Abstract
BACKGROUND
Solid-pseudopapillary neoplasms (SPNs) are rare pancreatic tumors with malignant potential. Clinicopathologic characteristics and outcomes of patients with SPN were reviewed.
STUDY DESIGN
Longterm outcomes were evaluated in patients with an SPN who were followed from 1970 to 2008.
RESULTS
Thirty-seven patients were identified with an SPN. Thirty-three (89%) were women, and median age at diagnosis was 32 years. Most patients were symptomatic; the most common symptom was abdominal pain (81%). Thirty-six patients underwent resection; one patient with distant metastases was not operated on. There were no 30-day mortalities. Median tumor size was 4.5 cm. Thirty-four patients underwent an R0 resection, 1 had an R1 resection, and 1 had an R2 resection. Two patients had lymph node metastases, and one patient had perineural invasion. After resection, 34 (94%) patients remain alive. One patient died of unknown causes 9.4 years after resection, and another died of unrelated causes 25.6 years after operation. The patient with widespread disease who didn’t have resection died 11 months after diagnosis. Thirty-five of the 36 patients having resection remained disease free, including those who died of unrelated causes (median followup, 4.8 years). One patient developed a recurrence 7.7 years after complete resection. She was treated with gemcitabine and remains alive 13.6 months after recurrence.
CONCLUSIONS
SPNs are rare neoplasms with malignant potential found primarily in young women. Formal surgical resection may be performed safely and is associated with longterm survival.
Solid-pseudopapillary neoplasms (SPNs) of the pancreas are rare neoplasms, comprising only 1% to 2% of all pancreatic tumors.1,2 There is a strong female preponderance, and most SPNs present in the third and fourth decade of life.3 The first published description of an SPN was by Frantz in 1959.4 This report consisted of a pathologic description of three patients with SPN. Hamoudi and colleagues5 added an additional patient to the literature in 1970 and detailed the electron microscopic appearance of the tumor. The first report in the surgical literature of an SPN was by Sanfey and associates in 1983.6 SPNs are also called solid and papillary tumors, papillary cystic tumors, solid cystic tumors, Frantz tumors, and Hamoudi tumors. SPN is synonymous with the preceding names and is the preferred terminology.7
SPNs are defined by their gross and histologic appearance.7 They are composed of discohesive polygonal cells that surround delicate blood vessels and form a solid mass, with frequent cystic degeneration and intracystic hemorrhage. The neoplastic cells have uniform nuclei, finely stippled chromatin, and nuclear grooves.7 Eosinophilic globules, foam cells, and cholesterol clefts are often present.
Symptoms of SPN are often nonspecific and include abdominal pain, dyspepsia, early satiety, and nausea and vomiting (41% to 64%).3,8 SPNs are usually localized pancreatic neoplasms, although 10% to 15% of patients will develop metastases.1,9 These metastases are often amenable to resection, and complete extirpation is associated with longterm survival.1,10 Reported clinical and histopathologic features predictive of recurrence or metastases include tumor size greater than 5 cm, venous invasion, nuclear grade, and prominent necrobiotic nests,9,11 but these features are not consistently reported in all large series.1,12,13
Because of the rarity of SPN, most of what is known about this disease is from small series or case reports, which include our previous reports of two and, later, seven patients.14,15 So the goal of this study was to determine the outcomes of a larger series of patients, from a single institution, who are undergoing resection of SPN.
METHODS
A review of our institution’s pathologic and surgical pancreatic databases from April 1970 to October 2008 was performed. Clinical, operative, pathologic, and survival data were obtained on all patients with a pathologic diagnosis of SPN. A pathologic review was performed on all patients included in this study for the verification of diagnosis. For purposes of inclusion in the study, SPN was defined as an epithelial neoplasm composed of discohesive polygonal cells that surround delicate blood vessels and form solid masses, with frequent cystic degeneration and intracystic hemorrhage.7 The neoplastic cells had to have uniform nuclei, finely stippled chromatin, and usually had nuclear grooves. In cases in which the diagnosis of SPN was unclear, immunolabeling was performed. Abnormal nuclear labeling with antibodies to β-catenin was considered to be strongly supportive of the diagnosis of SPN.
A perioperative surgical complication was defined as 1 occurring within 30 days of operation. A mortality occurring within 30 days of operation was considered a surgical mortality. Complications were classified from Grade I to IV, as previously described.16 Pancreatic fistula was defined using the recommendations of the International Study Group on Pancreatic Fistula.17 Longterm survival data and followup information were collected using the patients’ medical records and the US Social Security Death Index. Fisher’s exact test and a t-test were used for categorical and continuous data, respectively. Differences were considered significant if the p value was less than 0.05. This study was approved by the Institutional Review Board for Human Research and complied with the Health Insurance Portability and Accountability Act regulations.
RESULTS
Demographic characteristics
Thirty-seven patients with a diagnosis of SPN were identified from our pancreatic surgery and pathology databases. Thirty-six underwent a resection. Thirty-four underwent resection at our institution; this represents 0.9% of the total number of pancreatic resections at our institution during the time interval used in the study. Two of the 36 patients underwent resection elsewhere but were followed postoperatively at our institution. One patient had disseminated disease and was not operated on. There were 33 (89%) women in the entire cohort, yielding a female-to-male ratio of 8.25 to 1. The median age at presentation was 32 years (range, 13 to 75 years). Twenty-one patients were Caucasian, 12 patients were African American, 3 patients were Asian, and 1 patient was Hispanic (Table 1).
Table 1.
Demographic Information
| Characteristic | n (n = 37) | % |
|---|---|---|
| Age, median, y | 32 | |
| Female | 33 | 89 |
| Race | ||
| Caucasian | 21 | 57 |
| African American | 12 | 32 |
| Asian | 3 | 8 |
| Hispanic | 1 | 3 |
Presentation and workup
Preoperative data were available for 31 patients. Twenty-seven (87%) patients presented with symptoms. The most common presenting symptom was abdominal pain (n = 26; 84%), followed by nausea and vomiting (n = 6; 19%). A pancreatic mass was discovered incidentally on CT scan in two patients and during an open cholecystectomy in one patient. None of the patients presented with jaundice, and only three had significant weight loss (Table 2).
Table 2.
Presenting Symptoms
| Characteristic | n (n = 31) | % |
|---|---|---|
| Symptomatic | 27 | 87 |
| Abdominal pain | 26 | 84 |
| Jaundice | 0 | 0 |
| Fevers/chills | 1 | 3 |
| Nausea/vomiting | 6 | 19 |
| Pancreatitis | 3 | 10 |
| Weight loss | 3 | 10 |
The typical CT appearance of an SPN is shown in Figure 1 and is characterized by a heterogeneous pancreatic mass with cystic and solid components. The solid components enhance on arterial phase. All 36 patients who underwent a resection had a preoperative CT scan. Of these patients, 14 (39%) had a lesion in the pancreatic head alone, 7 (19%) patients had a lesion in the body of the pancreas alone, 14 (39%) patients had a lesion in the pancreatic tail alone, and 1 (3%) patient had multifocal disease (head and tail). Imaging further demonstrated calcifications in 6 (17%) patients and a dilated pancreatic duct in 5 (14%) patients. One patient demonstrated possible vascular invasion into the portal vein, but, at operation, no portal vein involvement was found. None of the patients was found to have lymphadenopathy on imaging. One patient was noted to have hepatic metastases at diagnosis. A preoperative percutaneous or endoscopic biopsy was performed in 13 patients (36%). Based on the preoperative biopsy, the pre-operative diagnosis of SPN was correctly made in 8 of 13 patients.
Figure 1.
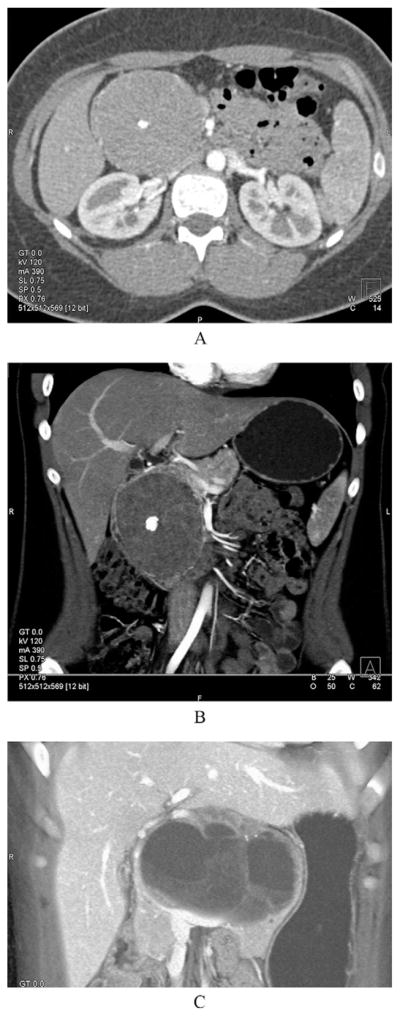
Cross-sectional imaging of a solid-pseudopapillary neoplasm (SPN). (A) Axial CT image of an SPN. (B) The same lesion but from a coronal view. Note that in the arterial phase, this particular lesion is mainly solid. A characteristic central calcification is clearly visible in both views. (C) A coronal CT image of an SPN that is mainly cystic. Note again the calcifications (white arrow).
In this population of SPN patients, the majority were nutritionally replete, with a serum albumin ranging from 3.3 to 5.1 g/dL (median, 4.35 g/dL). Median preoperative hemoglobin was 12.8 g/dL (mean, 12.8 g/dL; SD 0.2 g/dL). Three patients had an elevated serum amylase. Thirteen patients had CA-125, CA 19-9, or CEA levels drawn preoperatively, and none was elevated.
Operative and postoperative courses
Thirty-six patients in this series underwent resection of their SPN. Fourteen patients had a pancreaticoduodenectomy, 6 patients had a spleen-preserving distal pancreatectomy, 14 patients had a distal pancreatectomy with splenectomy, 1 patient had a total pancreatectomy, and 1 patient had an enucleation of 2 lesions. Two of these patients underwent a distal pancreatectomy at other hospitals. For the patients operated on at our institution, other procedures included transverse colectomy (n = 2), wedge gastrectomy (n = 1), Nissen fundoplication (n = 1), and oopherectomy (n = 1).
More detailed operative and perioperative data were available only for patients who underwent resection at our institution (n = 34). The median operative time, estimated blood loss, and intraoperative transfusion requirements were 4 hours and 45 minutes, 325 mL, and 0 U, respectively. There were no perioperative deaths. Postoperative complication data were available for 31 patients. The overall complication rate was 52% (n = 16). Grades I, II, IIIa, IIIb, and IV complication rates were 29% (n = 9), 3% (n = 1), 20% (n = 6), 10% (n = 3), and 0% (n = 0), respectively. The most common complication was development of a postoperative pancreatic fistula (n = 6, 20%) followed by delayed gastric emptying (n = 3; 10%) and pancreatitis (n = 3, 10%). Major therapeutic interventions for complications were necessary in three patients and included drainage of a pancreatic pseudocyst with a cystjejunostomy, a small bowel resection, and evacuation of an intraabdominal hematoma. Another patient required endoscopic dilation of the duodenojejunostomy. The median duration of postoperative hospital stay for all patients was 9 days (range, 5 to 48 days).
Pathology
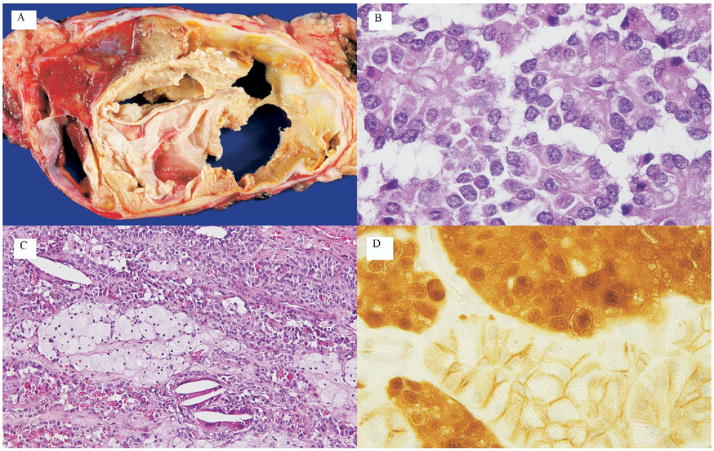
The typical gross and microscopic appearance of an SPN is shown in Figure 2. The majority of SPNs were well-demarcated masses and often demonstrated hemorrhagic areas. These neoplasms were composed of uniform poorly cohesive polygonal cells surrounding delicate blood vessels. Other common histologic findings included eosinophilic globules, foam cells, and cholesterol crystals. Immunohistologic analysis was performed in a subset of specimens for β-catenin, chromagranin, and synaptophysin. As expected, antibodies to β-catenin abnormally labeled neoplastic nuclei. The membranous labeling of nonneoplastic cells in the same slide served as an internal control (Fig. 2). Immunolabeling for chromogranin was focally positive in 2 of 15 labeled cases, and synaptophysin was focally positive in 7 of 14 labeled cases.
Figure 2.
Gross and histopathologic appearance of solid-pseudopapillary neoplasm (SPN). (A) Gross appearance of an SPN. Note the well-delineated mass with spaces (cysts) containing friable pale tan to yellow material. Hemorrhagic areas can be seen (arrow). (B) On microscopic examination, pseudopapillae are formed by loosely cohesive, monotonous short columnar cells surrounding delicate blood vessels. (C) Histologic patterns are variable. This particular example of an SPN exhibits islands of foamy macrophages (arrow), scattered cholesterol clefts with intracytoplasmic periodic-acid Schiff-diastase resistant hyaline globules. (D) Immunolabeling of the neoplasm for β-catenin exhibits both nuclear and cytoplasmic staining pattern (white arrow). Adjacent normal pancreas exhibits membranous pattern only (black arrow).
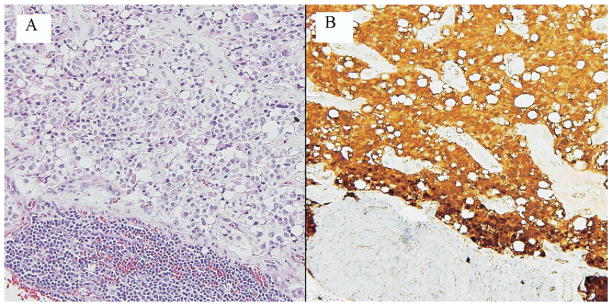
The median tumor size of resected specimens was 4.5 cm (range, 0.3 to 12.0 cm). Thirty-four patients underwent an R0 resection, 1 patient had an R1 resection, and 1 patient had an R2 resection. The patient with the R2 resection underwent a total pancreatectomy for a 12-cm body lesion with gross tumor left behind surrounding the superior mesenteric artery. The patient with an R1 resection underwent a pylorus-preserving pancreaticoduodenectomy and had microscopic disease at the pancreatic neck margin on final pathology. The median number of lymph nodes examined was 10 (range, 0 to 40 nodes). Two patients had metastatic spread to a single regional lymph node each. The histopathologic appearance of a lymph node metastasis is shown in Figure 3. One patient had perineural invasion.
Figure 3.
Solid-pseudopapillary neoplasm metastatic to a lymph node. (A) This lymph node contains metastatic solid-pseudopapillary tumor, leaving only a small contingent of lymphocytes (hematoxylin and eosin). (B) Resident lymphocytes stain pale blue with the counterstain (lower part of field); neoplastic cells exhibit nuclear and cytoplasmic labeling with an antibody for β-catenin.
Survival and followup
Median followup for this study was 4.8 years (range, 0.8 months to 27.4 years). One patient died of unknown causes 9.4 years after an R0 resection. This patient did not have a known recurrence for at least 5.7 years after resection. Another patient died 25.6 years after an R2 resection (total pancreatectomy). She did not have a recurrence and died from diabetes-related complications. A third patient who was not operated on because of widespread metastases died of her disease 11 months after presentation. A single patient developed a widespread intraabdominal recurrence of an SPN 7.7 years after pancreaticoduodenectomy and transverse colectomy. Her original pathology was a 5.2-cm SPN invading the duodenum. Perineural and duodenal invasion was identified, but all 14 lymph nodes were negative for metastases. She underwent gemcitabine-based chemotherapy and remains alive 13.6 months after recurrence. The remaining 33 patients remain alive and clinically disease free, including the 1 with a microscopically positive margin.
DISCUSSION
SPN is a rare tumor of the pancreas that most commonly affects young women. The number of reports about SPN has increased in the past decade, and, as of 2003, 718 cases had been reported. One-half of these reports were published between 1995 and 2003.3,8 This increase has also been seen at our institution, with 28 of our 37 patients with an SPN diagnosed after 1995.
The molecular events involved in the development of SPN have recently been reported. The genetic profile associated with SPN is distinct from that of pancreatic adenocarcinoma. Unlike pancreatic adenocarcinoma, SPNs typically do not harbor a KRAS gene mutation or exhibit genetic silencing of the DPC4 gene. SPNs are characterized by the presence of activating β-catenin gene mutations that interfere with phosphorylation of the protein product.18,19 β-catenin is a known downstream regulator of wnt signaling. Either physiologic signals or pathologic mutations that enhance β-catenin stability or disrupt adenomatosis polyposis coli (APC) function result in the accumulation of β-catenin within the cytoplasm. This results in the translocation of β-catenin into the nucleus, where it functions as a transcriptional regulator by binding to DNA regulatory elements. Known targets for this pathway include the growth regulatory genes cyclin D1 and c-myc. In addition, abnormal β-catenin function may also explain the poorly cohesive nature of SPN. It has been shown that β-catenin interacts with the cell adhesion molecule E-cadherin, preventing the formation of normal cell-to-cell interactions.20
The median age at diagnosis in our group was 32 years, which was comparable to other experiences reported in the US1 but older than those seen in several Asian series (median age in the range of 26 years).21–23 Although our findings were consistent with the previous notion that this entity is a disease of younger women, it should be noted that there was a wide age distribution among the women. Additionally, four of our patients were men. A recent study from Brazil described a difference in age at presentation between the genders, with men presenting at a younger age.13 Men in our series were actually older than the women, but we had fewer men to make a statistically meaningful comparison (40 years versus 32 years; p = 0.55).
The preoperative diagnosis of an SPN is often made based on clinical suspicion. Most of the patients in our series presented with abdominal pain or discomfort. In contrast to other reports, none of the patients in our study, even those with lesions in the head of the pancreas, had jaundice. The CT appearance of SPN can range from mostly solid to mostly cystic (Fig. 1). SPNs often display peripheral arterial enhancement and a central calcification. The differential diagnosis of a predominantly cystic SPN should include mucinous or serous cystic neoplasms and cystic degeneration of a typically solid neoplasm, such as a pancreatic neuroendocrine tumor, lymphoma, or acinar cell cancer. Of course, the possibility of pancreatic adenocarcinoma arising in a cystic neoplasm (intraductal papillary mucinous neoplasm or mucinous cystic neoplasm) must always be considered, particularly in an older patient. The utility of preoperative biopsy is not clearly defined by our study. Based on fine-needle aspiration, a correct diagnosis was made in only 62% of patients in our series.
The majority of patients in our study, and in other reports, are cured of their disease. But despite the indolent reputation of SPN, the potential for aggressive behavior exists. In our series, a total of 4 (11%) of the 37 patients presented with or developed metastases. These included 2 patients with lymph node metastases, a single patient who presented with a liver metastasis, and a patient who developed systemic disease nearly 8 years after a potentially curative resection. Similarly, other groups have reported that 10% to 15% of patients with SPN have metastases at the time of resection or develop metastases at some point in their course.1,9 In addition, several reports describe patients with locally advanced SPN, and we had one patient with involvement of the superior mesenteric artery and a second with invasion of the duodenum.
In light of the potentially aggressive behavior of SPN, it would be of interest to determine clinicopathologic factors that might predict outcomes. But this goal remains elusive. Because all but three patients in our series remain alive, statistical evaluation of risk factors is not possible. Two patients died of unknown causes and did not have evidence of recurrence near the time of their deaths. Additionally, a qualitative evaluation of potential risk factors lends little further insight into this issue. Two of the 36 surgically resected patients in our series had lymph node metastases present at the time of resection, and neither patient has evidence of recurrence at 6 months and 2.5 years after operation. One patient underwent an R2 resection and remained alive for more than 25 years after operation without evidence of recurrence. A single patient was found to have a microscopically positive margin on final pathology and has no evidence of recurrence 18.9 years later. The single patient who suffered recurrence, but remains alive, had node-negative disease and a margin-negative resection. She did have perineural invasion. Other investigators have reported clinical and histopathologic features predictive of recurrence or metastases that include tumor size greater than 5 cm, venous invasion, nuclear grade, and prominent necrobiotic nests.9,11 In our series, 14 of the 35 patients whose tumor sizes were available had lesions ≥ 5 cm, and only 1 of these had a recurrence. Other groups have found no relationship between clinicopathologic features (mitotic rate, nuclear pleomorphism, and vascular and perineural invasion) with patient survival.24
Various outcomes have been reported for patients with locally advanced or metastatic SPN. In the series from Memorial Sloan-Kettering, two patients were determined to be unresectable as a result of large-vessel invasion. Interestingly, both of these patients were alive at the time of publication of their series, with 1 having survived at least 13 years. Patients in that series were treated with 5-fluorouracil, doxorubicin, and streptozocin or interferon, cisplatin, and topotecan.1 Other investigators have reported longterm survival in patients undergoing synchronous or metachronous resection of liver metastases for SPN, with survival ranging from as many as 10 years25,26 to as few as 6 months.27 In the Memorial Sloan-Kettering series, four patients underwent simultaneous resection of their primary SPN and liver metastases. Two of these patients have no evidence of recurrence, and 1 is alive with recurrence at 6 years and another died of disease in 8 months. The single patient in our series with gross residual tumor left at the superior mesenteric artery survived more than 25 years without evidence of recurrence. She was not treated with adjuvant therapy.
Because longterm survival can be achieved after resection of metastatic SPN, it would seem prudent to take an aggressive surgical approach aimed at resection of the primary and all metastatic disease if possible. Some have argued that the low malignant potential of SPN and the increased morbidity of formal resection make enucleation the favorable operation for this disease.3 But the relative effectiveness of enucleation versus formal resection in the treatment of SPN is uncertain. The patient in our study who underwent enucleation has survived for at least 2 years.
The role of chemotherapy or chemoradiotherapy in the treatment of SPN is also unclear. A single case report noted radiation response in a locally advanced lesion involving the portal vein.28 One of our patients developed a recurrence, with widespread disease in her abdomen and lung. She has been treated with gemcitabine-based chemotherapy and remained alive at 13.6 months. Interestingly, Maffuz and coworkers29 reported a patient with successful reduction in tumor size using gemcitabine therapy who went on to resection. Small series or case reports have documented other successful regimens. For example, Rebhandl and associates30 demonstrated a response to ifosfamide, cisplatin, and VP16 in a patient who had an incomplete resection and a recurrence. Finally, a single case report of a 14-year-old girl with unresectable SPN demonstrated a significant response to a regimen consisting of cisplatin, ifosfamide, etoposide, and vincristine, but her SPN never became resectable.31
In conclusion, SPN is a rare neoplasm that is found primarily in young women. These lesions present with abdominal pain but not jaundice. Formal surgical resection may be performed safely and should be favored for patients with SPN. It is clear that although some SPNs behave aggressively, most do not, and we currently cannot predict outcomes for these patients.
Acknowledgments
Supported in part by the NIH SPORE (Specialized Programs of Research Excellence) in Gastrointestinal Cancer Grant CA62924.
Footnotes
Author Contributions
Study conception and design: Reddy, Cameron, Wolfgang
Acquisition of data: Reddy, Scudiere, Hruban, Fishman
Analysis and interpretation of data: Reddy, Cameron, Schulick, Wolfgang
Drafting of manuscript: Reddy, Ahuja, Edil, Wolfgang
Critical revision: Cameron, Ahuja, Pawlik, Schulick, Wolfgang
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 120th Annual Meeting, West Palm Beach, FL, December 2008.
References
- 1.Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9:35–40. doi: 10.1245/aso.2002.9.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Ng KH, Tan PH, Thng CH, Ooi LL. Solid pseudopapillary tumour of the pancreas. A N Z J Surg. 2003;73:410–415. doi: 10.1046/j.1445-2197.2003.t01-1-02634.x. [DOI] [PubMed] [Google Scholar]
- 3.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Frantz VK. Atlas of tumor pathology. Washington, DC: US Armed Forces Institute of Pathology; 1959. Tumors of the pancreas; pp. 32–33. [Google Scholar]
- 5.Hamoudi AB, Misugi K, Grosfeld JL, Reiner CB. Papillary epithelial neoplasm of pancreas in a child. Report of a case with electron microscopy. Cancer. 1970;26:1126–1134. doi: 10.1002/1097-0142(197011)26:5<1126::aid-cncr2820260524>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Sanfey H, Mendelsohn G, Cameron JL. Solid and papillary neoplasm of the pancreas. A potentially curable surgical lesion. Ann Surg. 1983;197:272–275. doi: 10.1097/00000658-198303000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hruban RH, Pitman MB, Klimstra DS. Fascile 6. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007. Solid pseudopapillary neoplasms. Atlas of tumor pathology. [Google Scholar]
- 8.de Castro SM, Singhal D, Aronson DC, et al. Management of solid-pseudopapillary neoplasms of the pancreas: a comparison with standard pancreatic neoplasms. World J Surg. 2007;31:1130–1135. doi: 10.1007/s00268-006-0214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang CM, Kim KS, Choi JS, et al. Solid pseudopapillary tumor of the pancreas suggesting malignant potential. Pancreas. 2006;32:276–280. doi: 10.1097/01.mpa.0000202956.41106.8a. [DOI] [PubMed] [Google Scholar]
- 10.Hassan I, Celik I, Nies C, et al. Successful treatment of solid-pseudopapillary tumor of the pancreas with multiple liver metastases. Pancreatology. 2005;5:289–294. doi: 10.1159/000085285. [DOI] [PubMed] [Google Scholar]
- 11.Nishihara K, Nagoshi M, Tsuneyoshi M, et al. Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Cancer. 1993;71:82–92. doi: 10.1002/1097-0142(19930101)71:1<82::aid-cncr2820710114>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Choi SH, Kim SM, Oh JT, et al. Solid pseudopapillary tumor of the pancreas: a multicenter study of 23 pediatric cases. J Pediatr Surg. 2006;41:1992–1995. doi: 10.1016/j.jpedsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Machado MC, Machado MA, Bacchella T, et al. Solid pseudo-papillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery. 2008;143:29–34. doi: 10.1016/j.surg.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Sanfey H, Mendelsohn G, Cameron JL. Solid and papillary neoplasm of the pancreas. A potentially curable surgical lesion. Ann Surg. 1983;197:272–275. doi: 10.1097/00000658-198303000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zinner MJ, Shurbaji MS, Cameron JL. Solid and papillary epithelial neoplasms of the pancreas. Surgery. 1990;108:475–480. [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y, Kato K, Notohara K, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401–8404. [PubMed] [Google Scholar]
- 20.Chetty R, Serra S. Membrane loss and aberrant nuclear localization of E-cadherin are consistent features of solid pseudopapillary tumour of the pancreas. An immunohistochemical study using two antibodies recognizing different domains of the E-cadherin molecule. Histopathology. 2008;52:325–330. doi: 10.1111/j.1365-2559.2007.02949.x. [DOI] [PubMed] [Google Scholar]
- 21.Sun CD, Lee WJ, Choi JS, et al. Solid-pseudopapillary tumours of the pancreas: 14 years experience. A N Z J Surg. 2005;75:684–689. doi: 10.1111/j.1445-2197.2005.03488.x. [DOI] [PubMed] [Google Scholar]
- 22.Goh BK, Tan YM, Cheow PC, et al. Solid pseudopapillary neoplasms of the pancreas: an updated experience. J Surg Oncol. 2007;95:640–644. doi: 10.1002/jso.20735. [DOI] [PubMed] [Google Scholar]
- 23.Peng CH, Chen DF, Zhou GW, et al. The solid-pseudopapillary tumor of pancreas: the clinical characteristics and surgical treatment. J Surg Res. 2006;131:276–282. doi: 10.1016/j.jss.2005.11.585. [DOI] [PubMed] [Google Scholar]
- 24.Adair CF, Wenig BM, Heffess CS. Solid and papillary cystic carcinoma of the pancreas: a tumor of low malignant potential. Int J Surg Pathol. 1995;2:326. [Google Scholar]
- 25.Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 2006;93:733–737. doi: 10.1002/bjs.5334. [DOI] [PubMed] [Google Scholar]
- 26.Nishihara K, Nagoshi M, Tsuneyoshi M, et al. Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Cancer. 1993;71:82–92. doi: 10.1002/1097-0142(19930101)71:1<82::aid-cncr2820710114>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Horisawa M, Niinomi N, Sato T, et al. Frantz’s tumor (solid and cystic tumor of the pancreas) with liver metastasis: successful treatment and long-term follow-up. J Pediatr Surg. 1995;30:724–726. doi: 10.1016/0022-3468(95)90701-7. [DOI] [PubMed] [Google Scholar]
- 28.Fried P, Cooper J, Balthazar E, et al. A role for radiotherapy in the treatment of solid and papillary neoplasms of the pancreas. Cancer. 1985;56:2783–2785. doi: 10.1002/1097-0142(19851215)56:12<2783::aid-cncr2820561211>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Maffuz A, Bustamante FT, Silva JA, Torres-Vargas S. Preoperative gemcitabine for unresectable, solid pseudopapillary tumour of the pancreas. Lancet Oncol. 2005;6:185–186. doi: 10.1016/S1470-2045(05)01770-5. [DOI] [PubMed] [Google Scholar]
- 30.Rebhandl W, Felberbauer FX, Puig S, et al. Solid-pseudopapillary tumor of the pancreas (Frantz tumor) in children: report of four cases and review of the literature. J Surg Oncol. 2001;76:289–296. doi: 10.1002/jso.1048. [DOI] [PubMed] [Google Scholar]
- 31.Hah JO, Park WK, Lee NH, Choi JH. Preoperative chemotherapy and intraoperative radiofrequency ablation for unresectable solid pseudopapillary tumor of the pancreas. J Pediatr Hematol Oncol. 2007;29:851–853. doi: 10.1097/MPH.0b013e318158159c. [DOI] [PubMed] [Google Scholar]