ABSTRACT
Inflammatory bowel diseases (IBDs) can be divided into two major disorders: ulcerative colitis and Crohn's disease. Although IBD-associated colorectal cancer (IBD-CRC) accounts for only 1–2% of all cases of colorectal cancer, IBD with colon involvement is among the top three high-risk conditions for colorectal cancer. Today, colorectal cancer accounts for approximately 10–15% of all deaths among IBD patients. Indeed, patients with IBD colitis are six times more likely to develop colorectal cancer than the general population and have a higher frequency of multiple synchronous colorectal cancers. Since IBD-CRC was first described in 1925, the colon remains the primary site of neoplasms in IBD patients today. Ulcerative colitis–associated colorectal cancer is most common in the rectum and sigmoid colon, whereas Crohn's disease–associated colorectal cancer is evenly distributed between the different colon segments. Chemoprevention of colorectal cancer remains an important goal, and colonoscopy surveillance programs are critical to early detection in these patients. Newer methods, such as chromoendoscopy, are currently being investigated as complementary techniques to enhance early detection of dysplasia and cancer in this high-risk population. We present a comprehensive review of the relationship between inflammatory bowel disease and colorectal cancer. Major themes covered include risk factors for IBD-CRC and the molecular pathobiology of progression from dysplasia to cancer, endoscopic surveillance and new methods for early detection of dysplasia, approaches to prevention of IBD-CRC, and current recommendations and controversies regarding the treatment of dysplasia. In particular, disagreement has arisen over optimal management of low-grade dysplasia, with some IBD experts now advocating close colonoscopic surveillance of patients with low-grade dysplasia rather then total colectomy.
Inflammatory bowel diseases (IBDs) are idiopathic inflammatory disorders of the gastrointestinal tract that can be subdivided into two major disorders: ulcerative colitis and Crohn's disease. In ulcerative colitis the disease extends proximally from the anal verge to involve all or part of the colon. Crohn's disease is typically a patchy disease that can affect the gastrointestinal tract anywhere from the mouth to the anus. Ulcerative colitis and Crohn's disease are characterized by episodes of remission and exacerbations in which the patient experiences abdominal pain, diarrhea, blood in the stool, and systemic symptoms. The peak incidence of IBD occurs in patients between the ages of 15 and 30 years; a second peak occurs between the ages of 50 and 80 years.1 The prevalence of IBD is higher in the westernized world compared to developing countries, with approximately 1.4 million Americans affected with ulcerative colitis or Crohn's disease.2
Since Crohn and Rosenberg first described IBD-associated colorectal cancer (IBD-CRC) in 1925,3 the colon remains the primary site of neoplasms in IBD patients today, and colorectal cancer accounts for approximately 10–15% of all deaths in IBD patients.4 Although IBD-CRC accounts for only 1–2% of all cases of colorectal cancer, IBD with colon involvement is among the top three high-risk conditions for colorectal cancer. Patients with IBD colitis are 6 times more likely to develop colorectal cancer than the general population and have a higher frequency of multiple synchronous colorectal cancers.5 Because IBD incidence is highest among young people, the mean age for developing IBD-CRC is lower than for sporadic colorectal cancer (40–50 years of age vs. 60 years of age).6 Ulcerative colitis–associated colorectal cancer is most common in the rectum and sigmoid colon, whereas Crohn's disease–associated colorectal cancer is evenly distributed between the different colon segments.7
RISK FACTORS FOR IBD-CRC
Patient risk stratification for developing IBD-CRC depends on the extent and duration of colonic disease, the co-existence of primary sclerosing cholangitis, a family history of sporadic colorectal cancer, and in some studies, young age at onset of colitis (Table 1). The risk of ulcerative colitis–associated colorectal cancer starts to increase after 7 years of extensive colonic disease, ie, disease extending from the anal verge to the splenic flexure (left-sided disease) or beyond the splenic flexure (pancolitis).8 One proposed mechanism is that the chronicity of bowel inflammation leads to colorectal dysplasia and eventually colorectal cancer.
Table 1.
Risk factors and protective factors
| Positive risk factors | Negative risk factors and protective agents |
|---|---|
| Active inflammation | Folic acid use |
| PSC | UDCA use (in patients with PSC) |
| Family history of colorectal cancer | Compliance with IBD treatment |
| Increased extent of disease | Compliance with colorectal cancer surveillance guidelines |
Abbreviations: IBD = inflammatory bowel disease; PSC = primary sclerosing cholangitis; UDCA = ursodeoxycholic acid.
The approximate cumulative incidence of colorectal cancer in patients with left-sided ulcerative colitis or pancolitis is 2% at 10 years, 8% after 20 years, and 18% after 30 years' duration of disease.9 These conclusions were derived from a mix of referral-center–based, hospital-based, and population-based studies. Patients with ulcerative proctitis and proctosigmoiditis are probably not at increased risk for colorectal cancer compared with the general population.10 Crohn's disease–associated colorectal cancer is observed in a similar time frame as in ulcerative colitis.11,12 This was illustrated in one series that included 80 patients with colorectal cancer complicating ulcerative colitis or Crohn's disease.13,14 The median duration of disease prior to the diagnosis of colorectal cancer was comparable for Crohn's disease and ulcerative colitis (15 and 18 years, respectively). The median age at diagnosis of colorectal cancer was 55 years in Crohn's disease and 43 years in ulcerative colitis.
In a more recent review by Lakatos et al,13 the authors discuss recent epidemiologic trends and causes for the observed changes. Population-based studies published within the last 5 years suggest that this risk has decreased over time, despite the low frequency of colectomies. The crude annual incidence rate of colorectal cancer in ulcerative colitis ranges from approximately 0.06% to 0.16%, with a relative risk of 1.0–2.75. The exact mechanism for this change is unknown; it may partly be explained by the more widespread use of maintenance therapy and surveillance colonoscopy.
The severity of inflammation may also be an important risk factor for IBD-CRC. A case–control study and a larger cohort study both found a significant correlation between the severity of inflammation as assessed by histology and the risk of colorectal neoplasia. 14–16 A case–control study by Rutter et al17 suggests that mucosal healing may decrease risk of cancer. This study concluded that a macroscopically normal-looking colonoscopy returns the cancer risk to that of the general population. However, some studies have found that colorectal cancer risk does not correlate with IBD disease activity and that patients with quiescent disease have a similar risk of developing IBD-CRC to those who have active disease.18,19
Another risk factor for IBD-CRC is the presence of colon pseudopolyps (or inflammatory polyps), which in themselves have no malignant potential. It is not clear whether this increased risk is due to a higher miss rate of dysplastic polyps difficult to distinguish from the benign pseudopolyps during surveillance colonoscopies or due to the fact that pseudopolyps are a historical marker of more severe inflammation.15
A population-based cohort study of 19,876 individuals with ulcerative colitis or Crohn's disease reported that nearly 10% of all cases of IBD-CRC occurred in patients with a family history of colorectal cancer. The magnitude of the association is similar to that observed among healthy individuals, though in patients with IBD, a family history of colorectal cancer will result in a doubling of the already increased risk of colorectal cancer.16
The association of primary sclerosing cholangitis (PSC) with ulcerative colitis was first shown in 1965 by Smith and Loe.18 Many studies have since confirmed the higher risk of ulcerative colitis–associated colorectal cancer in patients with PSC.19–22 In a study by Kornfeld et al,22 the cumulative colorectal cancer risk in patients with PSC was 33% at 20 years and 40% at 30 years after ulcerative colitis diagnosis. Interestingly, there was a higher prevalence of right-sided colorectal cancers in this population21. Alterations in the bile salt pool and a high concentration of bile acids in the colon may, at least partially, be responsible for this increased colorectal cancer risk.13
Young age at onset of colitis seems to increase risk of colorectal cancer. A population-based cohort of 3117 patients given a diagnosis of ulcerative colitis from 1922 through 1983 were followed through 1984 by Ekbom et al.23 Age at diagnosis and the extent of disease at diagnosis were strong and independent risk factors for colorectal cancer. For each increase in age group at diagnosis (<15 years, 15–29 years, 30–39 years, 40–49 years, 50–59 years, and ≥60 years), the relative risk of colorectal cancer, adjusted for the extent of disease at diagnosis, decreased by about half (adjusted standardized incidence ratio = 0.51; 95% confidence interval [CI], 0.46–0.56). The absolute risk of colorectal cancer 35 years after diagnosis was 30% for patients with pancolitis at diagnosis and 40% for those given this diagnosis at less than 15 years of age.
THE MOLECULAR PATHOBIOLOGY OF IBD-CRC
Inflammatory bowel disease is associated with a spectrum of environmental, genetic, and immunologic factors that lead to a multistage process of tumorigenesis within the colon.24 Carcinogenesis in the inflamed colon appears to follow a different sequence of genetic alterations than that observed in sporadic cancers in the uninflamed colon.25 As previously mentioned, there is an increased risk of colorectal cancer in patients with long-standing IBD, and a longer duration of colitis is associated with multifocal nature of tumors.26 It is suggested that, in addition to a series of genetic alterations, inflammation invokes a cascade within the abnormal epithelial proliferative zone, progressing through dysplasia, adenoma, and finally carcinoma.27
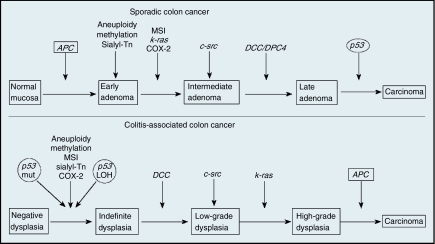
A study of multiple inflammation-linked cancers, including IBD-CRC, found increased levels of oxidative damage specifically at cancer sites. Chronic colonic inflammation causes oxidative DNA damage, which can result in neoplasia and cancer development when involving key growth regulatory genes.28 A gene encoding 8 oxoguanine DNA glycosylase (OGG1), which recognizes and excises oxidative damage within DNA, is also associated with an increased risk of developing colorectal cancer.29 In addition, it has been found that a polymorphism of the gene encoding TGFβ1 in patients without IBD leads to increased risk of colorectal cancer, highlighting the effect of inflammation as a significant variable in cancer.30,31 The progression of IBD-associated neoplasia appears to possess many of the same chromosomal and genetic abnormalities as sporadic colorectal cancer. However, it is the order and frequency of these mutations, and the fact that they often occur before a definite histologically defined dysplasia, that differentiates the two forms and their evolution within the tissue32 (Figure 1).
Figure 1.
Comparison of molecular alterations in sporadic colon cancer and colitis-associated colon cancer. (Adapted from Itzkowitz and Harpaz 2004.)
Chromosomal instability results from abnormal chromosomal segregation during mitosis and/or an aneuploidy state, in which there is a loss of chromosome material and heterozygosity due to abnormal DNA content. Conversely, microsatellite instability (MSI) is due to defects in mismatch repair gene products, such as the Mut S homologs or postmeiotic segregation factors (hMSH2, hMLH1, hPMS1, hPMS2, hMSH6, and hMLH3), which can result in a catastrophic frame shift during gene transcription.
The molecular pathobiology of IBD-CRC does possess many of the same abnormalities in cell cycle regulatory factors p53, APC, and Ki-ras and mismatch repair genes (eg, hMSH2) as in sporadic colorectal carcinoma. Moreover, the majority of colorectal cancer cases in both IBD-associated neoplasia and sporadic colorectal cancer (85%) result from chromosomal instability, followed by MSI (15%) and hypermethylation (<1%).31,33 However, despite such similarities, it is often the timing and frequency of these mutations that provide a unique profile of colorectal cancer risk factors in IBD.24,34 Of these key colorectal cancer markers, APC and p53 display dramatic differences in timing (progressive morphologic appearance of the neoplasia) of loss of function between sporadic colorectal cancer and IBD-associated neoplasm.
The development of a nonfunctional APC gatekeeper gene (5q21–q22), typically occurring prior to early adenoma in sporadic colorectal cancer, arises much later in IBD-associated neoplasia, just prior to carcinoma. Losing APC permits the accumulation of free β-catenin within the cytoplasm. This increase in free β-catenin results in a functional complex, β-catenin/TCF/LEF, which dramatically increases target genes (eg, Cyclin D1, L1, Nr-CAM) and cell cycle turnover.35 Here the APC loss of function can occur because of chromosomal instability or MSI abnormalities, presenting in either polypoid or flat lesions.
Historically known as tumor protein 53, p53 plays a pivotal role in regulating the cell cycle at the G1/S transition interface. Furthermore, mutations in p53 (chromosome 17p13.1) are highly associated with many cancers. Loss-of-function mutations in this gatekeeper protein occur in the same frequency and mechanism in both sporadic colorectal cancer and IBD-associated neoplasia. However, timing of the p53 mutations typically occur late in sporadic colorectal cancer (between the stages of late adenoma and carcinoma), while they develop much earlier in IBD-associated neoplasia (transition from negative dysplasia to indefinite dysplasia). In IBD patients, these p53 mutations are often found in grossly normal nondysplastic mucosa. However, in the case of sporadic colorectal cancer, we see an inverse relationship where the p53 loss of function is found in more morphologically aggressive-appearing lesions. This correlation between the timing of p53 loss of function and the gross morphologic appearance of the mucosal lesion is believed to be the defining molecular transitional characteristic from adenoma to carcinoma when comparing the neoplastic two forms.24
Among several less common mutations, the induction of the K-ras (12p12) oncogene does occur in IBD-associated colorectal cancer; however, it is found to be less likely in IBD-associated neoplasia than in sporadic colorectal cancer. The human mismatch repair gene hMSH2 (2p22) is commonly found at a high frequency in the transition from dysplasia to cancer, though it is not always a defining factor. Additionally, hypermethylation of CpG islands in several gene promoters, in particular the mismatch repair genes (eg, hMLH1, hMSH2) and p16, leads to methylation-induced silencing. The p27 protein, which binds and prevents both the activation of cyclin E-CDK2 or cyclin D-CDK4 complexes and the progression of G1-S, is often lost in ulcerative colitis–colorectal cancer.36,37
The identification of essential regulatory factors and crucial events in the development of IBD-CRC has had a significant effect on the understanding of the disease, specifically its progression as it correlates with inflammation and genetic mutation. However, changes in the frequency and timing of these mutational abnormalities continue to pose many unanswered questions. The ability to monitor these changes both clinically and in the laboratory provides medical scientists with the possibility of further delineating a clear pathobiologic cause of IBD-associated neoplasia and reasoning for such an inversion from sporadic colorectal cancer. Through careful investigation of these abnormalities, medical scientists hold the ability to develop novel screening techniques and new therapeutic advances.
ENDOSCOPIC SURVEILLANCE IN IBD
The goal of colorectal cancer screening colonoscopy in the general population and surveillance in IBD patients is to detect premalignant changes early enough that intervention can prevent complications of invasive cancer. The intervention mandated in IBD-associated dysplasia is colectomy, because of the high prevalence of synchronous or metachronous cancer, as well as the technical limitations of being able to identify confidently and completely resect dysplastic lesions in flat mucosa.
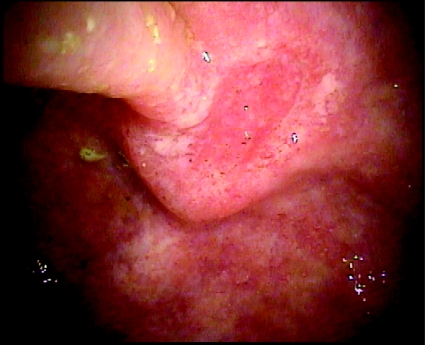
In the general population, the dysplastic or premalignant lesion is the colon polyp that can be easily visualized and resected at the time of the colonoscopy before it transforms into a malignant lesion. In contrast, dysplasia in IBD can be found at distant sites from the cancer itself or before the cancer develops and is difficult to recognize on colonoscopy, as it often arises from flat, normal-appearing mucosa (Figure 2).38 Dysplasia can also occur within or near plaque-like lesions or raised polypoid masses, defined as dysplasia-associated lesion or mass (DALM). Because IBD-CRC is preceded by dysplasia, finding dysplasia on random colon biopsies represents an increased risk of developing colorectal cancer in the near future. Dysplasia is classified as indefinite, low-grade, and high-grade. IBD-CRC occurs in areas of chronic inflammation and can be a polypoid, ulcerated, or plaque-like lesion. Most IBD-CRCs are adenocarcinoma, but there is a higher incidence of poorly differentiated, anaplastic, and mucinous carcinomas compared with sporadic colorectal cancer.
Figure 2.
A 28-year-old male with history of ulcerative pancolitis and primary sclerosing cholangitis diagnosed in 1997 who had no dysplasia on surveillance colonoscopy 2 years prior to this surveillance colonoscopy. This ulceration in the ascending colon revealed adenocarcinoma. This patient subsequently underwent total colectomy with ileostomy.
In a comprehensive review of 10 studies of dysplasia surveillance that included a total of 1225 patients with ulcerative colitis, the likelihood of finding concurrent colon cancer at the time of colectomy in patients with high- or low-grade dysplasia was 42% and 19%, respectively.39 Although prophylactic proctocolectomy can essentially eliminate the risk of cancer, most patients and their physicians opt for a lifelong program of surveillance. This entails regular medical follow-up, management with anti-inflammatory and potentially chemopreventive agents, as well as periodic colonoscopic examinations combined with extensive biopsy sampling throughout the colon.
Although a surveillance program is the best approach currently available, it has its limitations. Surveillance cannot guarantee against the development of colorectal cancer. It is important for both the patient and the physician to understand the risks of potentially missed lesions and the benefits of a strict surveillance program. Physician compliance in adhering to the surveillance biopsy recommendations is important. There should be regular call-back for all participating patients so that no patients are lost to follow-up. Patient noncompliance should trigger a series of letters, including a comment about the potential risk for developing colorectal cancer in the absence of follow-up.40
Based on previous epidemiologic data, guidelines from the Crohn's and Colitis Foundation of America (CCFA)40 and, more recently, from the European Crohn's and Colitis Organisation (ECCO)41 suggest a relatively strict surveillance policy. The American Gastroenterology Association (AGA) and most other gastrointestinal associations conclude that the risk of colorectal cancer–associated Crohn's colitis is similar to that of ulcerative colitis for comparable extent, duration, and age of onset of inflammatory disease. As a result, the surveillance strategy for ulcerative colitis also applies for Crohn's colitis. The recommended guidelines are as follows:40–44
Screening colonoscopy should be performed when the disease is in remission.
Initial surveillance colonoscopy should be performed in each patient beginning 8–10 years after symptom onset, partly to reassess disease extent.
Regular surveillance should begin on an annual or biannual basis beginning 8–10 years of disease for patients with left-sided or extensive colitis after symptom onset.43,44 There should be a decrease in the screening interval with increasing disease duration (from every other year to yearly). Patients with proctosigmoiditis, who have little or no increased risk of colorectal cancer compared with the general population, should be managed according to standard colorectal cancer prevention measures.
Patients with PSC represent a subgroup of IBD patients at higher risk for IBD-CRC, thus surveillance should be performed annually from the time of PSC diagnosis.
Two to four random biopsy specimens should be taken every 10 cm from the entire colon, with additional samples of suspicious areas. Particularly in ulcerative colitis, consideration should be given to taking 4-quadrant biopsies every 5 cm in the lower sigmoid and rectum, because the frequency of colorectal cancer is higher in this region.
Random biopsies visualize only 1% of total colonic mucosa surface area, promoting a high sampling error. In a retrospective review, the probability of detecting dysplasia was 90% if 33 and 95% if 56 random biopsies were taken.45 The cost of additional random biopsies to the current recommended 33 is hard to justify.13 To help increase the detection yield of random biopsies, jumbo biopsies can be used. In a prospective study by Elmunzer et al,46 jumbo forceps were found to be superior to standard large-capacity forceps in obtaining diagnostically adequate surveillance biopsy specimens. One problem with this study was the high interobserver variability between pathologists in analyzing the specimen. Nevertheless, jumbo biopsy forceps are recommended as part of colorectal cancer surveillance.
NEW METHODS FOR EARLY DETECTION OF DYSPLASIA
Targeted biopsies are an attractive alternative to random biopsies to increase the yield of dysplasia detection. Chromoendoscopy uses a dye sprayed on the colonic mucosa to enhance the visualization of subtle mucosal changes suggestive of neoplasia not visible with the white light of standard endoscopy. There are two main agents used for chromoendoscopy. Indigo carmine contrast dye is poorly absorbed by the normal alimentary epithelial cells.47 This method highlights irregularities in the mucosal architecture by the pooling of a blue dye solution in mucosal grooves, improving the precision of endoscopic diagnosis by defining minute and inconspicuous lesions that might be overlooked with conventional endoscopic methods. Methylene blue stains the normal absorptive epithelium of the small intestine and colon. The absence of staining in these tissues usually indicates the presence of metaplastic, neoplastic, or inflammatory change.
In a study by Rutter et al,26 the clinical accuracy of consecutive, random (n=2904) and targeted (indigo carmine, n=157) biopsies was compared. Nine dysplastic lesions were diagnosed at chromoendoscopy, whereas no dysplasia and no additional lesions were detected by random biopsies. Similar findings were noted in a prospective study by Kiesslich et al48 using methylene blue dye. A study by Hurlstone et al49 revealed a higher yield for detecting dysplasia using high-magnification chromoendoscopic colonoscopy than conventional optical colonoscopy with random biopsies. In 350 ulcerative colitis patients and 350 disease-extent-matched controls, 69 dysplastic lesions were identified by chromoendoscopy, compared with only 24 dysplastic lesions in the traditional surveillance group (P<.001). High-magnification chromoendoscopy increased the diagnostic yield of dysplastic lesion by 3.0–4.5 times.
Despite these results, chromoendoscopy is not routinely used for IBD-CRC surveillance. It is time consuming and requires an endoscopist who is familiar with the technique and who can identify various suspicious mucosal patterns. The natural history of dysplasia detected by chromoendoscopy or other advanced techniques is unknown, but it is not necessarily the same as dysplasia detected by random biopsy. Specific management recommendations for these patients are still evolving.
To assess the endoscopic visibility of dysplasia and colorectal cancer in ulcerative colitis using white light endoscopy, a retrospective review by Rubin et al50 was performed. All cases of dysplasia or colorectal cancer in ulcerative colitis between 1994 and 2004 were identified. Visible dysplasia was defined as a lesion reported by the endoscopist that led to directed biopsy and that was confirmed by pathology. Invisible dysplasia was defined as dysplasia diagnosed on pathology but not described on endoscopy. There were 1339 surveillance examinations in 622 patients with ulcerative colitis. Forty-six patients were found to have dysplasia or colorectal cancer at a median age of 48 years and with median duration of disease of 20 years. Of these patients, 77% had pancolitis, 21% had left-sided colitis, and 2% had proctitis.50 Thirty-eight of 65 dysplastic lesions (58.5%) and 8 of 10 cancers (80.0%) were visible to the endoscopist as 23 polyps and masses, 1 stricture, and 22 irregular mucosa. The per-patient sensitivities for dysplasia and for cancer were 71.8% and 100%, respectively. The overall per-lesion and per-patient sensitivities were 61.3% and 76.1%, respectively. This study concluded that dysplasia and cancer in ulcerative colitis are endoscopically visible in most patients and may be reliably identified during scheduled examinations.
Limited data regarding the role of newer endoscopic techniques for the detection of dysplasia is available. In a randomized controlled trial by Kiesslich et al,51 the value of combined chromoendoscopy and confocal laser endomicroscopy (which visualize the histology of the colonic mucosa in real time) for the diagnosis of intraepithelial neoplasia was assessed. By using chromoendoscopy with endomicroscopy, the diagnostic yield of neoplasia was increased by 4.75-fold compared with conventional colonoscopy with random biopsies (P=.005), though 50% fewer biopsy specimens were required.51
In a recent prospective, randomized, controlled study by Hurlstone et al,52 confocal chromoscopic endomicroscopy was superior to chromoendoscopy alone for detecting intraepithelial neoplasia. Endomicroscopy-targeted biopsies increased the diagnostic yield of intraepithelial neoplasia by 2.5-fold compared with chromoendoscopy-guided biopsies alone.52 Interobserver variability in interpreting real-time histology will be an important limitation of this modality. Even expert gastrointestinal pathologists frequently disagree with one another when interpreting dysplasia in IBD biopsies. This interobserver variability is magnified among gastroenterologists with much less experience at histologic interpretation.
The use of narrow band imaging (NBI, an optical filter technology that improves the visibility of vessels and other subtle tissue structures) does not improve the diagnostic accuracy compared with conventional colonoscopy. In a prospective randomized trial by Dekker et al,53 42 patients with long-standing ulcerative colitis underwent NBI and conventional colonoscopy with at least 3 weeks between the procedures. Although more lesions were identified using NBI, an almost equal number of dysplastic foci were identified and missed by both methods. More studies are needed before the use of these advanced techniques can be suggested in clinical practice.
Noninvasive approaches that are complementary to colonoscopy are being actively investigated. Stool DNA analysis is being studied as a colorectal screening method in the general population and may ultimately be used in IBD surveillance.
PREVENTION OF IBD-CRC
Surveillance colonoscopy has limitations for dysplasia detection. Intervening before the development of dysplasia can not only prevent cancer but also obviate colectomy. Given that chronic inflammation is the main driving force behind malignant transformation, the use of maintenance anti-inflammatory therapy should constitute primary chemoprevention. The ideal chemopreventive agent would be safe, effective at preventing neoplastic progression, inexpensive, and able to prevent flares and control disease activity and symptoms.13
5-Aminosalicylic Acid
5-Aminosalicylic acid (5-ASA) compounds are anti-inflammatory drugs used to treat inflammation of the digestive tract in IBD patients. Given that chronic inflammation is a plausible mechanism causing malignant transformation, the possibility exists for the use of maintenance anti-inflammatory therapy as primary chemoprevention. Various in vitro studies looking at the effect of 5-ASA on colonic epithelial cells have focused on its anti-inflammatory properties.
Aminosalicylates were found to specifically inhibit the NFκB pathway, which is associated with sustaining the chronic inflammatory processes in the gut.54–56 NFκB is also involved in tumor survival; by blocking the NFκB pathway, aminosalicylates induce apoptosis and thereby reduce tumor mass.6 It has previously been shown that aminosalicylates may induce apoptosis and inhibit proliferation of colonic epithelial cells in patients with sporadic polyps of the large bowel.57 Besides increasing apoptosis, mesalamine may also inhibit cell cycle progression by arresting cells in mitosis.58
A meta-analysis performed by Velayos et al59 supported the protective association of 5-ASA products and colorectal cancer in IBD patients. They systematically reviewed nine studies including 334 cases of colorectal cancer, 140 cases of dysplasia, and a total of 1932 patients with ulcerative colitis. Pooled analysis showed a protective association between use of 5-aminosalicylates and colorectal cancer (odds ratio [OR] = 0.51; 95% CI, 0.37–0.69) or a combined end point of colorectal cancer/dysplasia (OR = 0.51; 95% CI, 0.38–0.69). 5-ASA use was not, however, associated with a lower risk of dysplasia, though only two studies evaluated this outcome (OR = 1.18; 95% CI, 0.41–3.43).59
Another conflicting piece of data about whether 5-ASA has chemopreventive properties against IBD-related carcinogenesis was introduced by Terdiman et al.60 The objective of this observational study was to determine if an association between 5-ASA therapy and colorectal cancer risk exists in IBD patients. Adult patients with a new colorectal cancer diagnosis (n=18,440) were identified. For each case, 20 control patients with no record of colorectal cancer diagnosis or bowel surgery (n=368,800) were identified. An IBD diagnosis was associated with a six- to sevenfold increased risk of colorectal cancer, whether they had ulcerative colitis or Crohn's colitis. Among patients with IBD (364 colorectal cancer cases, 1172 controls), exposure to 5-ASA therapy of any dose or duration during the 12 months before colorectal cancer diagnosis was not associated with a reduced risk of colorectal cancer (OR = 0.97; 95% CI, 0.77–1.23). However, there was a trend toward a decreased risk of colorectal cancer, with increasing number of mesalamine prescriptions in the previous year, though statistical significance was not achieved (P=.08). This study concluded that treating IBD patients with 5-ASA medications did not have a protective effect against colitis-related colorectal cancer when assessed over a short period of exposure.
A case–control study by Pinczowski et al61 first described the possible chemoprotective role of sulfasalazine. The authors matched 102 cases of ulcerative colitis patients with colorectal cancer and 196 controls without cancer. Sulfasalazine treatment for at least 3 months was associated with a significant protective effect against ulcerative colitis–colorectal cancer, independent of disease activity (relative risk [RR] 0.38; 95% CI, 0.20–0.69). Another case–control study by Eaden et al,62 involved 102 cases of colorectal cancer from a population of ulcerative colitis patients treated at academic- and community-based gastroenterology practices. The use of any 5-ASA compound was associated with a 75% decreased risk of colorectal cancer (95% CI, 0.13–0.48). Among all 5-ASA formulations, mesalamine use was associated with the greatest degree of protection, providing the most benefit at doses greater than 1.2 g/d (OR = 0.09; 95% CI, 0.03–0.28). Sulfasalazine use was associated with a smaller protective effect, which was statistically significant only at dosages of 2 g/d or more (OR = 0.41; 95% CI, 0.18–0.92).
Ursodeoxycholic Acid
Protection against colonic exposure to bile acid by ursodeoxycholic acid (UDCA) may be a practical chemoprevention means in patients with altered bile pool, such as in patients with PSC. Secondary bile acids are carcinogenic, and their concentration is increased in patients with cholestatic liver disease, such as PSC. UDCA decreases the proportion of these injurious bile acids. Deoxycholic acid is one of the key cytotoxic bile acids involved in this process. In a retrospective cross-sectional study of patients with PSC and ulcerative colitis by Tung et al,63 UDCA use was strongly associated with decreased prevalence of colonic dysplasia (OR = 0.18; 95% CI, 0.05–0.61). The association between dysplasia and UDCA use remained after adjustment for sex, age at onset of colitis, duration of colitis, duration of PSC, severity of liver disease, and sulfasalazine use (adjusted OR = 0.14; 95% CI, 0.03–0.64).63 This chemopreventative effect of UDCA was also noted in a prospective placebo-controlled study by Pardi et al.64 Fifty-two patients with ulcerative colitis and PSC were followed for a total of 355 person-years. Those assigned to receive UDCA had a relative risk of 0.26 for developing colorectal dysplasia or cancer compared to those receiving placebo (95% CI, 0.06–0.92, P=.034).
There may be a secondary chemopreventive role for UDCA in patients with IBD without PSC but with a history of low-grade dysplasia and/or DNA aneuploidy. A prospective, double-blind, randomized, controlled pilot study by Sjoqvist et al65 introduced this possibility. Nineteen patients (13 ulcerative colitis, 6 Crohn's disease) with long-standing extensive IBD (median duration 21 years) with previous findings of low-grade dysplasia and/or DNA aneuploidy were randomized to receive either UDCA (500 mg bid, n=10) or placebo (n=9). In the placebo group, one patient's low-grade dysplasia progressed to high-grade dysplasia, and one patient with low-grade dysplasia developed a DALM; both underwent a colectomy. In contrast, no UDCA-treated patient had progression of their low-grade dysplasia.65
Although UDCA is the only agent for which we have prospective random controlled studies to support a chemopreventive effect, its use is not widely recommended because of potential detrimental effects on the liver. Larger studies, possibly exploring various UDCA doses, would be required before recommending secondary chemoprevention with UDCA.
Total Colectomy
Several studies from different geographic areas suggest the epidemiology of IBD-CRC is changing, and the incidence is likely not as high as previously reported in the Eaden meta-analysis. A cohort study conducted in Denmark between 1962 and 1987 reported only 13 cases of colorectal cancer among 1,160 patients with ulcerative colitis.66 The annual risk was 0.06%. The 30-year cumulative colorectal cancer risk was 2.1%. The rate of surgery for ulcerative colitis in Denmark is among the highest reported worldwide, making this a plausible explanation for the very low risk of colorectal cancer in this population. Besides the relatively higher rates of colectomy, there was an extensive use of continuous 5-ASA (70% of all patients) in this cohort. More recently, the Danish cohort study66 was extended for an additional 10 years, and the risk factors for colorectal cancer were reevaluated. The colorectal cancer risk was not increased, and this again was attributed to the aggressive surgical approach in medical treatment failures and to the widespread use of 5-ASA.
The role for prophylactic proctocolectomy in patients with PSC and IBD is still controversial. As mentioned previously, the cumulative colorectal cancer risk in patients with ulcerative colitis and PSC is 33% at 20 years and 40% at 30 years after diagnosis. This high incidence of colorectal cancer in patients with ulcerative colitis and PSC explains why some IBD experts recommend total colectomy after 15 years of disease in this subpopulation.
TREATMENT APPROACHES TO DYSPLASIA
In ulcerative colitis, findings of confirmed dysplasia usually lead to a total colectomy. Any biopsy revealing low-grade dysplasia, high-grade dysplasia, indefinite for dysplasia, or adenocarcinoma is considered an abnormal finding. Because dysplasia can be difficult to distinguish from epithelial regeneration because of inflammation, surveillance biopsies should be performed when the patient is in remission, and all abnormal biopsies should be reviewed by an experienced gastrointestinal pathologist.
If high-grade dysplasia is confirmed, total proctocolectomy should be performed, given the high rate of synchronous and metachronous adenocarcinoma in that context.39,40 Raised lesions on the background of colitis may resemble sporadic adenomas. Polypectomy is done, along with four biopsies taken from the adjacent colon. If complete polypectomy is confirmed and biopsies of the surrounding mucosa are negative for dysplasia, and in addition there is no dysplasia elsewhere in the colon, a follow-up examination should be performed within 6 months, with regular surveillance resumed if no dysplasia is found.40,67 If dysplasia is present in the surrounding mucosa, or if the dysplastic polypoid lesion is nonresectable or does not resemble a typical adenoma, this lesion is considered to be a DALM. The presence of a DALM with its high association with a synchronous colorectal cancer and the presence of overt adenocarcinoma should prompt a proctocolectomy.40
The management of low-grade dysplasia is more controversial. In a study published in 1994, almost one third of patients with low-grade dysplasia progressed to high-grade dysplasia or colorectal cancer during follow-up.39 A chart review by Ullman et al revealed neoplastic progression from low-grade dysplasia to advanced neoplasia as high as 53% at 5 years.68 There is evidence that an unrecognized synchronous colorectal cancer may already be present in up to 20% of individuals who undergo colectomy for low-grade dysplasia.39,68 These findings highlight the fact that low-grade dysplasia is an independent risk factor for colorectal cancer, and transformation into high-grade dysplasia is not a necessary step before the development of colorectal cancer. More recent studies have shown that patients with low-grade dysplasia have a lower rate of colorectal cancer than previously thought (2–10% during a 10-year follow-up),69 prompting certain IBD experts to recommend close colonoscopic surveillance of patients with low-grade dysplasia rather then total colectomy (Table 2).
Table 2.
Five and 10-year progression rates of LGD to HGD and colorectal cancer
| Trial (number of patients with LGD) | Five or 10-year progression to HGD and colorectal cancer |
|---|---|
| Connell et al 1994 (84) [70] | 54% 5-year progression |
| Ullman et al 2002 (18) [71] | 33% 5-year progression |
| Befrits et al 2002 (60) [69] | 3% 10-year progression |
| Ullman et al 2003 (46) [68] | 53% 5-year progression |
| Lim et al 2003 (40) [72] | 10% 10-year progression |
Abbreviations: HGD = high-grade dysplasia; LGD=low-grade dysplasia.
At this juncture, most experts agree that the presence of multifocal low-grade dysplasia should be managed with a total colectomy. When a single focus of low-grade dysplasia is found, both approaches (total colectomy vs. close colonoscopic surveillance) should be discussed with the patient. If the patient decides against total colectomy, then a repeat colonoscopy should be performed within 3 months and no later than 6 months from the discovery of the low-grade dysplasia.40 A subsequent surveillance exam revealing no dysplasia is not sufficient to return to routine surveillance. Continued exams should be performed every 6 months. Multiple biopsies, as previously described, need to be taken to decrease the sampling error during repeat surveillance.
SUMMARY
Ulcerative colitis and Crohn's disease are associated with an increased risk of colorectal cancer. Risk factors for cancer among IBD patients include young age at diagnosis, greater extent of colonic involvement, longer duration of disease, increased severity of inflammation, family history of sporadic colorectal cancer, and coexisting PSC. Tumorogenesis within the colon depends on unique environmental, genetic, and immunologic factors in IBD patients. Chemoprevention of colorectal cancer remains an important goal, and retrospective data suggest 5-aminosalicylates might play an important role in this context. Colonoscopy surveillance programs, with multiple biopsies performed every 1–2 years, are key components in early detection of colorectal cancer in these patients. Newer methods such as chromoendoscopy are currently being investigated as complementary techniques to enhance early detection of dysplasia and cancer in this high-risk population.
Footnotes
Disclosures of Potential Conflicts of Interest
The author indicated no potential conflicts of interest.
REFERENCES
- 1. Calkins BM, Lilienfeld AM, Garland CF, et al. : Trends in incidence rates of ulcerative colitis and Crohn's disease. Dig Dis Sci 29(10):913–920, 1984 [DOI] [PubMed] [Google Scholar]
- 2. Loftus EV, Jr: Management of extraintestinal manifestations and other complications of inflammatory bowel disease. Curr Gastroenterol Rep 6(6):506–513, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Crohn BB: The sigmoidoscopic picture of chronic ulcerative colitis (non-specific). Amer J Med Sci 170:220–228, 1925 [Google Scholar]
- 4. Munkholm P: Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther 18(suppl 2):1–5, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Lennard-Jones JE, Ritchie JK, Williams CB: Cancer surveillance in ulcerative colitis: experience over 15 years. Lancet 2:149–152, 1983 [DOI] [PubMed] [Google Scholar]
- 6. Munkholm P, Loftus EV, Jr, Reinacher-Schick A, et al. : Prevention of colorectal cancer in inflammatory bowel disease: value of screening and 5-aminosalicylates. Digestion 73(1):11–19, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Richards ME, Rickert RR, Nance FC: Crohn's disease-associated carcinoma: a poorly recognized complication of inflammatory bowel disease. Ann Surg 209(6):764–773, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gyde S, Dew NJ: Mortality in ulcerative colitis. Gastroenterology 83:36–43, 1982 [PubMed] [Google Scholar]
- 9. Eaden JA, Abrams KR, Mayberry JF: The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48(4):526–535, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levin B: Inflammatory bowel disease and colon cancer. Cancer 70(5 suppl):1313–1316, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Choi PM, Zelig MP: Similarity of colorectal cancer in Crohn's disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut 35(7):950–954, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gillen CD, Walmsley RS, Prior P, et al. : Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut 35(11):1590–1592, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lakatos PL, Lakatos L: Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol 14(25):3937–3947, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zisman TL, Rubin DT: Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol 14(17):2662–2669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Velayos FS, Loftus EV, Jr, Jess T, et al. : Predictive and protective factors associated with colorectal cancer in ulcerative colitis: a case–control study. Gastroenterology 130(7):1941–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Askling J, Dickman PW, Karlén P, et al. : Colorectal cancer rates among first-degree relatives of patients with inflammatory bowel disease: a population-based cohort study. Lancet 357(9252):262–266, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Rutter MD, Saunders BP, Wilkinson KH, et al. : Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut 53(12):1813–1816, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith MP, Loe RJ: Sclerosing cholangitis; review of recent case reports and associated diseases and four new cases. Am J Surg 110:239–246, 1965 [DOI] [PubMed] [Google Scholar]
- 19. Lakatos L, Mester G, Erdelyi Z, et al. : Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis 12(3):205–211, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Loftus EV, Jr, Harewood GC, Loftus CG, et al. : PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 54(1):91–96, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shetty K, Rybicki L, Brzezinski A, et al. : The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol 94(6):1643–1649, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Kornfeld D, Ekbom A, Ihre T: Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut 41(4):522–525, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ekbom A, Helmick C, Zack M, et al. : Ulcerative colitis and colorectal cancer: a population-based study. N Engl J Med 323(18):1228–1233, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Xie J, Itzkowitz SH: Cancer in inflammatory bowel disease. World J Gastroenterol 14(3):378–389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feagins LA, Souza RF, Spechler SJ: Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol 6(5):297–305, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Rutter M, Saunders B, Wilkinson K, et al. : Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126(2):451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Murthy S, Flanigan A, Clearfield H: Colorectal cancer in inflammatory bowel disease: molecular and clinical features. Gastroenterol Clin N Am 31(2):551–564, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Kawanishi S, Hiraku Y, Pinlaor S, et al. : Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem 387(4):365–372, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Liao J, Seril DN, Lu GG, et al. : Increased susceptibility of chronic ulcerative colitis-induced carcinoma development in DNA repair enzyme Ogg1 deficient mice. Mol Carcinog 47(8):638–646, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crivello A, Giacalone A, Vaglica M, et al. : Regulatory cytokine gene polymorphisms and risk of colorectal carcinoma. Ann N Y Acad Sci 1089:98–103, 2006 [DOI] [PubMed] [Google Scholar]
- 31. van Dieren JM, Wink JC, Vissers KJ, et al. : Chromosomal and microsatellite instability of adenocarcinomas and dysplastic lesions (DALM) in ulcerative colitis. Diagn Mol Pathol 15(4):216–222, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Willenbucher RF, Aust DE, Chang CG, et al. : Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am J Pathol 154(6):1825–1830, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tahara T, Inoue N, Hisamatsu T, et al. : Clinical significance of microsatellite instability in the inflamed mucosa for the prediction of colonic neoplasms in patients with ulcerative colitis. J Gastroenterol Hepatol 20(5):710–715, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Itzkowitz S: Colon carcinogenesis in inflammatory bowel disease: applying molecular genetics to clinical practice. J Clin Gastroenterol 36(5 suppl):S70–74; discussion S94-96, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Gavert N, Ben-Ze'ev A: beta-Catenin signaling in biological control and cancer. J Cell Biochem 102(4):820–828, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Hill KA, Wang KL, Stryker SJ, et al. : Comparative analysis of cell adhesion molecules, cell cycle regulatory proteins, mismatch repair genes, cyclooxygenase-2, and DPC4 in carcinomas arising in inflammatory bowel disease and sporadic colon cancer. Oncol Rep 11(5):951–956, 2004 [PubMed] [Google Scholar]
- 37. Bernstein CN: Neoplastic and other complications of inflammatory bowel disease. Curr Gastroenterol Rep 2(6):451–459, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Itzkowitz SH, Harpaz N: Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology 126(6):1634–1648, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Bernstein CN, Shanahan F, Weinstein WM: Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet 343(8889):71–74, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Itzkowitz SH, Present DH: Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis 11(3):314–321, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Biancone L, Travis S, Escher JC, et al. : European evidence-based consensus on the management of ulcerative colitis: special situations. J Crohn Colitis 2:63–92, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Eaden JA, Mayberry JF: Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut 51(suppl 5):V10–V12, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hanauer SB, Meyers S: Management of Crohn's disease in adults. Am J Gastroenterol 92(4):559–566, 1997 [PubMed] [Google Scholar]
- 44. Kornbluth A, Sachar DB: Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 99(7):1371–1385, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Rubin CE, Haggitt RC, Burmer GC, et al. : DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology 103(5):1611–1620, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Elmunzer BJ, Higgins PD, Kwon YM, et al. : Jumbo forceps are superior to standard large-capacity forceps in obtaining diagnostically adequate inflammatory bowel disease surveillance biopsy specimens. Gastrointest Endosc 68(2):273–278; quiz 334–336, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Mitooka H, Fujimori T, Ohno S, et al. : Chromoscopy of the colon using indigo carmine dye with electrolyte lavage solution. Gastrointest Endosc 38(3):373–374, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Kiesslich R, Hoffman A, Neurath MF: Colonoscopy, tumors, and inflammatory bowel disease: new diagnostic methods. Endoscopy 38(1):5–10, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Hurlstone DP, Sanders DS, Lobo AJ, et al. : Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy 37(12):1186–1192, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Rubin DT, Rothe JA, Hetzel JT, et al. : Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc 65(7):998–1004, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Kiesslich R, Goetz M, Lammersdorf K, et al. : Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology 132(3):874–882, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Hurlstone DP, Kiesslich R, Thomson M, et al. : Confocal chromoscopic endomicroscopy is superior to chromoscopy alone for the detection and characterisation of intraepithelial neoplasia in chronic ulcerative colitis. Gut 57(2):196–204, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Dekker E, van den Broek FJ, Reitsma JB, et al. : Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy 39(3):216–221, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Bantel H, Berg C, Vieth M, et al. : Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol 95(12):3452–3457, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Wahl C, Liptay S, Adler G, et al. : Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest 101(5):1163–1174, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kaiser GC, Yan F, Polk DB: Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology 116(3):602–609, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reinacher-Schick A, Seidensticker F, Petrasch S, et al. : Mesalazine changes apoptosis and proliferation in normal mucosa of patients with sporadic polyps of the large bowel. Endoscopy 32(3):245–254, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Reinacher-Schick A, Schoeneck A, Graeven U, et al. : Mesalazine causes a mitotic arrest and induces caspase-dependent apoptosis in colon carcinoma cells. Carcinogenesis 24(3):443–451, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Velayos FS, Terdiman JP, Walsh JM: Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol 100(6):1345–1353, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Terdiman JP, Steinbuch M, Blumentals WA, et al. : 5-Aminosalicylic acid therapy and the risk of colorectal cancer among patients with inflammatory bowel disease. Inflamm Bowel Dis 13(4):367–371, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Pinczowski D, Ekbom A, Baron J, et al. : Risk factors for colorectal cancer in patients with ulcerative colitis: a case–control study. Gastroenterology 107(1):117–120, 1994 [DOI] [PubMed] [Google Scholar]
- 62. Eaden J, Abrams K, Ekbom A, et al. : Colorectal cancer prevention in ulcerative colitis: a case–control study. Aliment Pharmacol Ther 14(2):145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Tung BY, Emond MJ, Haggitt RC, et al. : Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med 134(2):89–95, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Pardi DS, Loftus EV, Jr, Kremers WK, et al. : Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology 124(4):889–893, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Sjoqvist U, Tribukait B, Ost A, et al. : Ursodeoxycholic acid treatment in IBD-patients with colorectal dysplasia and/or DNA-aneuploidy: a prospective, double-blind, randomized controlled pilot study. Anticancer Res 24(5B):3121–3127, 2004 [PubMed] [Google Scholar]
- 66. Winther KV, Langholz E, Munkholm P, et al. : Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol 2:1088–1095, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Odze RD, Farraye FA, Hecht JL, et al. : Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol 2(7):534–541, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Ullman T, Croog V, Harpaz N, et al. : Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology 125(5):1311–1319, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Befrits R, Ljung T, Jaramillo E, et al. : Low-grade dysplasia in extensive, long-standing inflammatory bowel disease: a follow-up study. Dis Colon Rectum 45(5):615–620, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Connell WR, Lennard-Jones JE, Williams CB, et al. : Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology 107(4):934–944, 1994 [DOI] [PubMed] [Google Scholar]
- 71. Ullman TA, Loftus EV, Jr, Kakar S, et al. : The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol 97(4):922–927, 2002 [DOI] [PubMed] [Google Scholar]
- 72. Lim CH, Dixon MF, Vail A, et al. : Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut 52(8):1127–1132, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]