ABSTRACT
Background:
We performed a retrospective analysis of data from a phase II study evaluating sorafenib in patients with advanced hepatocellular carcinoma (HCC) to assess differences in safety and efficacy based on Child-Pugh (CP) status (A/B).
Methods:
Patients received sorafenib 400 mg PO bid. We analyzed safety, pharmacokinetic (PK), and efficacy data in the two CP groups.
Results:
Ninety-eight patients were CP A; 38 were CP B, with a median duration of therapy of 4 and 1.8 months, respectively. Grade 3/4 adverse events in the CP A and B groups, respectively, included hyperbilirubinemia (14% and 53%), ascites (3% and 5%), and encephalopathy (3% and 13%). Median overall survival (OS) in the CP A group was 9.5 months, compared with 3.2 months in the CP B population. Responses were limited in both groups. AUC and Cmax values were comparable between the two groups.
Conclusions:
Due to the lack of randomization against placebo or no therapy in this study, it is unclear if the more frequent worsening of liver cirrhosis and outcome of CP B patients are drug related or due to disease progression, or both. As expected, outcome was poorer in patients with CP B than in those with CP A cirrhosis. The hyperbilirubinemia seen in both groups may be at least partly related to inhibition of UGT1A1 by sorafenib. PK profiles were similar in the two groups. More data are needed to confirm and more fully understand the safety and efficacy of sorafenib in patients with advanced HCC and CP B cirrhosis.
Sorafenib, an oral multikinase inhibitor, has been studied extensively in patients with hepatocellular carcinoma (HCC). A large phase III study evaluated sorafenib versus placebo in patients with advanced HCC.1 Eligibility for this trial was restricted to patients with a Child-Pugh (CP) designation no worse than A (Table 1).2,3 This phase III trial demonstrated an overall survival advantage for sorafenib compared with placebo (10.7 vs. 7.9 months, P < .001). These results led to approval by the US Food and Drug Administration (FDA) for the use of sorafenib as first-line therapy in patients with unresectable HCC.4,5
Table 1.
Child-Pugh scoring
| Parameter | Points |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Albumin (g/dL) | >3.5 | 3.5–2.8 | <2.8 |
| Bilirubin (mg/dL) | <2 | 2–3 | >3 |
| Ascites | Absent | Slight | Moderate |
| Encephalopathy | None | I–II* | III–IV* |
| Prothrombin time (PT) (INR) | <1.7 | 1.8–2.3 | >2.3 |
| Score | A | B | C |
| Points | 5–6 | 7–9 | 10–15 |
INR = International Normalized Ratio.
Glasgow Coma Scale.
Although the phase III trial was limited to patients with a CP A status, the FDA approval of sorafenib was not. The safety and efficacy of sorafenib in patients with CP B or CP C cirrhosis has not been as fully defined as it has in those with CP A cirrhosis and needs to be further evaluated in the more severe CP strata. The pivotal phase III study was preceded by a large, nonrandomized phase II trial that evaluated sorafenib in patients with advanced HCC who had either CP A or B disease.6 We performed a retrospective, exploratory subanalysis of data from the phase II trial to evaluate safety and efficacy outcomes in patients with CP A versus CP B cirrhosis to understand better the effects of sorafenib in patients with CP B disease.
PATIENTS AND METHODS
The phase II study of sorafenib in HCC6 was an international, noncontrolled, single-arm trial. The study population was comprised of patients with advanced HCC and either CP A or B cirrhosis. The trial was approved by a human investigation committee at each center and was conducted in accordance with the United States Department of Health and Human Services guidelines. Informed consent was obtained from each participating patient.
Eligible patients had bidimensionally measurable, histologically proven, inoperable HCC, no prior systemic treatments for HCC, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and a CP score of either A or B. Estimated life expectancy was required to be ≥12 weeks, and adequate hematologic, hepatic, and renal function were required.
Patients received sorafenib 400 mg twice daily but were allowed up to two dose reductions (200 mg twice daily and 200 mg once daily) for drug-related toxicities (National Cancer Institute–Common Toxicity Criteria v2.0). Otherwise, treatment continued until disease progression or unacceptable drug-related toxicities. Investigator-assessed bidimensional tumor measurements were performed at baseline and every 8 weeks (two cycles) thereafter, according to modified World Health Organization criteria. Independent radiologic assessment was also performed for patients who had a baseline and at least one postbaseline imaging measurement.
For a patient to be regarded as achieving stable disease (SD), ≥16 weeks of documented nonprogression was required. The primary efficacy assessment was tumor response. Secondary assessments included progression-free survival (PFS), time to progression (TTP), overall survival (OS), and safety. The primary and all secondary end points were assessed in both patients with CP A and those with CP B cirrhosis. Blood samples were collected on day 1 of cycle 2 for pharmacokinetic analysis of sorafenib plasma concentrations using a validated liquid chromatography/mass spectrometry/mass spectrometry assay, with a lower limit of quantification of 1–10 μg/L, as previously reported.6
Statistical Analysis
We report the results of a retrospective, exploratory comparison. In the original study, no preplanned CP A/CP B stratification analysis was performed. All patients received at least one dose of study drug. Considering the retrospective, unplanned, exploratory nature of this analysis, and the inability to make formal comparisons between the CP A and CP B populations, formal statistical evaluations were not considered, and P values were not calculated. Descriptive statistics were used to compare outcomes in the two CP populations.
RESULTS
Demographics
Of 137 patients enrolled in the trial, 98 (71.5%) had a CP A designation, 38 (27.7%) were categorized as CP B, and 1 patient (0.73%) had a CP status described as “missing.” Baseline demographic and disease characteristics in the two CP groups are shown in Table 2. Some data points have been updated since the publication of the phase II study.
Table 2.
Baseline demographic and disease characteristics of patients by Child-Pugh (CP) status
| Baseline characteristic* | CP A (n = 98) | CP B (n = 38) |
|---|---|---|
| Age (years) | ||
| Median | 70.0 | 67.5 |
| Range | 29–86 | 28–79 |
| Gender, n (%) | ||
| Male | 70 (71) | 26 (68) |
| Female | 28 (29) | 12 (32) |
| ECOG performance status, n (%) | ||
| 0 | 53 (54) | 15 (39) |
| 1 | 45 (46) | 23 (61) |
| AFP > upper limit of normal, n (%) | ||
| Yes | 76 (78) | 26 (68) |
| No | 13 (13) | 4 (11) |
| Unknown | 9 (9) | 8 (21) |
| Positive hepatitis status, n (%) | ||
| Hepatitis B | 20 (20) | 7 (18.4) |
| Hepatitis C | 41 (42) | 13 (34) |
| TNM stage at study entry, n (%) | ||
| I | 5 (5) | 2 (5) |
| II | 9 (9) | 1 (3) |
| IIIA/IIIB | 41 (42) | 11 (29) |
| IVA/IVB | 37 (38) | 20 (53) |
| Unknown | 6 (6) | 4 (10) |
AFP = alpha-fetoprotein; AJCC = American Joint Committee on Cancer; ECOG = Eastern Cooperative Oncology Group; TNM = tumor nodes metastasis.
One patient had missing data regarding Child-Pugh classification.
Dose and Duration of Therapy
Median duration of therapy was 4 months (range, 0.14–60 months) for patients with a CP A designation and 1.8 months (range, 0–15 months) for those who were categorized as CP B. Patients in the CP A group received a median of five treatment cycles (range, 1–35 cycles), and those in the CP B group received a median of 3 treatment cycles (range, 1–17 cycles). Dose reductions were required in 31% and 21% of patients in the CP A and CP B groups, respectively.
Adverse Events
The most common adverse events (any grade) were dermatologic, constitutional, and gastrointestinal in nature (Table 3). Grade 3 toxicities included fatigue (CP A, 21.4%; CP B, 31.6%), diarrhea (CP A, 9.2%; CP B, 10.5%), and hand-foot syndrome (CP A, 5.1%; CP B, 5.3%). There were no grade 4 toxicities observed.
Table 3.
All-grade and grade-3 drug-related adverse events in ≥10% of patients in the Child-Pugh (CP) A and CP B groups*
| Adverse event, n (%) | All grades |
Grade 3 |
||
|---|---|---|---|---|
| CP A | CP B | CP A | CP B | |
| Dermatology | ||||
| Hand-foot skin reaction | 29 (30) | 5 (13) | 5 (5) | 2 (5) |
| Rash or desquamation | 12 (12) | (8) | 1 (1) | 0 |
| Alopecia | 11 (11) | 1 (3) | 0 | 0 |
| Constitutional symptoms | ||||
| Fatigue | 22 (22) | 6 (16) | 4 (4) | 3 (8) |
| Gastrointestinal | ||||
| Diarrhea (without colostomy) | 46 (47) | 13 (34) | 8 (8) | 3 (8) |
| Nausea | 16 (16) | 6 (16) | 0 | 0 |
| Anorexia | 14 (14) | 3 (8) | 2 (2) | 0 |
| Stomatitis | 12 (12) | 7 (18) | 0 | 0 |
| Vomiting | 11 (11) | 3 (8) | 0 | 0 |
No grade 4 adverse events were reported.
Certain adverse events were more frequent in the CP B group than in the CP A population (Table 4). All-grade hyperbilirubinemia was reported in 67% of patients with a CP A designation and 86% of those categorized as CP B. Grade 3/4 hyperbilirubinemia was reported in 14% and 53% of patients in the CP A and CP B groups, respectively. Three percent of patients in the CP A group and 5% of those in the CP B group developed or progressed to grade 3 or 4 ascites. Development of or worsening to grade 3 or 4 encephalopathy was reported in 3% of patients in the CP A group and 13% of those in the CP B group. One additional patient with CP A cirrhosis experienced grade 5 encephalopathy.
Table 4.
Grade 3–4 adverse events that occurred with greater frequency in patients with Child-Pugh (CP) B than in those with CP A cirrhosis
| Adverse event, n (%) | CP A | CP B |
|---|---|---|
| Bilirubin | 14 (14) | 20 (53) |
| Ascites | 3 (3) | 2 (5) |
| Encephalopathy* | 3 (3) | 5 (13) |
One additional patient with CP A cirrhosis had grade 5 encephalopathy.
There were four deaths secondary to adverse events. Two deaths (one in each CP class) occurred secondary to intracranial hemorrhage, and two deaths (one in each CP class) occurred as a result of gastrointestinal bleeding.
Efficacy
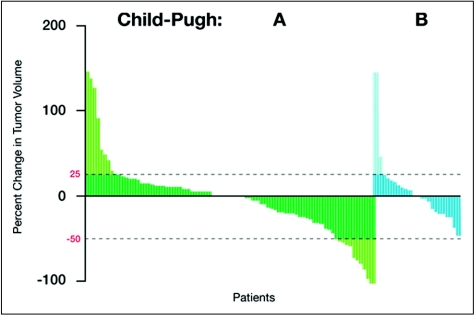
Response was independently assessed. Among patients with CP A, 3 (3.1%) sustained a PR, and 65 (66.3%) achieved SD. Among those with a CP B status, there were no responses, and 17 (44.7%) achieved SD. A waterfall plot depicts a continuum of tumor shrinkage in 33.3% and 50% of patients with CP A and CP B status, respectively (Figure 1).
Figure 1.
Waterfall plot showing degree of tumor shrinkage in patients with stable disease in the Child-Pugh (CP) A and CP B groups.
Based on investigator assessment, PFS in the CP A and CP B populations was 4.4 months (95% confidence interval [CI], 3.7–5.5 months) and 2.5 months (95% CI, 1.8–3.6 months), respectively. Median TTP was 5 months (95% CI, 3.8–5.9 months) and 3 months (95% CI, 2.0–4.2 months) in the CP A and CP B populations, respectively. Median OS in the CP A group was 9.5 months (95% CI, 8.5–14.8 months), compared with 3.2 months (95% CI, 2.7–6.0 months) in the CP B population.
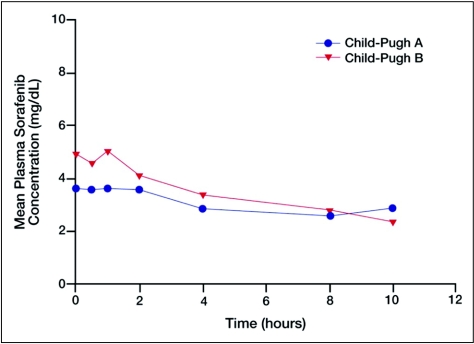
Pharmacokinetic Data
There was some variability in AUC and Cmax values, which were slightly greater in the CP B group than in the CP A group (Table 5 and Figure 2). Cmax, for example, was 4.9 and 6.0 mg/L in the CP A and CP B groups, respectively. These differences were not considered meaningful.
Table 5.
Sorafenib plasma pharmacokinetic parameters in cancer patients with hepatic impairment
| Child-Pugh (CP) status | AUC0–8* (mg · h/L) | Cmax (mg/L) | tmax† (h) |
|---|---|---|---|
| CP A (n = 14) | |||
| Geometric mean | 25.4 | 4.9 | 1.0 |
| Approximate CV (%) | (38.4) | (38.7) | (0–12) |
| CP B (n = 8) | |||
| Geometric mean | 30.3 | 6.0 | 0.5 |
| Approximate CV (%) | (82.1) | (73.8) | (0–8) |
CV = coefficient of variation.
AUC0–8 was reported because plasma samples were collected only up to 8 hours in all patients.
Median (instead of geometric mean) and range (instead of approximate CV) are reported for tmax.
Figure 2.
Geometric mean plasma concentrations of sorafenib following administration of 400 mg bid in patients with advanced hepatocellular carcinoma and either Child-Pugh (CP) A or CP B hepatic impairment.
DISCUSSION
Sorafenib is approved as a standard first-line therapy for patients with unresectable HCC, based largely on the results of a randomized, phase III study1 in which 95% and 98% of those randomized to sorafenib and placebo, respectively, were categorized as CP A. The phase II study of sorafenib in patients with advanced HCC6 is one of the few available sources of information on the safety and efficacy of sorafenib in patients with CP B cirrhosis. While data from the phase II study provide insight about the adverse-event profile of sorafenib and prognosis in patients with HCC and a CP B designation, they must be interpreted within the limitations of this retrospective and unplanned exploratory analysis. Furthermore, the phase II study was not controlled, so it is unclear whether the greater frequency of hyperbilirubinemia, ascites, and encephalopathy observed in the CP B group was a reflection of the patient population studied or due to a sorafenib-related effect, or both.
It is possible that the less favorable outcomes observed in patients with a CP B status are cirrhosis-related and due to the natural progression of cirrhosis, which, as expected, follows an exponential curve, with disease evolving at a faster rate than in patients categorized as CP A. This may partly explain the poorer median OS of 3.2 months in the CP B group, compared with 9.5 months in the CP A population. The similar TTP observed in the CP A and CP B groups also supports a cirrhosis-based explanation for differences in survival; however, it does not necessarily account for the shorter duration of therapy in the CP B group, which can be better explained by PFS.
In support of a non-drug effect on liver function is a phase I study evaluating the safety of sorafenib in 27 Japanese patients with advanced HCC and CP A or CP B cirrhosis.7 In this study, some differences in all-grade, drug-related adverse events between the CP A and CP B groups were observed, but these were not considered by the authors to be major. At a sorafenib dose level of 200 mg bid, rash or desquamation was reported in 50% of patients in the CP B group, compared with 29% of those in the CP A population. At the 400-mg bid dose level, differences in the rates of adverse events in the CP B and CP A groups, respectively, included diarrhea (63% vs. 33%), weight loss (50% vs. 17%), hypertension (38% vs. 17%), dry skin (38% vs. 0%), and fatigue (25% vs. 0%). The authors did not report any cirrhosis-related, non–drug-related events.
The geometric means of AUC0–12 and Cmax at steady state were slightly lower in the CP B group than in the CP A group, but the patient numbers in both groups were small, and these differences were not considered clinically relevant by the investigators. Considering the small sample size and the homogenous Japanese ethnicity, it would be difficult to extrapolate any explanations for these differences to a broader group of patients with HCC. It is also important to note that neither the published report of the phase II trial nor that of the Japanese phase I study specified the degree of CP B cirrhosis among the respective patient populations.
On the other hand, in support of a drug-related effect is a phase I and pharmacokinetic study evaluating sorafenib in 150 patients with organ dysfunction that included 17 patients with HCC.8 Treatment with sorafenib was associated with dose-limiting elevations in serum bilirubin concentration in 10 patients with hepatic dysfunction. The most commonly observed dose-limiting toxicity among patients with an elevated bilirubin concentration at baseline was, in fact, further elevation of bilirubin level. Based on their observations, the authors recommended a dosing schedule for sorafenib based on bilirubin level: 400 mg twice daily for bilirubin up to 1.5 times the upper limit of normal (ULN); 200 mg twice daily (or 400 mg daily) for bilirubin 1.5–3 times the ULN; and suspension of sorafenib dosing for bilirubin >3 times the ULN.
A recognized limitation of the study was that it was conducted in patients with a variety of different tumor types, including HCC. Obviously, the dose-limiting hyperbilirubinemia may have been due at least partly to the inhibition of uridine diphosphate-glucuronosyl-transferase (UGT1A1) by sorafenib.9 In this study, as in the reported phase II trial, no direct bilirubin measurements were collected; thus, it remains unclear if the elevations in bilirubin were due to worsening liver function caused by a toxic effect of sorafenib, a benign inhibitory effect of UGT1A1 leading to decreased bilirubin glucuronidation, disease progression, or some combination of these factors.
With the lack of firm data regarding the safety and efficacy of sorafenib in patients with HCC and advanced cirrhosis, one may best follow the recommendation of the FDA: “Given the paucity of treatment options and variability in CP scoring, the FDA approved the broad indication for therapy of unresectable HCC to facilitate clinical judgment for individual patients.”5 However, it is still the obligation of the scientific community to further clarify this matter.
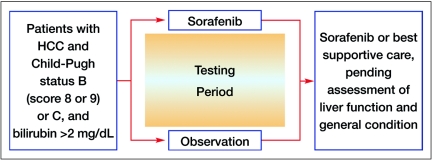
A potential resource for learning more about the safety and efficacy of sorafenib in patients with CP B cirrhosis is the phase IV Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of its Treatment with Sorafenib (GIDEON) study,10 which was initiated in December 2008 and has completed patient accrual. In addition, a proposed randomized, phase II study evaluating the safety and efficacy of sorafenib in patients with advanced HCC and CP status of B (score of 8 or 9) or C is currently under consideration (Figure 3). Patients would be randomized to sorafenib vs. best supportive care for a certain period of time, during which their liver-cirrhosis parameters would be recorded, and after which patients could continue treatment with sorafenib or best supportive care (pending assessment of liver function and general medical condition).
Figure 3.
Proposed schema for a randomized, phase II study to evaluate the safety and efficacy of sorafenib in patients with HCC and advanced cirrhosis.
The results of this exploratory subanalysis demonstrate that patients with CP B cirrhosis fared worse than those with CP A disease and experienced more frequent worsening of their cirrhosis. Further data are needed to confirm these results and more fully understand the safety and efficacy of sorafenib in patients with advanced HCC and CP B cirrhosis.
Footnotes
This work was supported by a research grant from Bayer HealthCare Pharmaceuticals.
Disclosures of Potential Conflicts of Interest
Dr. Abou-Alfa has received research funding from Bayer HealthCare Pharmaceuticals.
Drs. Anderson, Lathia, Moscovici, and Voiotis are employees of Bayer HealthCare Pharmaceuticals.
REFERENCES
- 1. Llovet JM, Ricci S, Mazzaferro V, et al. : Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R: Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649, 1973 [DOI] [PubMed] [Google Scholar]
- 3. Child CG., III The Liver and Portal Hypertension. Philadelphia, PA; WB Saunders; 1964:50 [Google Scholar]
- 4. US Food and Drug Administration Sorafenib. November 20, 2007. Available at: http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm129234.htm Accessed October 10, 2009
- 5. Kane RC, Farrell AT, Madabushi R, et al. : Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 14:95–100, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Abou-Alfa GK, Schwartz L, Ricci S, et al. : Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24:1–8, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K: Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci 99:159–165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller AA, Murry DJ, Owzar K, et al. : Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 27:1800–1805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miners JO, Mackenzie PI: Drug glucuronidation in humans. Pharmacol Ther 51:347–369, 1991 [DOI] [PubMed] [Google Scholar]
- 10. National Cancer Institute Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of Its Treatment with Sorafenib. March 18, 2009. Available at: http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=631237&version=HealthProfessional&protocolsearchid=6854751 Accessed April 19, 2011