Disorders of mitochondrial DNA (mtDNA) maintenance leading to multiple mtDNA deletions are a significant cause of inherited neurologic disease in adults, but the underlying nuclear gene defects remain elusive in many patients. Following the recent description of a truncating mutation in the RRM2B gene—encoding the small subunit, p53R2, of the p53-inducible ribonucleotide reductase protein—in 2 families with autosomal-dominant progressive external ophthalmoplegia (adPEO),1 we determined the frequency of RRM2B mutations in a large cohort of patients with chronic PEO and multiple mtDNA deletions in muscle in whom mutations in all known candidate genes (e.g., POLG, POLG2, SLC25A4, and PEO1) had been excluded.2
Methods.
We studied 75 unrelated probands with PEO, a mosaic defect of cytochrome c oxidase (COX) activity, and multiple mtDNA deletions in skeletal muscle who had been referred to Mitochondrial Diagnostic Centers at Newcastle, Oxford, or Munich for clinical assessment and histologic/molecular genetic analysis. The entire coding region, including intron–exon boundaries, of the RRM2B gene was determined as previously described.1 RRM2B exon copy number (exons 1–8) was assessed by MLPA (MRC-Holland kit P089-A1) in patients with single, heterozygous missense mutations.
Standard protocol approvals, registrations, and patient consents.
This study was approved and performed under the ethical guidelines issued by each institution for clinical studies, with written informed consent obtained from all subjects.
Results.
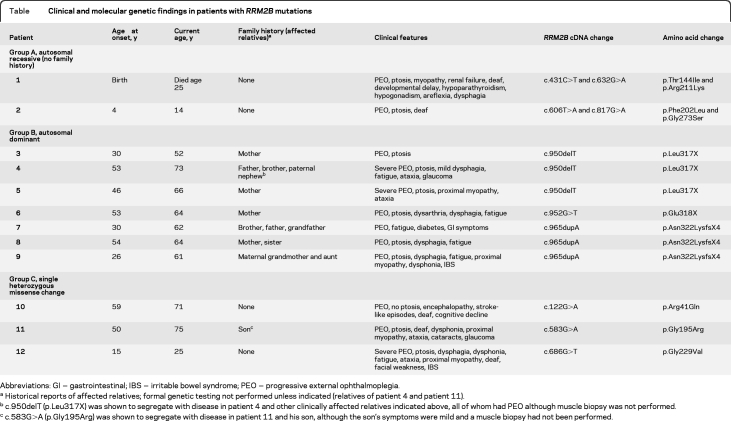
We identified 10 different RRM2B variants in 12 subjects, representing 16% of our undiagnosed cohort. The table summarizes these, together with details of the patients' clinical presentation. All 10 RRM2B changes were novel and were absent in 352 control alleles. All 7 missense variants altered amino acids conserved across mammalian species, and all except p.Gly273 were conserved to at least C elegans (figure e-1 on the Neurology® Web site at www.neurology.org).
Table.
Clinical and molecular genetic findings in patients with RRM2B mutations
Abbreviations: GI = gastrointestinal; IBS = irritable bowel syndrome; PEO = progressive external ophthalmoplegia.
Historical reports of affected relatives; formal genetic testing not performed unless indicated (relatives of patient 4 and patient 11).
c.950delT (p.Leu317X) was shown to segregate with disease in patient 4 and other clinically affected relatives indicated above, all of whom had PEO although muscle biopsy was not performed.
c.583G>A (p.Gly195Arg) was shown to segregate with disease in patient 11 and his son, although the son's symptoms were mild and a muscle biopsy had not been performed.
Two patients (group A, table) harbored compound heterozygous, missense variants implying autosomal recessive inheritance. Seven patients (group B) harbored single heterozygous, truncating mutations within exon 9 (figure e-2), all of whom had family histories consistent with autosomal dominant inheritance together with supportive genetic segregation data in one family (patient 4). We detected single heterozygous, missense variants in the 3 patients in group C (MLPA excluded exonic copy number variation in trans); however, the pathogenicity of these 3 variants is provisional in the absence of further supporting evidence.
Discussion.
Our data confirm a previous report describing dominant RRM2B mutations as an important cause of mtDNA maintenance disorders in adults1 and provide the first description of recessive RRM2B mutations associated with multiple mtDNA deletions and respiratory chain deficiency.
The clinical spectrum of disease associated with RRM2B gene mutations ranges from a fatal infantile neuromuscular syndrome with renal tubular insufficiency3–6 to late-onset PEO.1 Early-onset mtDNA depletion syndromes due to recessive RRM2B mutations are characterized by myopathy and renal proximal tubulopathy, often exhibit multisystem involvement, and are invariably fatal in early childhood.3–6 CNS features may include seizures,3,6 hearing loss,4,5 microcephaly, and global developmental delay.4 Respiratory insufficiency3,6,6 and gastrointestinal dysmotility3,6,6 are not infrequent. Our data confirm that adult-onset adPEO due to RRM2B mutations is associated with a more benign myopathic phenotype and characterized by muscle-restricted, mtDNA deletions. Ptosis and ophthalmoparesis are the predominant clinical characteristics of RRM2B defects in our adult-onset cohort accompanied by mild muscle-related symptoms such as fatigue, bulbar dysfunction, and proximal muscle weakness. In contrast, the 2 childhood onset recessive cases display a variable spectrum of clinical features of RRM2B mutations.
The 2 families previously reported with PEO and multiple mtDNA deletions due to RRM2B mutations were both found to have the same exon 9 truncating mutation, p.Arg327X.1 We identified 3 different truncating mutations in exon 9 (p.Leu317X, p.Glu318X, and p.Asn322LysfsX4) in 7 cases (group B), thereby establishing this class of mutation as an important cause of adPEO. Two further RRM2B mutations—p.Asn307IlefsX11 and p.Leu317Val—have previously been implicated in recessive mtDNA depletion, highlighting exon 9 as a mutation hotspot in mtDNA maintenance disorders.4
Mutations in POLG remain the major cause of multiple mtDNA deletion disorders, accounting for some 25% of all patients with mitochondrial disease presentations,7 followed by dominant PEO1 mutations (∼15% of our PEO/multiple mtDNA deletion cohort).2 We have now identified RRM2B mutations in ∼9% of patients, since the undiagnosed cohort studied here constitutes ∼55% of our total cohort with PEO and multiple mtDNA deletions. Consequently, mutations of RRM2B are more common than OPA1, SLC25A4, and POLG2 mutations, which together comprise less than 5% of our patient cohort, and as such sequencing of this gene should be considered in the diagnostic algorithm for multiple mtDNA deletion disorders once POLG and PEO1 mutations have been excluded.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the referring clinicians, the patients, and their families for contributing to this study. The mitochondrial diagnostic laboratories in Oxford, Newcastle, and London (UCLH/Institute of Neurology) are funded by the UK NHS Specialized Services to provide the “Rare Mitochondrial Disease of Adults and Children” service (http://www.mitochondrialncg.nhs.uk/.
Footnotes
Supplemental data at www.neurology.org
Disclosure: C. Fratter, P. Raman, C.L. Alston, Dr. Blakely, Dr. Craig, Dr. Smith, Dr. Evans, Dr. Seller, and Dr. Czermin report no disclosures. Dr. Hanna serves as Deputy Editor of the Journal of Neurology, Neurosurgery & Psychiatry and receives research support from the Medical Research Council UK. Dr. Poulton receives research support from the MRC UK and the Angus Memorial Mitochondrial Fund. Dr. Brierley and Dr. Staunton report no disclosures. Dr. Turnpenny receives publishing royalties for Emery's Elements of Medical Genetics (Elsevier, 2007) and has served as an expert witness in medico-legal cases. Dr. Schaefer reports no disclosures. Dr. Chinnery serves as an Associate Editor of Brain and receives research support from the Wellcome Trust (Senior Fellow in Clinical Science), the MRC UK, Parkinson's UK, the Association Française contre les Myopathies, and the UK NIHR Biomedical Research Centre for Ageing and Age-related Disease award to the Newcastle upon Tyne Foundation Hospitals NHS Trust. Dr. Horvath receives research support from the Deutsche Forschungsgemeinschaft, the Newcastle upon Tyne Hospitals NHS Charity, and the Academy of Medical Sciences UK. Dr. Turnbull receives research support from the Wellcome Trust and the MRC UK. Dr. Gorman receives research support from the UK NIHR Biomedical Research Centre for Ageing and Age-related Disease award to the Newcastle upon Tyne Foundation Hospitals NHS Trust. Dr. Taylor receives research support from the Wellcome Trust and the MRC UK.
References
- 1. Tyynismaa H, Ylikallio E, Patel M, Molnar MJ, Haller RG, Suomalainen A. A heterozygous truncating mutation in RRM2B causes autosomal-dominant progressive external ophthalmoplegia with multiple mtDNA deletions. Am J Hum Genet 2009;85:290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fratter C, Gorman GS, Stewart JD, et al. The clinical, histochemical and molecular spectrum of PEO1 (Twinkle)-linked adPEO. Neurology 2010;74:1619–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bourdon A, Minai L, Serre V, et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet 2007;39:776–780 [DOI] [PubMed] [Google Scholar]
- 4. Bornstein B, Area E, Flanigan KM, et al. Mitochondrial DNA depletion syndrome due to mutations in the RRM2B gene. Neuromuscul Disord 2008;18:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spinazzola A, Invernizzi F, Carrara F, et al. Clinical and molecular features of mitochondrial DNA depletion syndromes. J Inherit Metab Dis 2009;32:143–158 [DOI] [PubMed] [Google Scholar]
- 6. Kollberg G, Darin N, Benan K, et al. A novel homozygous RRM2B missense mutation in association with severe mtDNA depletion. Neuromuscul Disord 2009;19:147–150 [DOI] [PubMed] [Google Scholar]
- 7. Chinnery PF, Zeviani M. 155th ENMC workshop: polymerase gamma and disorders of mitochondrial DNA synthesis, 21–23 September 2007, Naarden, the Netherlands. Neuromuscul Disord 2008;18:259–267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.