from a discrete analogue of the Cox proportional hazards model containing the PCB score and anchored by the denominator of the PCB score, but otherwise unadjusted to facilitate comparison to observed values for the cohort stratified by PCB score and also for the entire cohort unstratified by PCB score. Predicted probability of pregnancy in each cycle was calculated by evaluating the model at the median PCB score within the 25th percentile observed in the analysis sample (0.38) compared to the median PCB score within the 75th percentile of the PCB score (0.70). Observed and predicted cumulative probability of pregnancy over thirteen menstrual cycles for daughters are plotted in Figure 1 for the 25th and 75th percentile of the PCB score in mother's serum samples in order to display the net effect of PCB exposures found in this study sample. A curve describing the cumulative probability of pregnancy for the entire study sample, unstratified by PCB exposure, is also plotted in Figure 1 for comparison to high and low values of the PCB score.
Table 2.
Associations of PCB congeners in mother's serum, which was collected 1-3 days after her daughter was born, with her daughter's fecundability up to 30 years later in Child Health and Development Studies (N=289). PCBs in red were associated with longer time to pregnancy in daughters. PCBs in blue were associated with shorter time to pregnancy in daughters. The fecundability ratio was estimated by a single discrete logistic model jointly adjusted for all individual PCB congeners shown entered as continuous variables, and also adjusted for whether mother breast fed her daughter (yes, no), for mother's race/ethnicity (African-American versus other) and for lipids (total cholesterol, total triglycerides). In the last column, the fecundability ratio is estimated for the median of the 75th percentile − the median of the 25th percentile as follows given in ng/mL: PCB 187, 0.135; PCB 105, 0.17; PCB 138, 0.54; PCB 99, 0.25; PCB 183, 0.16, PCB 156, 0.12. The fecundability ratio for the PCB score was additionally adjusted for the denominator of the score (sum of PCBs 105,138,183). The PCB score ranged from a median of 0.38 in the 25th percentile, to 0.70 in the 75th percentile of the score. For the PCB score, the median of the 75th percentile − the median of the 25th percentile was 0.32. The PCB score was defined after identifying the congeners that predicted fecundability and was composed of the ratio of the sum of congeners that were associated with longer time to pregnancy to the sum of congeners that were associated with shorter time to pregnancy. The purpose of the score was to describe the net effect of exposure found in the population.
| PCB Class [16] and Congener | Fecundability ratio per standard deviation (95% CI)) | Fecundability ratio, 75th percentile versus 25th percentile (95% CI) |
|---|---|---|
| Potentially estrogenic | ||
| PCB 187§ | 0.44 (0.28,0.69) | 0.59 (0.44,0.79) |
| Potentially Anti-Estrogenic | ||
| A. Mono-ortho, dioxin-like | ||
| PCB 105† | 1.21 (1.04,1.41) | 1.44 (1.09,1.91) |
| PCB 156† | 0.83 (0.71,0.97) | 0.78 (0.63,0.96) |
| B. Di-ortho, limited dioxin activity | ||
| PCB 138¶ | 1.99 (1.45,2.75) | 2.14 (1.50,3.05) |
| Phenobarbital, CYP1A and CYP2B inducers | ||
| PCB 99‡ | 0.75 (0.62,0.91) | 0.69 (0.53,0.89) |
| PCB 183* | 1.21 (0.99,1.48) | 1.27 (0.99,1.63) |
| PCB Score: | ||
|
|
0.80 (0.70,0.92) | 0.62 (0.47, 0.83) |
p < 0.10.
p < 0.05.
p <0.01.
p <0.001.
p <0.000.
Figure 1. Predicted (lines) and observed (points) cumulative probability of pregnancy after 13 menstrual cycles for 289 daughters according to the PCB score measured in their mothers' preserved serum, which was collected 1-3 days after the daughters were born.
A low PCB score is defined as the 25th percentile observed in maternal samples (blue curves at top of figure). A high PCB score is defined as the 75th percentile observed in maternal samples (red curves at bottom of figure). Observed values for the entire sample are shown by the middle curve (black open triangles). Definition of the PCB score, percentiles and methods for drawing these curves are described in the text.
We also performed supplementary analysis, using linear regression, to determine whether women who never conceived (nulliparas) had higher prenatal PCB exposure and longer attempt times than women reporting on their first pregnancy (primiparas) or women reporting on a higher order pregnancy (multiparas).
3. Results
PCBs were detected in all maternal serum samples (Table 1).
Table 1.
PCB congeners observed in maternal serum in the Child Health and Development Studies, collected in 1960-1963, 1-3 days after their daughters were born (N=289). PCBs in red were significantly associated with longer time to pregnancy in daughters. PCBs in blue were significantly associated with shorter time to pregnancy in daughters (see Table 2 for details). The PCB score was defined after identifying the congeners that predicted time to pregnancy in daughters and was composed of the ratio of the sum of congeners that were associated with longer time to pregnancy to the sum of congeners that were associated with shorter time to pregnancy (sum of PCBs 187,156,99/ sum of PCBs 105,138,183). The purpose of the score was to describe the net effect of exposures found in the population. See text for details.
| PCB Class [16] and Congener | Median (ng/mL) | Minimum (ng/mL) | Maximum (ng/mL) | SD (ng/mL) | %> LOD |
|---|---|---|---|---|---|
| Potentially Estrogenic | |||||
| 101 | 0.08 | 0.01 | 3.34 | 0.24 | 54 |
| 187 | 0.17 | 0.05 | 3.48 | 0.21 | 98 |
| 201 | 0.11 | 0.01 | 1.04 | 0.09 | 77 |
| Potentially Anti-Estrogenic | |||||
| A. Non-ortho, mono-ortho, dioxin-like | |||||
| 66 | 0.14 | 0.03 | 1.68 | 0.35 | 85 |
| 74 | 0.17 | 0.01 | 3.52 | 0.25 | 93 |
| 105 | 0.14 | 0.01 | 0.74 | 0.09 | 89 |
| 118 | 0.44 | 0.14 | 2.46 | 0.27 | 100 |
| 156 | 0.07 | 0.01 | 0.94 | 0.09 | 57 |
| 167 | 0.07 | 0.01 | 0.83 | 0.09 | 57 |
| B. Di-ortho, limited dioxin activity | |||||
| 138 | 0.57 | 0.11 | 7.03 | 0.49 | 100 |
| 170 | 0.17 | 0.01 | 1.59 | 0.12 | 97 |
| Phenobarbital, CYP1A and CYP2B inducers | |||||
| 99 | 0.17 | 0.01 | 2.57 | 0.19 | 88 |
| 153 | 0.79 | 0.36 | 8.24 | 0.55 | 100 |
| 180 | 0.43 | 0.15 | 5.74 | 0.37 | 100 |
| 203 | 0.13 | 0.01 | 1.24 | 0.09 | 94 |
| 183 | 0.09 | 0.01 | 1.93 | 0.13 | 68 |
| PCB Score | 0.51 | 0.25 | 1.12 | 0.15 | NA |
PCB, polychlorinated biphenyl; LOD, limit of detection; NA, not applicable
One potentially estrogenic congener (PCB187), four potentially anti-estrogenic congeners (PCB105, PCB118, PCB156 and PCB138), and two congeners from among the Phenobarbital, CYP1A, and CYB2B inducers (PCB99 and PCB183), and were retained as significant predictors of TTP (see Methods for details). When we removed the non-significant congeners (PCB101, PCB201, PCB66, PCB74, PCB167, PCB170 PCB153, PCB180 and PCB203 and PCB118) there were no substantial changes in the size of estimates for significant congeners. Adjustment for maternal lipids did not substantially influence the magnitude or significance of PCB associations (Supplemental Table A).
The final model is shown in Table 2. In utero exposure to higher levels of PCBs 187, 156 and 99 was significantly associated with longer TTP. In contrast, in utero exposure to PCBs 105, 138 and 183 was associated with shorter TTP.
The PCB score, defined post hoc as the ratio of the sum of congeners associated with longer TTP to the sum of congeners associated with shorter TTP, predicted longer TTP as expected (Table 2, last row, see Methods for details). We do not provide significance probabilities (p-values) for the score because it is a post hoc construction. Women in the analysis sample had a considerable range of values for the PCB score, ranging from a median of 0.38 in the 25th percentile of the PCB score to 0.70 in the 75th percentile of the PCB score, demonstrating that mothers of our subjects showed substantial variability on the mix of PCB congeners found in their prenatal serum.
Additional adjustment for maternal age, and maternal body mass index and maternal p,p′-DDT and p,p′-DDE did not substantially influence associations shown in Table 2 for the PCB score (Details are found in Supplemental Table B) or for individual congeners (Details are found in Supplemental Table C). The p,p′-DDT and p,p′-DDE associations with TTP that we previously reported for this same study sample [6] remained after adjustment for PCBS (Details are found in Supplemental Table B).
Women who failed to conceive (nulliparas) had significantly longer attempt times, higher in utero exposure to PCB congeners associated with longer TTP, and a higher PCB score than either primiparas or multiparas (Table 3). These findings are consistent with results from the logistic model shown in Table 2).
Table 3. Mean number of cycles of unprotected intercourse and prenatal PCB exposure for nuligravidas (women who failed to conceive) compared to women reporting on their first pregnancy (primigravidas) and women reporting on higher order pregnancies (multigravidas).
| Mean | |||
|---|---|---|---|
| Nulligravidas (N=54) |
Primagravidas (N=70) |
Mutligravidas (N=159) |
|
| A. Number of cycles at risk for pregnancy | 6.50 | 3.87** | 3.60** |
| B. Sum of pcbs 187,99,156 (ng/mL) | 0.64 | 0.49* | 0.43** |
| C. Sum of pcbs 105,138,183 (ng/mL) | 1.03 | 0.92 | 0.86 |
| D. PCB Score (Ratio of B. to C.) | 0.62 | 0.54** | 0.51** |
p<0.05 for difference compared to nuligravidas
p<0.01 for difference compared to nuiligravidas
6 individuals are missing information about number of pregnancies
Figure 1 summarizes the overall effects of developmental exposure to PCBs for the daughters in our cohort. TTP increased and cumulative conception rates decreased for women whose mothers were in the 75th percentile of the PCB score compared to the entire study sample. In contrast, TTP decreased and cumulative conception rates increased for women whose mothers were in the 25th percentile of the PCB score in maternal serum compared to the entire study sample. The longer TTP and higher frequency of infertility (percent who had not conceived by 13 cycles) was substantial for women whose mothers were in the 75th percentile of the PCB score compared to the 25th percentile. We note that these percentiles were based on scores observed in the analysis sample and therefore apply to the women in our study. We also note that Figure 1 indicates that TTP estimated by the model corresponds fairly well with observed TTP.
4. Discussion
We found that 1) Prenatal exposure to some PCB congeners was associated with longer TTP; 2) Prenatal exposure to other PCB congeners was associated with shorter TTP; 3) The ratio of these groups of congeners in prenatal serum showed considerable variability among mothers in this study population; 4) The upper quartile of the ratio was associated with longer TTP and increased frequency of infertility, compared to the lower quartile.
The findings of this study have broad relevance. PCB exposure continues globally due to widespread soils contamination and bio-accumulation. More than two thirds of 1.2 million tons of PCBs produced worldwide is either still in use or waiting for disposal, presenting a significant, global, public policy challenge. [19] Recent studies document continuing perinatal exposure to PCBs. [20] The PCB dilemma could be a model for other contemporary persistent organic pollutants, particularly those that share structural similarities with PCBS, such as polybrominated diphenyl ethers, PBDEs. [21-22]
The rationale for the study of developmental exposure to PCBs is based on animal studies that suggest that PCB exposure during early development can disrupt adult reproductive function. [18, 23-24] Exposures in utero could affect reproduction and fertility through multiple alternative mechanisms which operate during fetal life. Among the many potential mechanisms are influences on the structure and function of the uterus or fallopian tubes as was observed following prenatal exposure to the potent estrogen diethylstilbestrol [25]; impacts on oogenesis and cell death in the human ovary during fetal life that could alter the size of the ovarian reserve in adult life and possibly the quality of the oocyte destined to become the conceptus; [26] disturbance of maternal and therefore fetal thyroid function that persists and later disrupts menstrual cycling and fertility in the daughter.[27] or disruption of sexually dimorphic patterns in the hypothalamus-pituitary-gonadal axis during early development. [23]
Using a derived, post-hoc, score to describe net effects of PCB congener mixtures is crude, but may be useful, given that PCB classification for the purpose of predicting effects in human populations yields inconsistent results. It seems clear that the functional classification we used is not sufficient for understanding the PCB effects that we observed. PCBs in different functional classes had similar effects; PCB 187, and PCB 99 were both associated with longer TTP, even though these were not originally classified together. Moreover, some effects are contrary to expectations.
Given that dioxin activity is associated with reproductive toxicity, [18] we expected PCB 105 to be associated with longer TTP. Instead, PCB 105 was associated with shorter TTP. This contradiction might be explained by anti-androgenic activity of PCB 105. [28] This hypothesis is supported by our previous finding that in utero exposure to the potent anti-androgen, p,p′-DDE, [29] was also associated with shorter TTP. [6] We had speculated that this effect is explained by protection against germ cell loss in utero, due to antagonism of androgen action. [6] In support of this hypothesis, in the present study we also observed shorter TTP for in utero exposure to the di-ortho congener, PCB 138, which has also been reported to have anti-androgenic activity. [30] However, it is unknown whether shorter TTP is benign or is associated with other unexpected effects in the mother or subsequent generations. A growing literature that endeavors to unravel the process of oogenesis and folliculogenesis and their origins in utero [26, 31] is likely to eventually shed some light on whether shorter TTP is associated with health hazards for mother or offspring over the life-course.
The substantial increase in TTP associated with PCB 187, classified as potentially estrogenic, [16] is in accord with observations that estrogenic compounds interfere with reproduction in females. [32] We note that PCB 99, which can also have estrogenic activity [17], was also significantly associated with longer TTP. However, others have reported that PCB 187 has anti-estrogenic activity.[33] Our results defy consistent classification, providing further evidence that PCB exposures may have multiple and varying effects depending on outcomes examined. [18] Moreover, it is unlikely that all biological effects of PCB congeners and their metabolites are fully known. Future experimental studies may help resolve the paradoxical findings we report in this study.
The hydroxylated PCB metabolite of PCB 187 (4-OH-CB 187) could have been the underlying risk factor for the PCB 187 effects we observed. We recently reported substantial levels of 4-OH-PCB 187 in maternal pregnancy serum in this study population and we also observed that it was positively correlated with PCB 187. [34] 4-OH-CB 187 may interfere with fetal thyroid metabolism [35] and has been shown to have anti-estrogenic properties, [36-37] or may exert other undefined effects. The hydoxylated metabolite of PCBs 105 and 118, 4-OH-CB 107, which has been reported to show anti-estrogenicity in fish liver cell culture [38] was also identified as a major OH-PCB congener in this population. In a pilot assay study of CHDS maternal serum for OH-PCBs, (N=510, unpublished data) levels were comparable to mothers residing near a PCB contaminated area in Slovakia. [39] These high levels in the CHDS probably reflect the effects of bioaccumulation of PCBs in the1960's, prior to the ban on manufacture. [40] The transfer of OH-PCBs from the maternal to fetal compartment may be greater than for parent compounds,[41] suggesting that fetal exposure to OH-PCB metabolites should be further investigated.
Chance remains an alternative explanation of study findings, despite fairly low p-values for associations with individual congeners observed (Table 2). In addition, we note that our results apply to the women for whom TTP could be calculated, a limitation in human studies where many external characteristics are related to reproductive behavior, including education, occupation, and cultural norms.
We were unable to determine whether number of prior pregnancies modified associations of prenatal PCB exposure with time to pregnancy due to sample size limitations. Since pregnancy can reduce maternal body burdens of PCBs, it is possible that including multiparas in our analysis may have biased estimates of effects of prenatal PCB exposure downwards.
Time to pregnancy studies can be subject to several other types of bias. In this study, where we asked women to recall TTP, recall errors are possible. However, women in our study were interviewed close to the time of their pregnancies (ages 28-31 years) minimizing recall errors. By including women in our study who became pregnant without planning, we have reduced one important source of bias that could occur if unplanned pregnancies are correlated both with in utero PCB exposure and TTP.[7] Our questionnaire probed women to respond to TTP whenever possible, including cases of contraceptive failure or omission of birth control, even if for only one cycle. In our study population there were 129 women of 289 who did not plan their most recent pregnancy. These women did not differ from planners for the prenatal PCB score or its components. This finding suggests that bias due to contraceptive practice is an unlikely explanation of our study findings. However, there were still some women who could not report on their TTP and were therefore excluded from our study, so we cannot rule out selection bias. We note that unmeasured sources of bias would have to have very strong associations both with TTP and with in utero exposure to the different PCB congeners in varying directions because some PCBs were positively correlated with TTP and others were negatively correlated.
We cannot rule out the possibility that effects observed are explained by continuing postnatal PCB exposure, although we did account for whether subjects were breastfed. The effects we report could still have been due to exposure in infancy or childhood if maternal pregnancy PCB levels reflect a continuing exposure source for their daughters after birth. It is also possible that some other unmeasured exposures could co-vary with PCBs we measured. These might include factors that are beneficial, or factors that are hazardous. Dietary factors, such as fish consumption, that correlate with various PCB congeners could be involved. It is also possible that host factors, related to selective exposure or retention of the various PCBs, could explain the associations we observed.
Prior studies of PCB exposure in adult women, near the time of conception, vary in their design and in their results. The existing literature is comprised of heterogenous study designs and analytic methods. These include studies that measure exposure via recall of fish consumption [42-44] and studies that measure exposure in serum samples typically collected from adult women sometime after pregnancy or pregnancy attempts at various intervals ranging from months to years.[45-47] Methods for defining or classifying PCB exposure varies among the studies including investigation of only one measured congener, summing total PCBs, investigation of several single congeners, and summing subgroups of congeners. Analysis strategies usually did not include mutual adjustment for multiple congeners or multiple congener classes and this has been suggested to be an important limitation of prior studies.[48] Most studies were based on retrospective designs requiring recall of exposure (dietary studies), or recall of TTP at various ages.[2] One prospective study of adult women exposed to PCBs via contaminated cooking oil and where serum levels of PCBs were also measured, reported no associations with inability to conceive for greater than one year or fertility problems, although women did not report on time to pregnancies they had conceived. [49] One other prospective study reported on serum pre-conceptual exposure to PCBs in adult women who were planning pregnancy.[48] Although PCB associations were not statistically significant, some PCB congeners were associated with shorter TTP and others (estrogenic and anti-estrogenic groups) were associated with longer TTP. None of these prior studies measured prenatal PCB exposure and are therefore not directly comparable to the present study, particularly if the fetal period represents a critical period of vulnerability.
The stronger effect for developmental exposure, which we have observed in the present study, is in keeping with animal and human studies where the fetus has been shown to be more vulnerable than the adult. [18] For example, prenatal exposure to the synthetic estrogen, diethylstilbestrol (DES), had greater consequences for daughters exposed in utero than for their mothers who were exposed during pregnancy. [50]
Long-term and transgenerational follow-up studies in human populations will be required to fully determine the public health significance of developmental exposures. Such studies are especially important in light of the recent report that developmental PCB exposure could result in transgenerational effects on reproductive regulation in animals. [51] Animal studies of DES, [52] and the anti-androgen, vinclozolin [53] further support the concept that developmental exposures can have transgenerational effects in subsequent, unexposed generations. However, transgenerational effects in humans will be difficult to study. More than 50 years of follow-up are required to observe at least four human generations. Existing, long-term pregnancy cohorts are best suited to yield timely answers. Of particular concern is whether altered TTP reflects altered thyroid, ovarian or neuro-endocrine function or structural changes in the breast or other endocrine responsive organs with implications for health conditions in midlife including obesity, diabetes, heart disease and breast cancer.
5. Conclusion
This study supports the hypothesis that in utero exposure to some PCB congeners may impact human reproduction, either by increasing or decreasing time to pregnancy (TTP). Until the consequences of shorter TTP are better understood, the findings regarding longer TTP have more important public health significance. Longer TTP carries psychosocial as well as clinical consequences for women and is a marker for reproductive hazard. Overall, in this population, about one quarter of women were exposed to a mix of PCB congeners before birth that predicted longer TTP and a reduced probability of conception thirty years later. Whether these results are explained by chance, PCB exposure directly, underlying host factors, or other exposures that co-vary with prenatal PCB exposure is not known.
Supplementary Material
Acknowledgments
We acknowledge the late Jacob Yerushalmy for founding the Child Health and Development Studies, Barbara J. van den Berg and Roberta E. Christianson and the late Frank Oechsli for making this study possible by preserving the CHDS data and cohort, Mary S. Wolff for conducting PCB assays and for comments on earlier versions of this manuscript, J. Richard Udry for collecting the TTP data in collaboration with one of us (P.S) and making it available for this study. This research was funded by The National Institute of Environmental Health Sciences (R01 ES08345) and The National Institute of Child Health and Development (N01 DK63422 and N01 HD 1 3334). The point of view and conclusions expressed in this paper are those of the authors and do not necessarily represent the official position or policies of the Department of Health and Human Services.
Abbreviations
- CHDS
Child Health and Development Studies
- DDT
trade name of a pesticide used widely in the United States from 1945 to 1972
- PCBs
polychlorinated biphenyls
- p,p′-DDT
1,1,1-Trichloro-2,2-bis(p-chlorophenyl)ethane, the primary component of DDT
- p,p′-DDE
1,1′-Dichloro-2, 2′-bis(p-chlorophenyl)ethylene, the most persistent DDT metabolite
- 95% CI
95 percent confidence interval
- TTP
Time to Pregnancy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baird DD, Wilcox AJ, Weinberg CE. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–80. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- 2.Buck Louis G, Lynch C, C MA. Environmental Influences on Female Fecundity and Fertility. Seminars in Reproductive Medicine. 2006;24:147–55. doi: 10.1055/s-2006-944421. [DOI] [PubMed] [Google Scholar]
- 3.Mendola P, Messer L, Rappazzo K. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult female. Fertil Steril. 2008;89:e81–e94. doi: 10.1016/j.fertnstert.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988;2:265–82. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 5.Udry JR, Morris NM, Kovenock J. Androgen effects on women's gendered behaviour. J Biosoc Sci. 1995;27:359–68. doi: 10.1017/s0021932000022884. [DOI] [PubMed] [Google Scholar]
- 6.Cohn BA, Cirillo PM, Wolff MS, Schwingl PJ, Cohen RD, Sholtz RI, et al. DDT and DDE exposure in mothers and time to pregnancy in daughters. Lancet. 2003;361:2205–6. doi: 10.1016/S0140-6736(03)13776-2. [DOI] [PubMed] [Google Scholar]
- 7.Baird DD, Weinberg CR, Schwingl P, Wilcox AJ. Selection bias associated with contraceptive practice in time-to-pregnancy studies. Ann N Y Acad Sci. 1994;709:156–64. doi: 10.1111/j.1749-6632.1994.tb30395.x. [DOI] [PubMed] [Google Scholar]
- 8.Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW. Serial levels of serum organochlorines during pregnancy and postpartum. Arch Environ Health. 1999;54:110–4. doi: 10.1080/00039899909602244. [DOI] [PubMed] [Google Scholar]
- 9.Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst. 1993;85:648–52. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- 10.Wolff M, Rivera M, Baker D. Detection limits of organochlorine pesticides and related compounds in blood serum. Bull Environ Contam Toxicol. 1991;47:499–503. doi: 10.1007/BF01700936. [DOI] [PubMed] [Google Scholar]
- 11.Gammon MD, Wolff MS, Neugut AI, Eng SM, Teitelbaum SL, Britton JA, et al. Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiol Biomarkers Prev. 2002;11:686–97. [PubMed] [Google Scholar]
- 12.Brock JW, Burse VW, Ashley DL, Najam AR, Green VE, Korver MP, et al. An improved analysis for chlorinated pesticides and polychlorinated biphenyls (PCBs) in human and bovine sera using solid-phase extraction. J Anal Toxicol. 1996;20:528–36. doi: 10.1093/jat/20.7.528. [DOI] [PubMed] [Google Scholar]
- 13.Long G, Winefordner JD. Limit of detection. A closer look at the IUPAC definition. Anal Chem. 1983;55:712A–24A. [Google Scholar]
- 14.Taylor J. Quality Assurance of Chemical Measurements. Chelsea, Michigan: Lewis Publishers, Inc.; 1987. pp. 125–6. [Google Scholar]
- 15.Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129:1072–8. doi: 10.1093/oxfordjournals.aje.a115211. [DOI] [PubMed] [Google Scholar]
- 16.Wolff MS, Camman D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 1997;105:13–4. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106:171–89. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, et al. Fifteen Years after “Wingspread”--Environmental Endocrine Disrupters and Human and Wildlife Health: Where We are Today and Where We Need to Go. Toxicol Sci. 2008;105:235–59. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holoubek I. Polychlorinated Biphenyl (PCB) Contaminated Sites Worldwide. In: Robertson LW, Hansen LG, editors. PCBs Recent Advances in Environmental Toxicology and Health Effects. Lexington: The University Press of Kentucky; 2001. pp. 17–26. [Google Scholar]
- 20.Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, et al. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ Health Perspect. 2007;115:1794–800. doi: 10.1289/ehp.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lilienthal H, Hack A, Roth-Harer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2, 4,4, 5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodavanti PRS, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, et al. Developmental Exposure to a Commercial PBDE Mixture, DE-71: Neurobehavioral, Hormonal, and Reproductive Effects. Toxicological Sciences. 116:297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- 23.Dickerson S, Gore A. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Reviews in Endocrine & Metabolic Disorders. 2007;8:143–59. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 24.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122:778–88. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Hartshorne GM, Lyrakou S, Hamoda H, Oloto E, Ghafari F. Oogenesis and cell death in human prenatal ovaries: what are the criteria for oocyte selection? Mol Hum Reprod. 2009;15:805–19. doi: 10.1093/molehr/gap055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poppe K, Velkeniers B. Female infertility and the thyroid. Best Pract Res Clin Endocrinol Metab. 2004;18:153–65. doi: 10.1016/j.beem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Schrader TJ, Cooke GM. Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reproductive Toxicology. 2003;17:15–23. doi: 10.1016/s0890-6238(02)00076-x. [DOI] [PubMed] [Google Scholar]
- 29.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–5. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 30.Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC. Polychlorinated Biphenyls Interfere with Androgen-Induced Transcriptional Activation and Hormone Binding. Toxicology and Applied Pharmacology. 2002;179:185–94. doi: 10.1006/taap.2002.9371. [DOI] [PubMed] [Google Scholar]
- 31.Krysko DV, Diez-Fraile A, Criel G, Svistunov AA, Vandenabeele P, D'Herde K. Life and death of female gametes during oogenesis and folliculogenesis. Apoptosis. 2008;13:1065–87. doi: 10.1007/s10495-008-0238-1. [DOI] [PubMed] [Google Scholar]
- 32.Soto AM, Rubin BS, Sonnenschein C. Endocrine Disruption and the Female. In: Gore AC, editor. Endocrine-Disrupting Chemicals From Basic Research to Clinical Practice. Totowa: Humana Press; 2007. pp. 9–31. [Google Scholar]
- 33.Plísková M, Vondrácek J, C RF, Nera J, Kocan A, Petrík J, et al. Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environ Health Perspect. 2005;113:1277–84. doi: 10.1289/ehp.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JS, Petreas M, Cohn BA, Cirillo PM, Factor-Litvak P. Hydroxylated PCB metabolites (OH-PCBs) in archived serum from 1950-60s California mothers: a pilot study. Environment International. 2009;35:937–42. doi: 10.1016/j.envint.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otake T, Yoshinaga J, Enomoto T, Matsuda M, Wakimoto T, Ikegami M, et al. Thyroid hormone status of newborns in relation to in utero exposure to PCBs and hydroxylated PCB metabolites. Environ Res. 2007;105:240–6. doi: 10.1016/j.envres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura S, Jinno N, Suzuki T, Sugihara K, Ohta S, Kuroki H, et al. Thyroid hormone-like and estrogenic activity of hydroxylated PCBs in cell culture. Toxicology. 2005;208:377–87. doi: 10.1016/j.tox.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Machala M, Bláha L, Lehmler HJ, Plísková M, Májková Z, Kapplová P, et al. Toxicity of Hydroxylated and Quinoid PCB Metabolites: Inhibition of Gap Junctional Intercellular Communication and Activation of Aryl Hydrocarbon and Estrogen Receptors in Hepatic and Mammary Cells. Chem Res Toxicol. 2004;17:340–7. doi: 10.1021/tx030034v. [DOI] [PubMed] [Google Scholar]
- 38.Braathen M, Mortensen AS, Sandvik M, Skare JU, Arukwe A. Estrogenic effects of selected hydroxy polychlorinated biphenyl congeners in primary culture of Atlantic Salmon (Salmo salar) hepatocytes. Arch Environ Contam Toxicol. 2009;56:111–22. doi: 10.1007/s00244-008-9163-0. [DOI] [PubMed] [Google Scholar]
- 39.Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, et al. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in pregnant women from eastern Slovakia. Environ Health Perspect. 2007;115:20–7. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutz FW, Wood PH, Bottimore DP. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev Environ Contam Toxicol. 1991;120:1–82. doi: 10.1007/978-1-4612-3080-9_1. [DOI] [PubMed] [Google Scholar]
- 41.Park JS, Bergman K, Linderholm L, Athanasiadou M, Kocan A, Petrik J, et al. Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from eastern Slovakia. Chemosphere. 2008;70:1676–84. doi: 10.1016/j.chemosphere.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Axmon A, Rylander L, Stromberg U, Hagmar L. Time to pregnancy and infertility among women with a high intake of fish contaminated with persistent organochlorine compounds. Scand J Work Environ Health. 2000;26:199–206. doi: 10.5271/sjweh.532. [DOI] [PubMed] [Google Scholar]
- 43.Buck GM, Vena JE, Schisterman EF, Dmochowski J, Mendola P, Sever LE, et al. Parental consumption of contaminated sport fish from Lake Ontario and predicted fecundability. Epidemiol. 2000;11:388–93. doi: 10.1097/00001648-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 44.McGuinness BM, Buck GM, Mendola P, Sever LE, Vena JE. Infecundity and consumption of polychlorinated biphenyl-contaminated fish. Arch Environ Health. 2001;56:250–3. doi: 10.1080/00039890109604449. [DOI] [PubMed] [Google Scholar]
- 45.Axmon A, Rylander L, Stromberg U, Jonsson B, Nilsson-Ehle P, Hagmar L. Polychlorinated biphenyls in serum and time to pregnancy. Environ Res. 2004;96:186–95. doi: 10.1016/j.envres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Law DC, Klebanoff MA, Brock JW, Dunson DB, Longnecker MP. Maternal serum levels of polychlorinated biphenyls and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and time to pregnancy. Am J Epidemiol. 2005;162:523–32. doi: 10.1093/aje/kwi240. [DOI] [PubMed] [Google Scholar]
- 47.Toft G, Axmon A, Giwercman A, Thulstrup AM, Rignell-Hydbom A, Pedersen HS, et al. Fertility in four regions spanning large contrasts in serum levels of widespread persistent organochlorines: a cross-sectional study. Environ Health. 2005;4:26. doi: 10.1186/1476-069X-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buck Louis GM, Dmochowski J, Lynch C, Kostyniak P, McGuinness BM, Vena JE. Polychlorinated biphenyl serum concentrations, lifestyle and time-to-pregnancy. Hum Reprod. 2009;24:451–8. doi: 10.1093/humrep/den373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu ML, Guo YL, Hsu CC, Rogan WJ. Menstruation and reproduction in women with polychlorinated biphenyl (PCB) poisoning: long-term follow-up interviews of the women from the Taiwan Yucheng cohort. Int J Epidemiol. 2000;29:672–7. doi: 10.1093/ije/29.4.672. [DOI] [PubMed] [Google Scholar]
- 50.Giusti R, Iwamoto K, Hatch E. Diethylstilbestrol revisited : A review of the longterm health effects. Annals of Internal Medicine. 1995;122:778–88. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 51.Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: Development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78:1091–101. doi: 10.1095/biolreprod.107.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newbold R, Hanson R, Jefferson W, Bullock B, Haseman J, McLachlan J. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19:1655–63. doi: 10.1093/carcin/19.9.1655. [DOI] [PubMed] [Google Scholar]
- 53.Anway MD, Skinner MK. Epigenetic Transgenerational Actions of Endocrine Disruptors. Endocrinology. 2006;147:s43–9. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.