Abstract
Amoeboid protists that harbor bacterial pathogens are of significant interest as potential reservoirs of disease-causing organisms in the environment, but little is known about them in marine and other saline environments. We enriched amoeba cultures from sediments from four sites in the New England estuarine system of Mt. Hope Bay, Massachusetts and from sediments from six sites in the Great Salt Lake, Utah. Cultures of amoebae were enriched using both minimal- and non-nutrient agar plates, made with fresh water, brackish water or saltwater. Recovered amoeba cultures were assayed for the presence of Legionella species using nested polymerase chain reactions (PCR) and primers specific for the genus. Positive samples were then screened with nested amplification using primers specific for the macrophage infectivity potentiator surface protein (mip) gene from L. pneumophila. Forty-eight percent (185 out of 388) of isolated amoeba cultures were positive for the presence of Legionella species. Legionella pneumophila was detected by PCR in 4% of the amoeba cultures (17 out of 388), and most of these amoebae were growing on marine media. Our results show that amoebae capable of growing in saline environments may harbor not only a diverse collection of Legionella species, but also species potentially pathogenic to humans.
Keywords: Amoeba, diversity, Legionella, marine, salt
Introduction
Species of the gram-negative bacterial genus Legionella can cause outbreaks and random cases of pneumonia-like infections in humans (Fields et al. 2002, Lau & Ashbolt 2009). The severe infection is known as Legionnaires’ disease, while the less acute, flu-like disease is called Pontiac Fever. The CDC estimates that between 8,000–18,000 hospitalized cases of Legionnaire’s disease occur in the U.S. each year, with a fatality rate of 5–40% (http://www.cdc.gov/legionella/top10.htm). Approximately half of the more than 50 recognized species have been associated with human infections, but about 90% of the cases of Legionnaires’ disease in the U.S. are caused by Legionella pneumophila, specifically serogroup 1 strains (Yu et al. 2002, Lau & Ashbolt 2009, Newton et al. 2010). Diagnosis of infections is difficult, particularly in the absence of outbreaks, due to the lack of distinctive symptoms, and it is likely that Legionella infections are significantly under-diagnosed and under-reported (Lau & Ashbolt 2009).
Species of Legionella are found worldwide in freshwater environments, especially in man-made water structures including hot water systems, cooling towers and even sewage (Fields et al. 2002, Wullings & van der Kooij 2006), but have been reported rarely in the marine environment (Ortiz-Roque & Hazen 1987, Palmer et al. 1993, Sinigalliano et al. 2007). The marine sites positive for legionellae were associated with sewage, suggesting repeated contamination was likely rather than the persistence of these organisms in the environment. The traditional view has been that legionellae, especially L. pneumophila, do not survive or persist in saline waters. Studies with culture media supporting the growth of legionellae in the laboratory reported that the presence of NaCl inhibited growth of the virulent form of the organism (Catrenich & Johnson 1989). Prior to that, Dutka (Dutka 1984) showed that while L. pneumophila was more susceptible to inactivation by sunlight in saltwater than in freshwater, it was more robust than Escherichia coli, Staphylococcus aureus and Pseudomonas under the same conditions. A study by Heller et al. (Heller et al. 1998) found that between 4–20°C, the die off of L. pneumophila in saline media was not much different than in distilled water, but die off was enhanced at 37°C in the presence of 1.5–3% NaCl (15‰ – 30‰ salinity). When they tested autoclaved natural seawater corresponding to ca. 15‰ salinity (Baltic Sea) and 30‰ salinity (North Sea), the effect of temperature on die off was reduced, and even after 7 days viable L. pneumophila could be recovered at 37°C. These later results strongly suggest that legionellae could persist in viable forms in saline environments, such as coastal marine sites and estuaries.
Legionellae are fastidious organisms that require a unique mixture of nutrients for laboratory growth (Fields et al. 2002). Their presence in aquatic environments and in biofilms is often linked to the presence of free-living protists (Fields et al. 2002, Greub & Raoult 2004), and the infection of humans is considered an opportunistic relationship and is associated with the presence of amoebae in water (Bichai et al. 2008). Amoebae are most commonly observed as hosts of Legionella, but ciliates and the slime mold Dictyostelium are implicated as well (Fields 1996, Hagele et al. 2000, Solomon et al. 2000, Barker & Brown 1994). Acanthamoebae, the amoebae most commonly associated with legionellae, are considered freshwater organisms despite multiple reports of their recovery from marine environments by cultivation on brackish media and subsequent growth on full-strength seawater media (Davis 1976, Daggett et al. 1982, Sawyer et al. 1977). Acanthamoebae often do not compete successfully against other marine amoebae in enrichment culture using full-strength seawater (Sawyer et al. 1977), which may have led to the opinion that they were not active participants in the marine environment. Their ability to form dormant, resilient cysts when stressed due to lack of food or shifts in temperature, also may have contributed to this view. Acanthamoebae have been demonstrated to be present in the marine environment, along with several other types of amoebae (Davis 1976, Page 1983). Therefore, it is possible that there are organisms present in coastal marine systems that may be capable of supporting the intracellular growth of legionellae.
The goal of our research was to examine whether legionellae, and specifically L. pneumophila, could be detected in amoeba cultures recovered from saline aquatic environments. Additionally, if the pathogen could be detected, we wanted to determine which amoebae were potentially harboring it. The coastal environments that were chosen for sampling were ones that had some form of anthropogenic impact, either treated sewage, freshwater runoff or thermal input. Amoebae were cultured from sediment samples and cultures were harvested and tested for the presence of Legionella by polymerase chain reaction (PCR) amplification. Samples positive for L. pneumophila were also amplified for eukaryotic small subunit (18S) ribosomal genes to determine the potential amoeba host.
Methods
Sample sites and sediment collection
Sediment was collected at 4 sites in Mt Hope Bay, Massachusetts (Figure 1A; 41.7°N −71.2°W) and 6 sites in the Great Salt Lake, Utah (Figure 1B; 41.0°N −112.2°W). Mt. Hope Bay (MHB) is located within the Narragansett Bay Watershed in southeastern Massachusetts and eastern Rhode Island. The bay is impacted by urban activities, including secondary sewage outfalls, runoff from impervious surfaces of the cities that surround it, and thermal discharge from a seawater-cooled, coal-fired power plant. The 4 sampling sites were selected to reflect the range of impacts on the bay. Common Fence Point (Site 1; Figure 1A) was chosen to represent the normal residential impact on the water and sediments within the bay, with a year round range of surface water temperature between 7–24°C and salinity between 23–29‰. The Brayton Point location (Site 2; Figure 1A) is impacted by heated water from the power plant discharge (temperature 4–29°C; salinity 27–29‰), while Braga Bridge (Site 3; Figure 1A) was selected near an underwater sewage outfall pipe (temperature 4–26°C; salinity 22–29‰). Dighton Bridge (site 4; Figure 1A) was chosen to represent the input from the brackish water of the Taunton River (between November and August temperature range 16–27°C; salinity 2–8‰). MHB was sampled seven times from November 2004 until June 2006. Sediment samples were collected in MHB using a VanVeen grab with solid “hatch” covers to help reduce contamination from the water column. Surface sediment was collected using sterile spoons or scooped directly into sterile 50 ml centrifuge tubes. These were stored in a cooler for transport back to the lab where they were kept at 4°C for less than 24 hours before processing.
Figure 1.
Sampling sites. A) Mt Hope Bay, MA (Google Earth image). Site 1 is Common Fence Point; Site 2 is Brayton Point; Site 3 is Braga Bridge; Site 4 is Dighton Bridge. B) Great Salt Lake (NASA image). Site 1 is ca. 250 m from the discharge of the Salt Lake City Sewage Canal (GSLSC); Site 2 is the Farmington Bay Wildlife Refuge (GSLR); Site 3 is Farmington Bay Causeway (GSLB); Site 4 is the Bridger Bay swimming beach on Antelope Island in hypersaline Gilbert Bay (GSLFB; sampled only in June 2006); Site 5 is the Bear River Bay Bridge (BRBB); and Site 6 is within Bear River Bay (BRB).
The Great Salt Lake (GSL) was sampled in August 2005 and June 2006. Three of the sample sites were located in hypereutrophic Farmington Bay that receives considerable secondary-treated sewage from metropolitan Salt Lake City (Marcarelli et al. 2006). Two of the sites (Figure 1B, 1 and 2) were located at the south end of the bay in a channel that receives effluents from the Salt Lake City Sewage Canal, and from another wastewater treatment plant. A third site in Farmington Bay was sampled 200 m SW of the breach that joins Farmington and Gilbert Bays. Farmington Bay has a gradient of salinity from nearly fresh water at the southern end, to salinities of ca. 30‰ at the north end (when samples were taken). Another site was sampled at a swimming beach on the NW side of Antelope Island State Park in mesotrophic Gilbert Bay where the salinity was 140‰. This site is only 2.5 km from the breach to Farmington Bay, and less saline water from the bay sometimes overflows the dense Gilbert Bay water and reaches the swimming beach. Two other sites were sampled in mesotrophic Bear River Bay which has highly variable salinities, but they were near 3‰ when sampled during spring runoff in June 2006 when water levels were higher than shown in Figure 1B. Great Salt Lake samples were collected in water < 0.5 m deep by pushing sterile, 60-ml centrifuge tubes into the soft mud sediments. They were shipped on ice to Woods Hole within 24 hours of collection.
Amoeba enrichment
Amoebae were enriched on agar plates made with fresh water, brackish water (mixture) or salt water. Low nutrient plates were made following the recipes for Marine Ameba Medium (32‰ salinity; ATCC 994; 0.1 g malt extract, 0.1 g yeast extract, 10 g agar, 1 L seawater), Brackish Water Ameba Medium (6.4‰ salinity; ATCC 996; 0.1 g malt extract, 0.1 g yeast extract, 10 g agar, 167 ml seawater, 833 ml distilled water) and Fresh Water Ameba Medium (0‰ salinity; ATCC 997; 0.1 g malt extract, 0.1 g yeast extract, 10 g agar, 1 L distilled water) from the American Type Culture Collection. Non-nutrient agar plates were made by mixing full-strength seawater or freshwater with Bacto -agar (10 g/L; Becton Dickinson). Seawater (32‰ salinity) was obtained from Vineyard Sound, Cape Cod, MA.
Dead bacteria were used as food for the amoebae, and were made by growing E. coli in a liter of LB (Bertani 1951) overnight. The cells were collected by centrifugation at 5000 × g for 20 min at 4°C, washed 2 times with sterile 1X phosphate buffered saline (PBS), resuspended in 20 ml 1X PBS and autoclaved. The dead bacteria were streaked in the shape of a plus-sign on the plates, and a 5mm size portion of sediment was aseptically placed at the center. Plates were sealed with Parafilm M® and incubated right side up at 15–18°C. Plates were observed at one-week intervals for 4 weeks using an inverted microscope at 200X. When amoebae were detected, they were recovered from the plates using pipet tips and transferred to a well in a 12–well culture dish containing liquid medium of the same kind as the plate they were growing on. A drop (approximately 20 μl) of dead bacteria was added as food. When the amoebae formed a monolayer in the well of the culture dish (after 4–5 days), they were recovered by scraping the bottom of the well. A small aliquot was used to transfer the culture to a new well, and the remaining material (approximately 1 ml) was collected for nucleic acid extraction.
DNA isolation, PCR amplification, cloning and sequencing
Amoebae were collected by centrifugation at 10,000 × g for 5 minutes, the supernatant was removed and the pellet resuspended in 100 μl lysis buffer. The lysis procedure followed that previously described (Gast et al. 2004). Recovered nucleic acids were resuspended in 20 μl of 0.01M Tris (pH 7.0) and stored at −20°C.
Genus-specific nested amplifications for Legionella were accomplished using the 16S ribosomal RNA (rRNA) gene primer sets Leg225/Leg858 (Miyamoto et al. 1997) and p1.2/cp3.2 (Jonas et al. 1995). Samples positive for the Legionella 16S rRNA gene were then amplified using primers targeting the gene encoding the macrophage infectivity potentiator (mip) surface protein of L. pneumophila. Nested amplifications were conducted using mipf1/m1548r (Koide et al. 1993) and mipf1/mipr1 (Templeton et al. 2004). Samples were considered positive for the Legionella genus or L. pneumophila only if they showed a band of the correct size (Figure 2A&B), which had to be conscientiously assessed because products of other sizes did occur in some samples (Figure 2B). Sequencing of the appropriately sized bands helped to confirm that the products were correct, especially in the case of the mip gene. 18S rRNA genes of amoebae were amplified using primers Euk A and Euk B (Medlin et al. 1988) from the original amoeba culture extract of samples that were positive for the Legionella genus. No template negative controls were run for every set of amplification reactions to monitor for contamination, and details of the PCR reactions are presented in Supplemental Materials.
Figure 2.
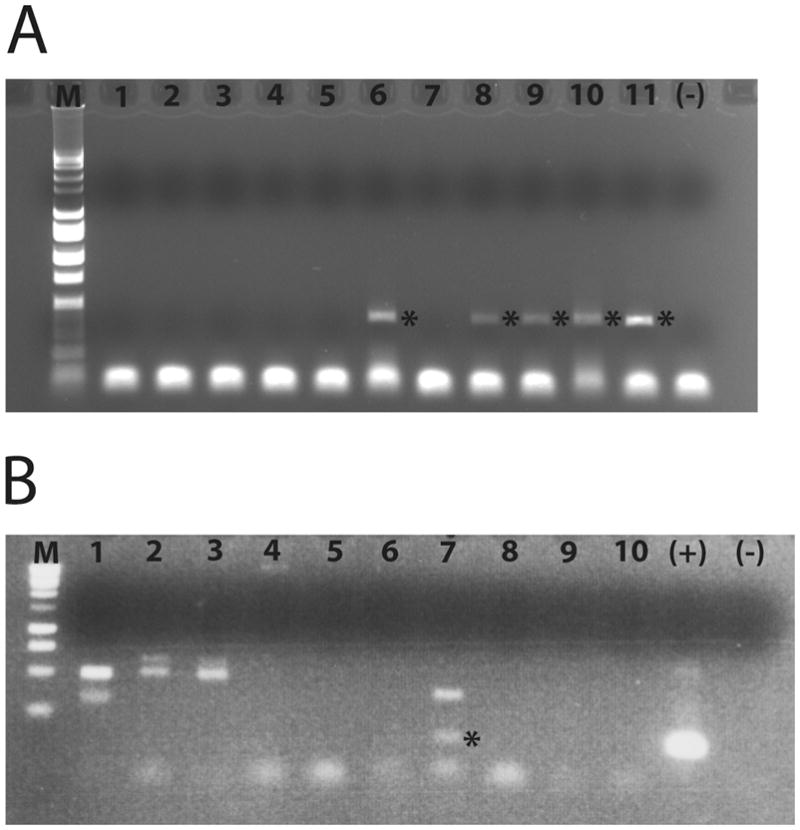
Nested amplification products. A) Legionella genus-specific 16S amplification reactions for eleven July 2005 Mt Hope Bay amoeba culture extracts. M=Promega 1Kb Ladder; lane 1 - 0705 DB997.1; lane 2 - 0705 DB997.2; lane 3 – 0705 DB994.1; lane 4 – 0705 DB994.3; lane 5 – 0705 CO997.1; lane 6 – 0705 BPMNN.3; lane 7 – 0705 BP994.1; lane 8 – 0705 BP996.1; lane 9 – 0705 BP997.1; lane 10 0705 BP997.3; lane 11 – 0705 BP997.4; (−) no template negative control. Asterisks (*) indicate positive amplification result. B) Mip gene amplification reactions for ten June 2006 MHB amoeba culture extracts. M = Promega 1Kb Ladder; lane 1 – 0606 BRMNN.6; lane 2 – 0606 CO996.2; lane 3 – 0606 COFFN.1; lane 4 – 0606 COFFN.2; lane 5 – 0606 COMNN.2; lane 6 – 0606 DBFNN.1; lane 7 – 0606 DBFNN.2; lane 8 – 0606 DBMNN.3; lane 9 – 0606 BPFNN.1; lane 10 – 0606 BPMNN.6; (+) positive control, L. pneumophila genomic DNA; (−) no template negative control. The asterisk (*) in lane 7 indicates a positive amplification product
PCR reactions to determine the relative limit of detection were composed of a dilution series of L. pneumophila subsp pneumophila genomic DNA (ATCC 33152; 1 ng, 100 pg, 10 pg, 1 pg, 100 fg, 10 fg and 1 fg) spiked with either water or sediment extract (1 μl) that was negative for amplification of either the Legionella genus or L. pneumophila. These reactions were then taken through the nested amplification process for the detection of each target.
We directly sequenced Legionella genus-specific 16S amplification products and 3 of the mip products from MHB. We did not sequence GSL Legionella genus-specific amplicons. Four mip products (0805GSLBMNN.1, 0805BP994.2, 0705BR994.1, 0605CO997.3) were cloned for sequencing.
Specific descriptions of the cloning and sequencing are in Supplemental Materials.
Eighty-nine Legionella genus-specific 16S products and 3 of the mip products (0805GLSR996.2, 0805DB996.2, 0805DB994.5) were successfully sequenced directly as PCR products using the amplification primers. Seven mip clones (representing 4 samples) were sequenced using M13F primer, and 35 amoeba clones were sequenced using internal ribosomal primers (Weekers et al. 1994). All sequences recovered and reported in this manuscript have been submitted to GenBank (Legionella genus GU185941-GU186030, Legionella pneumophila mip GU186031-41, amoeba cultures GU320576-GU320610).
Phylogenetic analysis
Sequencher™ (Gene Codes Corporation, Ann Arbor, MI, USA) was used to identify groups of Legionella genus 16S rDNA PCR product sequences that were <1% different, and only one representative from each group was included in the alignment (Table 1). The fragment of the 16S rRNA gene for the Legionella genus recovered by PCR was aligned using ClustalW (http://align.genome.jp/, Kyoto University Bioinformatics Center) with sequences from GenBank, and the alignment was trimmed so that all sequences were of similar size (<400 bp). Sequences <75% of the fragment length were removed from the alignment, yielding a final dataset composed of 157 sequences with 408 sites (nucleotides). The phylogenetic reconstruction was determined using RAxML bootstrapping (Stamatakis 2006, Stamatakis et al. 2008) via the CIPRES portal (Miller et al. 2009). RaxML was run with maximum likelihood search after the bootstrap, the proportion of invariant sites estimated and the number of bootstrap iterations automatically determined by the program.
TABLE 1.
Groups of Legionella genus 16S rDNA sequences from amoeba cultures identified at <1% difference.
| Blast identification of contig1 | Members of leg group2 |
|---|---|
| L. drancourtii LLAP12 98% coverage/99% ID | 1, 2, 84, 85, 87 |
| Unc. Legionella SEC27 100% coverage/96% ID | 5, 10, 12, 13 |
| Unc. Legionella Tang2-4 98% coverage/98% ID | 32, 101, 102, 103 |
| Unc. Legionella Tsw8-2 100% coverage/97% ID | 3, 7, 8, 11, 18, 19, 20, 21 |
| Unc. Legionella Tsw5-3 99% coverage/98% ID | 22, 23, 25, 29, 35, 37, 38, 42, 47, 48, 49, 50, 57, 61, 62, 65, 66, 69, 70, 71, 72 |
| L. anisa 100% coverage/99% ID | 46, 54, 63 |
| Unc. Legionella Tang1-1 | 39, 68 |
| L. moravica 100% coverage/ 99% ID | 73, 74, 78 |
| L. impletisoli 100% cov/ 94% ID | 75, 76 |
| Unc. Legionella SAM09-34 99% coverage/ 96% ID | 33, 93 |
contig based upon all sequences in group, generated by Sequencher ™, used for Blast search of GenBank
number in bold is the sequence used in 16S phylogeny
Mip sequences were initially identified via submission at the UK Health Protection Agency website (http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1195733805138) using their mip-based Legionella species identification program. Based upon that information, Legionella mip sequences were downloaded from GenBank and aligned using ClustalW. The alignment was trimmed so that all the sequences were of a similar size, yielding a dataset of 39 sequences and 212 sites (nucleotides). The phylogenetic reconstruction was determined using RaxML bootstrapping (Stamatakis et al. 2008) via the CIPRES portal (Miller et al. 2009) as described above.
Results
Cultures
Three hundred sixteen amoeba cultures were recovered throughout the sampling period from 27 MHB sediment samples, and 72 were recovered from 8 GSL samples (Table 2). Although cultures were transferred to new medium every 2–3 weeks, many of the cultures were no longer viable after 1 year. The three L. pneumophila amoeba cultures that remained viable one year later, were no longer positive for the presence of the bacterium.
TABLE 2.
Number of amoeba cultures recovered from Mt. Hope Bay and Great Salt Lake sediments.
| Sample location | Date of collection
|
||||||
|---|---|---|---|---|---|---|---|
| Nov 04 | Apr 05 | Jun 05 | Jul 05 | Aug 05 | Feb 06 | Jun 06 | |
|
Mt. Hope Bay
| |||||||
| Braga Bridge | 19 (13) | 10 (5) | 11 (5) | 18 (4/1) | 7 (7/1) | 14 (5) | 14 (3) |
| Brayton Point | 1 (0) | 21 (1) | 12 (4) | 17 (6) | 7 (4/1) | 2 (0) | 15 (7) |
| Common Fence | 0 | 20 (2) | 11 (8/1) | 15 (1) | 9 (9) | 9 (8) | 12 (7) |
| Dighton Bridge | 4 (1) | 12 (0) | 11 (5/1) | 14 (4) | 20 (17/6) | na | 11 (6/2) |
|
| |||||||
|
Great Salt Lake
| |||||||
| Farmington Bay Sewage Canal | 6 (1) | 13 (8/1) | |||||
| Farmington Bay Wetlands | 11 (9/1) | na | |||||
| Farmington Bay Causeway | 8 (4/2) | 7 (7) | |||||
| Swimming Beach Antelope Island | na | 11 (8) | |||||
| Bear River Bay | na | 10 (10) | |||||
| Bear River Bay Bridge | na | 6 (6) | |||||
Number in parenthesis indicates the number of amoebae positive for the genus Legionella by 16S PCR amplification and sequencing. Number after slash indicates number of amoebae positive for L. pneumophila by mip gene amplification. na = not assessed.
Limit of detection by PCR
For water, we were able to recover a product from 10 fg of template for the Legionella genus-specific 16S amplification, and 100 fg of template for the mip amplification. For sediment, we were able to recover a mip product from 10 fg of template and from 1 fg of template for the genus-specific 16S amplification. Each Legionella cell carries about 3 fg of genomic DNA, so we were theoretically able to detect a minimal cell number between 1 and 33 cells per reaction, depending upon the sample type and the target (ribosomal vs single copy mip).
Legionella PCR amplification from amoeba cultures
One hundred thirty-two of the MHB cultures (42%) and 53 of the GSL cultures (74%) were positive for the presence of the Legionella genus by PCR amplification (Table 2). There was no obvious trend between the recovery of Legionella products and the season of sample collection or the location of sample collection. Thirteen of the 132 MHB Legionella-positive amoeba cultures were positive for L. pneumophila mip genes (9.8%) by PCR (Table 2), and 4 out of 53 Great Salt Lake Legionella positive cultures were mip positive (7.5%; Table 2). A list of the cultures considered positive is given in Table 3. Most of the mip positive cultures occurred in summer (June, July and August), and in amoebae that were growing on brackish (996) or full strength seawater (994, MNN), although three of the MHB mip positive amoebae were growing on freshwater media (997, FNN).
TABLE 3.
Amoeba cultures from Mt Hope Bay and the Great Salt Lake that were positive for the Legionella mip gene.
| Culture Name | Sample location | Sample date | Medium | Confirmed by sequence as L. pneumophila |
|---|---|---|---|---|
|
Mt. Hope Bay
| ||||
| 06/05CO997.3 | Common Fence Point | June 2005 | Freshwater, minimal media | yes |
| 06/05DB997.3 | Dighton Bridge | June 2005 | Freshwater, minimal media | |
| 07/05BR994.1 | Braga Bridge | July 2005 | Saltwater minimal media | yes |
| 08/05BP994.2 | Brayton Point | August 2005 | Saltwater, minimal media | yes |
| 08/05BR996.1 | Braga Bridge | August 2005 | Brackish water, minimal media | |
| 08/05DB994.2 | Dighton Bridge | August 2005 | Saltwater, minimal media | |
| 08/05DB994.3 | Dighton Bridge | August 2005 | Saltwater, minimal media | |
| 08/05DB994.5 | Dighton Bridge | August 2005 | Saltwater, minimal media | yes |
| 08/05DBMMN.5 | Dighton Bridge | August 2005 | Saltwater, non-nutrient | |
| 08/05DB996.1 | Dighton Bridge | August 2005 | Brackish water, minimal media | |
| 08/05DB996.2 | Dighton Bridge | August 2005 | Brackish water, minimal media | yes |
| 06/06DB996.2 | Dighton Bridge | June 2006 | Brackish water, minimal media | |
| 06/06DBFNN.2 | Dighton Bridge | June 2006 | Freshwater, non-nutrient | |
|
| ||||
|
Great Salt Lake
| ||||
| 08/05GSLR996.2 | Farmington Bay wetlands | August 2005 | Brackish water, minimal media | yes |
| 08/05GSLB996.1 | Farmington Bay Causeway | August 2005 | Brackish water, minimal media | |
| 08/05GSLBMNN.1 | Farmington Bay Causeway | August 2005 | Saltwater, non-nutrient | yes |
| 06/06GSLFB996.2 | Farmington Bay Causeway | June 2006 | Brackish water, minimal media | |
Culture Name indicates collection date (month/year), location (CO - Common Fence Point, DB - Dighton Bridge, BR – Braga Bridge, BP – Brayton Point, GSLR – wildlife refuge, GSLB – causeway breach, GSLFB – beach at Antelope Island), and culture medium with isolate number (e.g. 996.3).
Legionella genus diversity associated with amoeba cultures
Eighty-nine of the amplification products of the Legionella genus 16S rRNA gene fragments were successfully directly sequenced (Table 4). BLAST similarities to Legionella sequences in GenBank ranged from 93%–100% (Table 4). The fragments were all relatively short (~ 400bp), so some of the percent similarities represent only a small number of differences (e.g. 96% similarity would be about 16 base pair differences). Eleven of the 89 sequences were possibly not legionellae, and had top BLAST hits to Rheinheimera aquimaris (7 of 11), uncultured bacteria or betaproteobacteria (2 of 11), or an uncultured bacterium sequence from sediment 103 meters below the surface of Lomonosov Ridge (2 of 11).
TABLE 4.
Amoeba cultures and corresponding Legionella 16S sequence identification and amoeba 18S sequence identification.
| Amoeba culture number | Culture Name | Putative Legionella-like 16S sequence | Bacterial Blast Result | Putative amoeba 18S sequence | Amoeba Blast resul |
|---|---|---|---|---|---|
| 1 | 04/05 CO 996.3 | L. lyticum | 100% cov/ 100% ID | Cercozoan | 100% cov/ 97% ID |
| 2 | 04/05 CO MNN.2 | L. lyticum | 98% cov/ 99% ID | ||
| 3 | 06/05 CO 997.1 | Unc. Legionella Tsw8-2 | 100% cov/ 97% ID | ||
| 5 | 06/05 CO 997.3 | Unc. Legionella clone 20 | 100% cov/ 96% ID | ||
| 6 | 06/05 CO 994.1 | Unc. Legionella clone 20 | 98% cov/ 98% ID | ||
| 7 | 06/05 CO FNN.1 | Unc. Legionella Tsw8-2 | 100% cov/ 97% ID | Platyamoeba sp. | 99% cov/ 93% ID |
| 8 | 06/05 CO FNN.2 | L. pneumophila | 100% cov/ 96% ID | ||
| 9 | 06/05 BR MNN.1 | L. worsliensis | 100% cov/ 97% ID | Reticulamoeba sp. | 92% cov/ 90% ID |
| 10 | 06/05 BR 996.1 | Unc. Legionella Sb1-1 | 100% cov/ 96% ID | Flabellula sp | 91% cov/ 99% ID |
| 11 | 06/05 BR 996.2 | Unc. Legionella Tsw8-2 | 100% cov/ 97% ID | Paratetramitus sp. | 100% cov/ 99% ID |
| 12 | 06/05 BR FNN.2 | Unc. LegionellaSb1-1 | 100% cov/ 96% ID | ||
| 13 | 06/05 BP MNN.2 | Unc. Legionella; Sb1-1 | 100% cov/ 96% ID | ||
| 14 | 06/05 BP MNN.3 | Unc. Legionella S4-7 | 100% cov/ 98% ID | ||
| 15 | 06/05 BP 997.1 | Singhamoeba sp. | 100% cov/ 98% ID | ||
| 16 | 06/05 BP FNN.2 | L. londiniensis | 100% cov/ 96% ID | ||
| 17 | 06/05 DB MNN.1 | Unc. Legionella clone 27 | 100% cov/ 95% ID | Cercozoan/Protaspis | 100% cov/ 90% ID |
| 18 | 06/05 DB 997.1 | L. pneumophila | 100% cov/ 96% ID | ||
| 19 | 06/05 DB 997.3 | Unc. Legionella Tsw8-2 | 100% cov/ 97% ID | ||
| 20 | 06/05 DB 994.2 | Unc. Legionella Tsw8-2 | 100% cov/ 96% ID | ||
| 21 | 06/05 DB 996.2 | Unc. Legionella Tsw8-2 | 100% cov/ 97% ID | Acanthamoeba polyphaga | 99% cov/ 99% ID |
| 22 | 07/05 BR 994.1 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | Didymium sp. | 99% cov/ 87% ID |
| 23 | 07/05 BR MNN.1 | Unc. LegionellaTsw5-3 | 99% cov/ 98% ID | ||
| 24 | 07/05 BR MNN.2 | Unc. Legionella clone 45 | |||
| 25 | 07/05 BR MNN.3 | Unc. Legionella clone 46 | 100% cov/ 99% ID | Cercozoan | 92% cov/ 99% ID |
| 26 | 07/05 DB 997.1 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | Acanthamoeba sp. | 99% cov/ 98% ID |
| 28 | 07/05 DB 994.1 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | ||
| 29 | 07/05 DB 994.3 | Unc. LegionellaTsw5-3 | 100% cov/ 98% ID | ||
| 31 | 07/05 BP MNN.3 | Unc. Legionella clone LS-16 | 100% cov/ 96% ID | ||
| 32 | 07/05 BP 994.1 | Unc. Legionella clone LS-16 | 98% cov/ 99% ID | ||
| 33 | 07/05 BP 997.2 | Unc. Legionella clone SAM09-34 | 99% cov/ 97% ID | ||
| 34 | 07/05 BP 997.3 | Unc. Legionella clone LS-16 | 100% cov/ 97% ID | ||
| 35 | 08/05 BP 994.2 | Unc. Legionella Sb1-2 | 99% cov/ 98% ID | ||
| 36 | 08/05 BP 994.3 | Unc. Legionella Tag4-3 | 100% cov/ 98% ID | Putative cercozoan | 87% cov/ 86% ID |
| 37 | 08/05 BP 994.4 | Unc. Legionella Tsw5-3 | 100% cov/ 97% ID | ||
| 38 | 08/05 BP MNN.1 | Unc. Legionella Sb1-2 | 99% cov/ 97% ID | Neoparamoeba sp. | 100% cov/ 97% ID |
| 39 | 08/05 BR MNN.1 | Unc. Legionella Tang1-1 | 100% cov/ 98% ID | ||
| 40 | 08/05 BR MNN.2 | Unc. Legionella Tsw5-3 | 99% cov/ 95% ID | ||
| 42 | 08/05 BR MNN.4 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | ||
| 43 | 08/05 BR 996.1 | Rheinheimera aquimaris | 98% cov/ 99% ID | Acanthamoeba sp. | 99% cov/ 99% ID |
| 45 | 08/05 BR 996.3 | Rheinheimera aquimaris | 98% cov/ 99% ID | Acanthamoeba sp. | 100% cov/ 99% ID |
| 46 | 08/05 CO 994.1 | L. anisa | 99% cov/ 99% ID | ||
| 47 | 08/05 CO 994.2 | Unc. Legionella Tsw5-3 | 100% cov/ 98% ID | ||
| 48 | 08/05 CO 994.3 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | Cercozoan | 96% cov/ 98% ID |
| 49 | 08/05 CO 994.4 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | ||
| 50 | 08/05 CO 994.5 | Unc. Legionella Clone 46 | 100% cov/ 98% ID | ||
| 52 | 08/05 CO MNN.2 | Unc. Legionella S5-1 | 98% cov/ 96% ID | ||
| 54 | 08/05 CO MNN.5 | L. anisa | 100% cov/ 99% ID | ||
| 55 | 08/05 DB 996.1 | Rheinheimera aquimaris | 99% cov/ 99% ID | Acanthamoeba lugdunensis | 100% cov/ 99% ID |
| 56 | 08/05 DB 996.2 | Rheinheimera aquimaris | 98% cov/ 99% ID | Acanthamoeba sp. | 99% cov/ 99% ID |
| 57 | 08/05 DB 996.3 | Unc. Legionella Tsw5-3 | 99% cov/ 97% ID | Acanthamoeba lugdunensis | 100% cov/ 99% ID |
| 58 | 08/05 DB FNN.1 | Unc. Legionella S6-1 | 99% cov/ 96% ID | ||
| 59 | 08/05 DB 994.1 | Rheinheimera aquimaris | 99% cov/ 99% ID | ||
| 60 | 08/05 DB 994.2 | Rheinheimera aquimaris | 98% cov/ 98% ID | ||
| 61 | 08/05 DB 994.3 | Unc. Legionella Sb1-2 | 99% cov/ 98% ID | Vannella sp. | 99% cov/ 99% ID |
| 62 | 08/05 DB 994.5 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | Vannella sp. | 99% cov/ 99% ID |
| 63 | 08/05 DB 997.2 | L. anisa | 100% cov/ 99% ID | Hartmannella sp. | 99% cov/ 99% ID |
| 64 | 08/05 DB 997.3 | Unc. Legionella Tang2-1 | 98% cov/ 98% ID | Naegleria sp. | 99% cov/ 99% ID |
| 65 | 08/05 DB 997.4 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | ||
| 66 | 08/05 DB 997.5 | Unc. Legionella Sb1-2 | 99% cov/ 98% ID | ||
| 67 | 08/05 DB MNN.1 | Unc. Legionella Clone 20 | 100% cov/ 97% ID | ||
| 68 | 08/05 DB MNN.2 | Unc. Legionella Tang1-9 | 100% cov/ 97% ID | ||
| 69 | 08/05 DB MNN.3 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | ||
| 70 | 08/05 DB MNN.4 | Unc. Legionella Sb1-2 | 99% cov/ 98% ID | ||
| 71 | 08/05 DB MNN.5 | Unc. Legionella Tsw5-3 | 99% cov/ 98% ID | ||
| 72 | 08/05 DB 994.4 | Unc. Legionella Sb1-2 | 99% cov/ 98% ID | Vannella sp. | 99% cov/ 99% ID |
| 73 | 06/06 BR 996.1 | L. moravica | 100% cov/ 99% ID |
Paraflabellula sp. (73) Filamoeba sp. (73b) |
100% cov/ 98% ID 99% cov/99% ID |
| 74 | 06/06 BR 996.2 | L. moravica | 100% cov/ 99% ID | ||
| 75 | 06/06 CO 994.5 | L. impletisoli | 100% cov/ 94 % ID | ||
| 76 | 06/06 CO 994.6 | L. impletisoli | 100% cov/ 94 % ID | ||
| 77 | 06/06 CO 994.7 | Unc. Bacteria UC04 | 99% cov/ 97% ID | ||
| 78 | 06/06 DB 996.2 | L. moravica | 100% cov/ 99% ID | ||
| 79 | 06/06 DB 996.3 | L. longbeachae | 100% cov/ 92% ID | ||
| 80 | 06/06 DB 997.2 | Unc. Legionella Tang7-1 | 99% cov/ 97% ID | ||
| 81 | 06/06 BP 994.7 | Unc. Beta-proteobacteria | 99% cov/ 99% ID | ||
| 82 | 06/06 BP 994.8 | Unc. Legionella S6-1 | 99% cov/ 98% ID | ||
| 84 | 06/06 BP 997.2 | L. drancourtii LLAP 12 | 100% cov/ 99% ID | Hyperamoeba sp. | 97% cov/ 91% ID |
| 85 | 06/06 BP 997.3 | L. drancourtii LLAP 12 | 100% cov/ 99% ID | ||
| 87 | 06/06 CO 996.2 | L. drancourtii LLAP 12 | 100% cov/ 99% ID | Filamoeba sp. | 99% cov/ 98% ID |
| 88 | 06/06 CO FNN.1 | Rheinheimera sp. | 99% cov/ 100% ID | Filamoeba sp. | 99% cov/ 98% ID |
| 89 | 06/06 CO FNN.2 | Unc. Legionella Tsw8-5 | 99% cov/ 99% ID | ||
| 90 | 06/06 CO MNN.2 | Unc. Legionella Tag6-1 | 98% cov/ 97% ID | ||
| 92 | 06/06 DB FNN.2 | L. drancourtii LLAP 12 | 100% cov/ 99% ID | ||
| 93 | 06/06 DB MNN.3 | Unc. Legionella SAM09-34 | 99% cov/ 96% ID | ||
| 94 | 06/06 BP FNN.1 | Unc. Legionella Sb1-7 | 100% cov/ 98% ID | ||
| 96 | 02/06 BR 997 | L. quateirensis | 100% cov/ 98% ID | Reticulamoeba sp. | 96% cov/ 96% ID |
| 98 | 02/06 BR MNN.5 | Unc. Bacteria 103mbsf_2_8A | 94% cov/ 99% ID | Reticulamoeba sp. | 98% cov/ 96% ID |
| 99 | 02/06 BR MNN.7 | Unc. Bacteria 103mbsf_2_8A | 98% cov/ 99% ID |
Reticulamoeba sp. (99) Nolandella sp. (99a) |
98% cov/ 95% ID 97% cov/ 99% ID |
| 101 | 02/06 CO FNN.9 | L. worsliensis | 100% cov/ 99% ID | Filamoeba sp. | 99% cov/ 99% ID |
| 102 | 02/06 CO FNN.2 | LLAP 10 | 100% cov/ 100% ID | ||
| 103 | 02/06 CO FNN.4 | L. quateirensis | 99% cov/ 98% ID | Naegleria sp. | 100% cov/ 99% ID |
Culture Name indicates collection date (month/year), location (CO - Common Fence Point, DB - Dighton Bridge, BR – Braga Bridge, BP – Brayton Point), and culture medium with isolate number (e.g. 996.3).
The fragments were placed in an alignment with Legionella 16S rRNA gene sequences from GenBank to establish the general relationship to other sequence types. The Legionella sequences recovered from amoebae were all novel and distributed throughout the ribosomal phylogeny for the genus (Figure 3). Generally, they clustered with other environmental isolates from the database, but in some instances they clustered with potentially pathogenic isolates, such as Legionella-like amoebal pathogen 10 (Group B, Figure 3). The Rheinheimera-like sequences all cluster together in Group G (Figure 3) on one of the longest branches in the reconstruction, suggesting that they may not be Legionella. In contrast, the Lomonosov Ridge bacterium-like sequences appear to cluster more closely with other legionellae (Group H, Figure 3).
Figure 3.
Legionella 16S ribosomal gene phylogeny. Bootstrapped RAxML phylogeny of the Legionella 16S ribosomal gene fragment sequences recovered from MHB and GSL amoeba cultures. The leg1 – leg103 taxon labels are derived from the amoeba culture number in column one of Table 4. Bootstrap values are shown at the nodes, and only values >50% are indicated. The scale bar indicates the number of substitutions per site.
Mip gene amplification
We successfully recovered sequences from 7 of the 17 mip products (0605COMNN.2, 0705BR994.1, 0805BP994.2, 0805DB996.2, 0805DB994.5, 0805GSLR996.1. 0606GSLFB996.2). All of the recovered mip sequences cluster significantly with the mip sequences from L. pneumophila (Figure 4). For three samples where we cloned PCR products, clones from the same mip product differed by one or more bases (Figure 4). This could indicate the presence of a mixture of strains in the sample, or PCR and sequencing errors.
Figure 4.
mip gene phylogeny reconstruction. Bootstrapped RAxML phylogeny of mip gene fragment sequences recovered from MHB and GSL amoeba cultures. Mip gene taxon labels are derived from amoeba culture names indicated in Table 3. Bootstrap values are shown at the nodes, and only values >50% are indicated. The scale bar indicates the number of substitutions per site.
Amoeba types potentially harboring L. pneumophila in marine environment
Multiple 18S rDNA clones were recovered and partially sequenced, and only those that yielded putative amoeba sequences were pursued further. Our recovery of amoeba 18S sequences was low relative to the total number of cultures (35/132 positive for legionellae). We, and others, have had difficulty recovering ribosomal sequences from environmental amoebae, even when in laboratory cultures (personal observations). The amoeba types identified by BLAST are presented in Table 4 and include typical freshwater amoebae (Acanthamoeba and Naegleria), as well as commonly occurring marine amoebae (Vannella and Platyamoeba). Most of the amoeba culture extracts yielded only one amoeba-like 18S sequence, but we recovered two each from cultures 73 and 99 (Table 4). We also recovered other protistan 18S sequences from some of the cultures, and while most of these were from flagellates (bodonid, kinetoplastid, dinoflagellate, euglenozoa, bicosoeicid), ciliate sequences (Engelmanniella, Homalogastra, Parauronema) were found in two cultures, and algal sequences (Chlorella and Nanochlorum) in two other cultures.
Discussion
The most significant result in this study was that Legionella pneumophila, and other potentially pathogenic Legionella species, can be detected in amoeba cultures recovered from widely different saline environments and grown in saline conditions. Although only a small proportion of our amoeba cultures were positive for potential pathogens, this observation is of importance not only because it indicates that the pathogen can persist in saline environments, but that there is the potential for growth in those environments. Studies have indicated that the virulence of some Legionella species increased after intracellular growth (Lau & Ashbolt 2009, Cirillo et al. 1999, Cirillo et al. 1994), and if these organisms are able to grow intracellularly in the marine environment, they could represent a human health risk. Unfortunately, it was not possible for us to confirm, either by Giemsa staining or in situ hybridization, the presence of L. pneumophila within isolated amoebae. By the time we determined that L. pneumophila sequences were present in a particular culture, the culture was either dead, or no longer positive for the presence of legionellae. Legionellae, especially L. pneumophila, lyse their host cells, and this may account for the inability to recover infected amoebae from the cultures at a later stage.
Our study shows that amoebae isolated from the marine environment appear to harbor a diverse collection of Legionella species. This diversity of sequence types has been reported previously from freshwater environments (Wullings & van der Kooij 2006, Sheehan et al. 2003, Calvo-Bado et al. 2003, Wéry et al. 2008), but not from saline environments. Most of the Legionella sequence types recovered from sediment were distinct from each other and from previously reported sequences, but similar (<1% difference) sequence types were recovered from amoeba cultures from the different sampling sites in MHB (Table 4). Often, the samples sharing sequence types were from the same collection date, indicating a potential seasonal pattern to species distributions. It was not possible to determine whether human impact played a role in the presence of legionellae in these samples as all of the sites sampled had some anthropogenic contribution, usually either treated sewage or urban runoff. Future studies of saline environments that lack these inputs would be useful for understanding whether Legionella species are a natural component of salt water microbial communities, or if they are introduced through human activity, but have managed to persist.
Amoeba isolates carrying similar Legionella sequence types often grew on the same media (brackish/freshwater vs marine), but not exclusively (Table 4). This implies the potential for Legionella-like bacteria to inhabit both freshwater and saltwater amoebae. It is also very likely that amoebae are able to adapt to growth at different salinities, but due to difficulty in recovering sequences from cultured amoebae (Table 4), we cannot make an extensive comparison between the amoeba types, media salinity and Legionella sequence types.
Forty-eight percent of our amoeba cultures were positive for sequences from the genus Legionella. Although L. pneumophila mip sequences were not abundant they were detected (9% of Legionella-positive amoebae, 4% of all amoebae), as were sequences related to a variety of other potential pathogens such as Legionella-like amoebal pathogens (LLAP; 18% of Legionella-positive amoebae, 9% of all amoebae). LLAP are bacteria that cause the lysis of their amoebal host in a manner similar to that of Legionella (Rowbotham 1986). These organisms may be important as potential human pathogens as the strategy by which they avoid protistan digestion and multiply intracellularly could also be employed in evading destruction by the human innate immune response.
We found that there is the potential for mixed Legionella populations in the amoeba cultures. Mip-positive samples did not yield L. pneumophila sequences by the Legionella genus 16S rRNA gene amplifications. This suggests that there may be mixtures of Legionella species present in the amoebae or the sample, and that L. pneumophila was not necessarily the dominant organism. Co-infection of a single host is a possibility as it has been shown that the presence of other bacteria does not inhibit infection with L. pneumophila (Declerck et al. 2005). It seems unlikely that L. pneumophila was growing in the cultures on its own, but that cannot be ruled out. Also, we cannot rule out that another amoeba was present and harboring one of the two types. Although most of the cultures appeared to be a single amoeba type based upon shape and size (Figure 5), amoeba morphology is not exact and there may have been mixtures present, with the L. pneumophila infected (and susceptible) amoeba making up a small portion of the whole population. Recent research has indicated that different amoebae show different susceptibility to infection by L. pneumophila (Dey et al. 2009). Although the study was conducted with three different genera, there is the possibility that different species and strains of the same genus could show different susceptibility to infection as well. There is also the possibility that another protist in the culture could have harbored the bacterium. Although we did recover sequences for other protists in our amplification and cloning, ciliates, the only other protist considered to potentially harbor Legionella, were not observed visually or by sequence in the mip positive cultures.
Figure 5.
Microscope images of select amoeba cultures. All images were taken at a magnification of 400X under phase contrast illumination. A) Culture from November 2004, Common Fence Point, Marine Non-Nutrient media (1104COMNN.3). B) Culture from April 2005, Common Fence Point, ATCC 996 media (0405CO996.3; Cercozoan sequence). C) Culture from June 2005, Brayton Point, Marine Non-Nutrient media (0605BPMNN.3). D) Culture from June 2005, Braga Bridge, ATCC 997 media (0605BR997.1; Singhamoeba sequence). Scale bar in all figures is approximately 30 microns.
This is one of the first attempts made to identify the potential interactions between Legionella-like bacteria and salt-tolerant hosts. It has been reported that 13 species of amoebae can harbor Legionella bacteria (Fields 1996). In this study, we recovered sequences related to several of these amoebae (Acanthamoeba, Naegleria, Hartmannella), as well sequences that represent potentially new associations with this bacterium (Paratetramitus, Reticulamoeba, Singhamoeba, Platyamoeba, Filamoeba, and unidentified cercozoa). We usually only recovered one amoeba-like 18S sequence from a culture, but there were two instances (amoeba 73 and amoeba 99) wherein two different amoebal sequences were obtained when screening the clones. For amoeba culture 99, it may have been that only Reticulamoeba harbored the Legionella-like bacteria, as Nolandella was not recovered from our other sequencing efforts. It may also be that any of the amoebae detected were capable of harboring the bacteria, and because we were not successful in recovering amoeba sequences from all of our cultures we missed other occurrences of amoebae in our survey.
Conclusion
In this project, we have shown that Legionella pneumophila is present at low levels in saline environments. They may be harbored in amoebae that can grow in salt water, which could lead to the growth and persistence of this pathogen in the environment. The overall diversity of Legionella-like sequences we recovered, as well as the amoeba sequence types that could harbor them, suggest that these bacteria/protist associations are not uncommon, even in the marine realm.
Supplementary Material
Acknowledgments
This work was funded by NSF OCE-0430724 and NIEHS P50ES012742 grants to the Woods Hole Center for Oceans and Human Health. We acknowledge the assistance of Hilary Morrison from the COHH Genomics Core Facility at the MBL. We want to thank Roger Williams University and the Woods Hole US Geological Survey for providing access to boats for sampling the MHB. We also want to thank Amy Marcarelli and Phillip Brown for assistance in collecting samples in the Great Salt Lake. Funding there was provided by the Utah Division of Water Quality and the Central Davis Sewer Improvement District.
References
- Barker J, Brown MRW. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology. 1994;140:1253–1259. doi: 10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Eschericia coli. Journal of Bacteriology. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichai F, Payment P, Barbeau B. Protection of waterborne pathogens by higher organisms in drinking water: a review. Canadian Journal of Microbiology. 2008;54:509–524. doi: 10.1139/w08-039. [DOI] [PubMed] [Google Scholar]
- Calvo-Bado LA, Morgan JA, Sergeant M, Pettitt TR, Whipps JM. Molecular characterization of Legionella populations present within slow sand filters used for fungal plant pathogen suprresion in horticultural crops. Applied and Environmental Microbiology. 2003;69:533–541. doi: 10.1128/AEM.69.1.533-541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrenich CE, Johnson W. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton Medium. Infection and Immunity. 1989;57(6):1862–1864. doi: 10.1128/iai.57.6.1862-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo JD, Cirillo SLG, Yan L, Bermudez LE, Falkow S, Tompkins LS. Intracellular growth of Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infection and Immunity. 1999;67:4427–4434. doi: 10.1128/iai.67.9.4427-4434.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo JD, Falkow S, Tompkins LS. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infection and Immunity. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daggett PM, Sawyer TK, Nerad TA. Distribution and possible interrelationships of pathogenic and nonpathogenic Acanthamoeba from aquatic environments. Microbial Ecology. 1982;8:371–386. doi: 10.1007/BF02010676. [DOI] [PubMed] [Google Scholar]
- Davis PG. Oceanic amoebae from the North Atlantic: culture, distribution, and taxonomy. University of Rhode Island; 1976. [Google Scholar]
- Declerck P, Behets J, Delaedt Y, Margineanu A, Lammertyn E, Ollevier F. Impact of non-Legionella bacteria on the uptake and intracellular replication of Legionella pneumophila in Acanthamoeba castellanii and Naegleria lovaniensis. Microbial Ecology. 2005;50:536–549. doi: 10.1007/s00248-005-0258-0. [DOI] [PubMed] [Google Scholar]
- Dey R, Bodennec J, Mameri MO, Pernin P. Free-living freshwater amoebae differ in their suceptibility to the pathogenic bacterium Legionella pneumophila. FEMS Microbiology Letters. 2009;290:10–17. doi: 10.1111/j.1574-6968.2008.01387.x. [DOI] [PubMed] [Google Scholar]
- Dutka BJ. Sensitivity of Legionella pneumophila to sunlight in fresh and marine waters. Applied and Environmental Microbiology. 1984;48(5):970–974. doi: 10.1128/aem.48.5.970-974.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. The molecular ecology of legionellae. Trends in Microbiology. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- Fields BS, Benson RF, Besser RE. Legionella and legionnaires’ disease: 25 years of investigation. Clinical Microbiology Reviews. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast RJ, Dennett MR, Caron DA. Characterization of Protistan Assemblages in the Ross Sea, Antarctica by Denaturing Gradient Gel Electrophoresis. Applied and Environmental Microbiology. 2004;70:2028–2037. doi: 10.1128/AEM.70.4.2028-2037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clinical Microbiology Reviews. 2004;17:413–433. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagele S, Hacker J, Brand BC. Dictyostelium discoideum: a new host model system for intracellular pathogens of the genus Legionela. Cell Microbiology. 2000;2:165–171. doi: 10.1046/j.1462-5822.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- Heller R, Holler C, Sussmuth R, Gundermann KO. Effect of salt concentration and temperature on survival of Legionella pneumophila. Letters in Applied Microbiology. 1998;26:64–68. doi: 10.1046/j.1472-765x.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- Jonas D, Rosenbaum A, Weyrich S, Bhakdi S. Enzyme-linked immunoassay for detection of PCR-amplified DNA of legionellae in brochoalveolar fluid. Journal of Clinical Microbiology. 1995;33(5):1247–1252. doi: 10.1128/jcm.33.5.1247-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Saito A, Kusano N, Higa F. Detection of Legionella spp. in coooling tower water by the polymerase chain reaction method. Applied and Environmental Microbiology. 1993;59:1943–1946. doi: 10.1128/aem.59.6.1943-1946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HY, Ashbolt NJ. The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water. Journal of Applied Microbiology. 2009;107(2):368–378. doi: 10.1111/j.1365-2672.2009.04208.x. [DOI] [PubMed] [Google Scholar]
- Marcarelli AM, Wurtsbaugh WA, Griset O. Salinity controls pyhtoplankton response to nutrient enrichment in the Great Salt Lake, Utah, USA. Canadian Journal of Fisheries and Aquatic Science. 2006;63:2236–2248. [Google Scholar]
- Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T. The CIPRES Portals. 2009 [online] http://www.phylo.org/sub_sections/portal2009-08-04]
- Miyamoto H, Yamamoto H, Arima K, Fujii J, Maruta K, Izu K. Development of a new seminested PCR method for detection of Legionella species and its application to surveillance of legionellae in hospital cooling tower water. Applied and Environmental Microbiology. 1997;63:2489–2494. doi: 10.1128/aem.63.7.2489-2494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton HJ, Ang DKY, van Driel IR, Hartland EL. Molecular Pathogenesis in Infections Caused by Legionella pneumophila. Clinical Microbiology Reviews. 2010;23(2):274–298. doi: 10.1128/CMR.00052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Roque CM, Hazen TC. Abundance and distribution of legionellacea in Puerto Rican waters. Applied and Environmental Microbiology. 1987;53(9):2231–2236. doi: 10.1128/aem.53.9.2231-2236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page FC. Marine Gymnamoebae. Institute of Terrestrial Ecology; Cambridge: 1983. [Google Scholar]
- Palmer CJ, Tsai YL, Paszko-Kolva C, Mayer C, Sangermano LR. Detection of Legionella species in sewage and ocean water by polymerase chain reaction, direct fluorescent- antibody, and plate culture methods. Applied and Environmental Microbiology. 1993;59:3618–3624. doi: 10.1128/aem.59.11.3618-3624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham TJ. Current views on the relationships between amoebae, legionellae and man. Israeli Journal of Medicine and Science. 1986;22:678–689. [PubMed] [Google Scholar]
- Sawyer TK, Visvesvara GS, Harke BA. Pathogenic amoebas from brackish and ocean sediments, with a description of Acanthamoeba hatchetti, n.sp. Science. 1977;196:1324–1325. doi: 10.1126/science.867031. [DOI] [PubMed] [Google Scholar]
- Sheehan KB, Fagg JA, Ferris MJ, Henson JM. PCR detection and analysis of the free-living amoeba Naegleria in hot springs in Yellowstone and Grand Teton National Parks. Applied and Environmental Microbiology. 2003;69(10):5914–5918. doi: 10.1128/AEM.69.10.5914-5918.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigalliano CD, Gidley ML, Shibata T, Whitman D, Dixon TH, Laws E, Hou A, BAchoon D, Brand L, Amaral Zettler LA, Gast RJ, Steward GF, Nigro OD, Fujioka RS, Betancourt WQ, Vithnage G, Mathews J, Fleming LE, Solo-Gabriele HM. Impact of hurricanes Katrina and Rita on the microbial landscape of the New Orleans area. Proceedings of the National Academy of Sciences. 2007;104:9029–9034. doi: 10.1073/pnas.0610552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JM, Rupper A, Cardelli JA, Isberg RR. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infection and Immunity. 2000;68:2939–2947. doi: 10.1128/iai.68.5.2939-2947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web-Servers. Systematic Biology. 2008;75(5):758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Templeton KE, Scheltinga SA, Beersma MFC, Kroes ACM, Claas ECJ. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3 and 4. Journal of Clinical Microbiology. 2004;42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weekers PHH, Gast RJ, Fuerst PA, Byers TJ. Sequence variations in small-subunit ribosomal RNAs of Hartmannella vermiformis and their phylogenetic implications. Molecular Biology and Evolution. 1994;11:684–690. doi: 10.1093/oxfordjournals.molbev.a040147. [DOI] [PubMed] [Google Scholar]
- Wéry N, Bru-Adan V, Minervini C, Delgénes J-P, Garrelly L, Godon J-J. Dynamics of Legionella spp. and bacterial populations during the proliferation of L. pneumophila in a cooling tower facility. Applied and Environmental Microbiology. 2008;74(10):3030–3037. doi: 10.1128/AEM.02760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullings BA, van der Kooij D. Occurrence and genetic diversity of uncultured Legionella spp. in drinking water treated at temperatures below 15C. Applied and Environmental Microbiology. 2006;72:157–166. doi: 10.1128/AEM.72.1.157-166.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Chereshsky A. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: and international collaborative survey. Journal of Infectious Disease. 2002;186:127–128. doi: 10.1086/341087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






