Abstract
Background:
There is little published information regarding postoperative management of patients with Chronic Exertional Compartment Syndrome (CECS). Reports of recurrence of symptoms following surgical decompression exist, and are not uncommon depending on the specific technique used. Recurrence suggests that more time and effort may need to be spent on implementing strategic post-operative rehabilitation management in order to avoid repeat surgical intervention or prolonged symptoms.
Objective:
To summarize relevant literature regarding CECS and propose scientifically-based guidelines for rehab following compartment release with the rationale based on tissue healing, muscle loading, and scar tissue formation and consideration of all tissues contained in the involved compartment.
Literature review:
A literature search was performed in PubMed, SPORTDiscus, CINAHL, PEDRO, and Google Scholar using the phrase: “chronic exertional compartment syndrome.”
Results:
No specific rehabilitation guidelines following surgical compartment release for lower extremity CECS were found in the literature search performed for this clinical commentary.
Discussion:
The development of the proposed post-operative guidelines may allow for improved long-term outcomes following anterior compartment release.
Summary:
Adequate description of long-term follow-up of outcomes following compartment release for CECS is lacking in current literature. The proposed guidelines for rehab following compartment release include consideration of tissue healing, muscle loading, scar tissue formation, and consideration of soft tissues contained in the involved compartment. Utilization of the proposed guidelines may allow for future research to be performed in order to assess outcomes following surgical intervention for CECS.
Keywords: chronic exertional compartment syndrome, tissue healing parameters
BACKGROUND/INTRODUCTION
Compartment syndrome occurs when increased intramuscular pressure (fluid hydrostatic pressure in the interstitial space of a skeletal muscle tissue) impedes local muscle blood flow thereby impairing neuromuscular function of tissues within the specific compartment.1 Chronic Exertional Compartment Syndrome (CECS) is a reversible form of abnormally increased intramuscular pressure that occurs during exercise/exertion secondary to osteofascial tissues that are noncompliant with muscle volume expansion during exercise.1,2 Other terms used to describe the condition may include: anterior tibial pain, anterior compartment syndrome, recurrent compartment syndrome, idiopathic compartment syndrome, non-traumatic compartment syndrome of the lower extremity, fasciitis, pain in limb, swelling in limb, transient paralysis of limb, and disorder of soft tissue.1 Although compartment syndrome can exist wherever a compartment is present (thigh, forearm, upper arm, foot, hand, lumbar spine, abdomen, buttock), the anterior and lateral compartments of the lower leg are most commonly affected. This clinical commentary will focus on the anterior compartment of the lower leg, and on the open fasciotomy surgical technique. The principles described herein may, however, be applied to other lower leg compartments and surgical techniques.
CECS is often initially treated conservatively with rehabilitation. However, surgical decompression of the fascial compartment is often the subsequent treatment of choice for active individuals. There is little evidence in the literature on postoperative management of patients with CECS. Recurrence rates following various decompression techniques range from 3-17%. Over 35% of patients who undergo partial fasciectomy have reoccurrence of compartment syndrome or development of compartment syndrome in a different lower leg compartment, causing a reduction in exercise levels.3,4,5 This recurrence rate suggests that more time and effort needs to be spent on implementing strategic post-operative rehabilitation management after compartment release, in order to avoid additional surgical intervention or prolonged symptoms. The objective of this clinical commentary is to summarize the published evidence and propose scientifically-based guidelines for rehab following anterior compartment release with the rationale based on tissue healing, muscle loading, scar tissue formation, and consideration of all tissues contained in the involved compartment. Utilization of the proposed guidelines may allow for future research to be performed in order to assess outcomes following surgical intervention for CECS.
LITERATURE REVIEW
Published research and expert commentary articles were identified using both medical and rehabilitation electronic databases. All relevant articles regarding surgical rehabilitation following decompression of the anterior compartment were reviewed. A literature search was performed utilizing PubMed, SPORTDiscus, CINAHL, PEDRO, and Google Scholar. All references that were available by the first week of October 2010 were included. Initial searches were performed using the phrase: “chronic exertional compartment syndrome.” Attempts to perform searches without the entire phrase resulted in access to a wide variety papers that addressed varied compartments as well as those addressing acute compartment syndromes. Therefore, the results from the searches with use of the quotation separated phrase were then combined using the Boolean connectors “or” and “and” followed by (1) “rehabilitation,” (2) “treatment,” or (3)”postoperative.” From each database, titles, abstracts, and articles were reviewed in order to identify any articles that included information regarding postoperative rehabilitation or outcomes following surgical decompression of CECS of the anterior compartment of the lower leg. Additional citations were identified by assessing the references contained within each article. Finally, an attempt was made to perform the same search while using the other terms used to describe the condition including: anterior tibial pain, anterior compartment syndrome, recurrent compartment syndrome, idiopathic compartment syndrome, non-traumatic compartment syndrome of the lower extremity, fasciitis, pain in limb, swelling in limb, transient paralysis of limb, and disorder of soft tissue.
RESULTS
The electronic searches produced the following number of articles on each of the following databases: PubMed (99), SPORTDiscus (67), CINAHL (43), PEDRO (1), and Google Scholar (306). Articles that did not fit the objective of the clinical commentary were omitted from the review. These included articles regarding acute compartment syndrome as well as chronic exertional compartment syndromes occurring in the foot, other lower leg compartments, thigh, forearm, hand, and lumbar spine.
No specific criterion-based guidelines following surgical compartment release for lower extremity CECS (in any compartment) were found. Some time-based guidelines for general return to sport/play after CECS were found however there was some inconsistency regarding both the timelines of treatment and type of activity/sport being described. Furthermore, none of the guidelines specifically referenced or referred to time required for tissue healing, demands of eccentric muscle loading, effects of scar tissue formation, and consideration for all tissues (particularly nerve) contained within a compartment.
The U.S. Department of Health & Human Services' Agency for Healthcare Research and Quality has a searchable database of clinical practice guidelines called the National Guidelines Clearinghouse. The guidelines represented there are linked to a particular term derived from the U.S. National Library of Medicine's classification for diseases/conditions and treatments/interventions. Searching the National Guidelines Clearinghouse also yielded no guidelines for this diagnosis.
ANATOMY
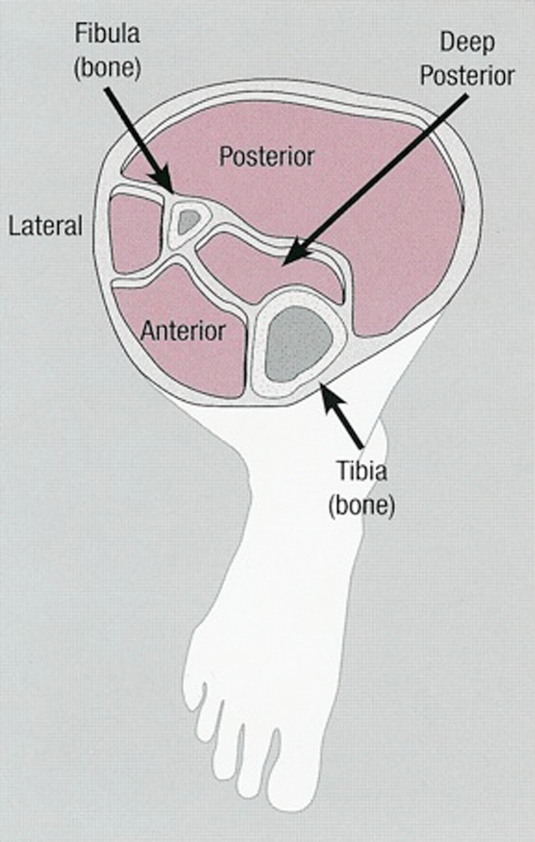
The lower leg is comprised of four universally described compartments (anterior, lateral, superficial posterior, and deep posterior). Figure 1 shows a cross section of the compartments of the lower leg. Each compartment is surrounded by osseofascial structures that define the various compartments in the extremities. The osseofascial compartments have relatively fixed volumes. The anterior compartment contains the tibialis anterior, extensor digitorum longus, extensor hallucis longus and peroneus tertius muscles in addition to the anterior tibial artery and vein and deep peroneal nerve. The lateral compartment contains the peroneus longus and brevis muscles, a branch of the anterior tibial artery and vein as well as the superficial peroneal nerve. The superficial posterior compartment contains the gastrocnemius, soleus, and plantaris muscles as well as a branch of the tibial artery and vein and the sural nerve whereas the deep posterior compartment contains the tibialis posterior, flexor digitorum longus, flexor hallucis longus, and popliteus muscles in addition to the posterior tibial artery and vein and tibial nerve.6
Figure 1.
Cross section of compartments in the lower extremity. Property of UW Health Sports Medicine Clinic, used with permission.
ETIOLOGY AND PATHOPHYSIOLOGY
The exact physiological cause of CECS remains unclear but it is thought to be multi-factorial. Contributors to CECS may include: muscle hypertrophy, fascial thickness or stiffness, stimulation of fascial sensory stretch receptors, decreased venous return, microtraumatic muscular injuries, and clinical myopathies.7 Intrinsic factors are likely contributory and may include leg length discrepancy and varus or valgus malalignment. Extrinsic factors are also likely contributory and may include: decreased strength, endurance or flexibility; incorrect motor control patterns; and inappropriate training volume, intensity, or frequency.8
Turnipseed, Hurschler, and Vanderby (1995)9 found muscle fascia surrounding the anterior compartment in those with CECS to be stiffer and thicker than those unaffected by the condition. Approximately 10-60% of athletes with CECS symptoms have small fascial defects in the lower leg.10 Birtles, Rayson, Jones, Padhiar, et al11 compared high force eccentric contractions of the anterior tibialis muscles in patients with CECS to healthy controls. The CECS patients had increased mean pain with dorsiflexion and palpation 24 and 48 hours following exercise, suggesting that some patients with CECS may be more susceptible to pain with eccentric contractions. Eccentric activity may be the cause of damage and inflammation of connective tissue given that myofibril change and edema of fast-twitch fibers occurs following eccentric activity.1 Brennan and Kane documented that in post-pubertal athletes, exercise that biases eccentric contraction (downhill running for example) may decrease fascial compliance over time and contribute to CECS.10
Brennan and Kane also deomostrated that muscle volume increases by 20% during cardiovascular exercise, and lower extremity intramuscular pressure can exceed 500 mm Hg during contractions.10 Peripheral muscles perfuse oxygen when muscle is relaxed and the arterial/venous gradient subsequently increases. The gradient must be at least 30 mm Hg to overcome intramuscular resting pressure. If compartment pressure exceeds 30 mm Hg the perfusion gradient does not exist.10
Strenuous exercise can cause microtrauma to muscle tissue. Those affected by CECS do have greater deoxygenation of muscle during exercise and delayed reoxygenation of muscle after exercise.5 Styf describes how muscle and capillary bed inflammation may increase fluid flow from the capillaries to the interstitial space thereby increasing the volume and fluid pressure of the space.1 When increased compartment pressure persists, pain, paresthesia, and muscle weakness can develop. The degree of elevated pressure however does not correlate with the degree of symptoms or predict outcomes following surgical fasciotomy.10 Hutchinson and Ireland concluded that elevated compartment pressure increases venous pressure, which in turn decreases the arterial-venous gradient thereby reducing blood flow within the compartment.12 Reduced blood flow can lead to ischemia however this is not universally accepted as the cause for CESC.10,12,13,14,15,16,17 Other theories include increased facial thickness and stiffness, increased pressure as a source for pain receptor stimulation in the fascia and periosteum, and increased lactate in the bloodstream secondary to decreased perfusion within a compartment.10,13,14
PREVALENCE
The incidence of CECS in those with chronic exercise-induced anterior lower leg pain ranges from 14-27%.18 Seventy percent of patients with chronic exertional compartment syndrome in the anterior compartment are runners.1,10,13 The condition is nearly evenly split between males and females; early reports showed predominance among men but the proportion of females with CECS is rising.13 Women may respond less well to operative measures.13 Incidence of CECS is nearly equal in recreational and elite athletes12 and the median age of those who present with CECS is 20 years.13 The anterior and lateral compartments of the lower leg are most commonly affected and have better outcomes with surgical treatment, in comparison to the posterior compartment.10
DIAGNOSIS
Careful evaluation is vital when assessing an athlete with complaints of “shin pain”. Differential diagnosis may include muscle/tendon involvement (CECS, muscle strain, fascial hernia, tendinopathy), bone involvement (medial tibial stress syndrome, stress fracture), vascular involvement (popliteal artery entrapment syndrome, intermittent claudication, venous insufficiency), neurologic involvement (peripheral or central nerve entrapment/impingement, referred symptoms from proximal joint), tumor, or infection.
CECS has been reported in the forearm, thigh, hand and foot, however, 95% of cases occur in the lower leg.13 Bilateral symptoms in the legs occur in 85-95% of those affected.10
Subjectively, those affected with CECS often complain of dull, aching, or cramping pain localized to the compartment affected in the lower extremity at the same duration of time (minutes) following the initiation of each episode of exercise.14 On clinical examination, muscle hypertrophy and pallor may be noted.12,13 Compartment muscles may be firm to palpation,19 while distal pulses are typically normal.1,12 Passive stretching can increase pain when pressures are elevated in the affected compartment.10,12,14 Nerve dysfunction may impair sensation to light touch as well as strength particularly following an exercise test; paresthesia may also be present.1,12 The gold standard test used to confirm the diagnosis of CECS is intracompartmental pressure testing, performed first at rest and then following exercise.5,10,13,14 Normal resting intracompartmental pressure is between 0-8 mm Hg.12 Resting compartment pressure greater than 15 mm Hg, post-exercise compartment pressure (measured one minute after exercise cessation) greater than 30 mm Hg, or post-exercise compartment pressure (measured five minutes after exercise cessation) of greater than 20 mm Hg are diagnostic for CECS in all compartments.5,10,12,13,14 Furthermore, compartment pressures that fail to return to baseline within 15 minutes post-exercise are considered borderline for CECS diagnosis.10
TREATMENT
Many physical therapists are first contact providers who provide direct access (DA) intervention. They, along with other primary care providers (PCP), may benefit from direction regarding optimal care for CECS.
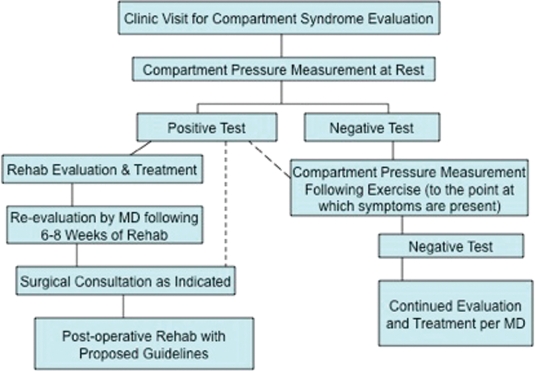
CECS often is initially treated conservatively with rehabilitation, ranging in duration from a few weeks to several months. Surgical decompression of the fascial compartment may be the treatment of choice for active individuals if conservative intervention fails. Although conservative treatment appears to have minimal long-term value in published reports in the literature, there are no randomized controlled studies to investigate the effectiveness of conservative management.8 The proposed treatment algorithm, found in Figure 2, outlines management of CECS. If CECS is suspected by the PCP or physical therapist, a clinic visit for compartment syndrome evaluation by a Sports Medicine or Vascular Physician specialist may be warranted.
Figure 2.
Proposed treatment algorithm for treatment of Chronic Exertional Compartment Syndrome
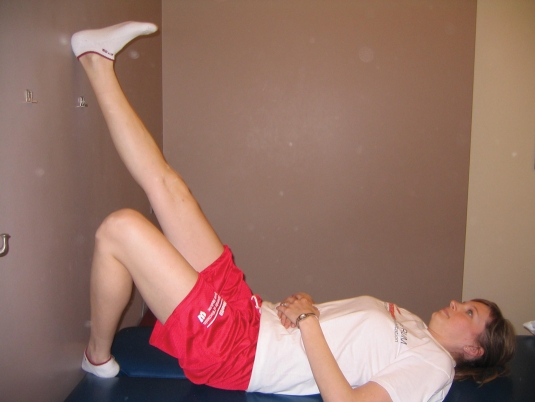
Figure 3.
Active muscle pumping exercise at ankle joint with lower extremity elevated on wall to assist with venous return and swelling
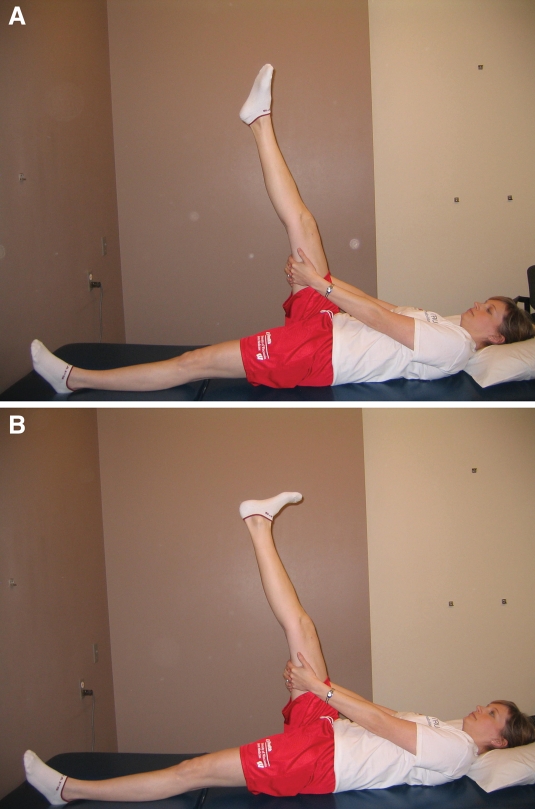
Figure 4.
A & B. Nerve mobilization exercise in supine. The lower extremity is placed in a straight-leg raise position, nerve gliding is performed by actively and alternately plantarflexing in inversion (A) and dorsiflexing (B) the ankle. Note: ankle plantarflexion with inversion places tension on the common peroneal nerve tract
Figure 5.
Gait drill involving forward marching
Figure 6.
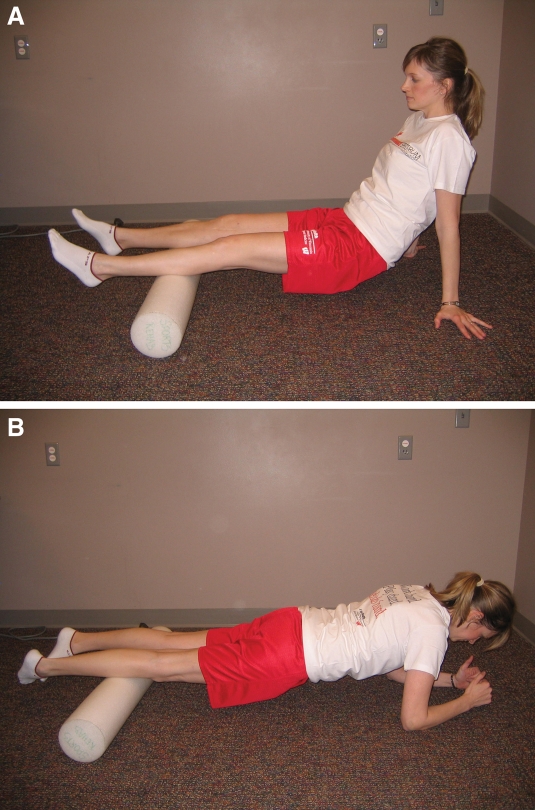
A & B. Lower extremity deep massage using a foam roller to improve flexibility and soft tissue mobility for posterior (A) and anterior (B) aspects of the lower leg
Figure 7.
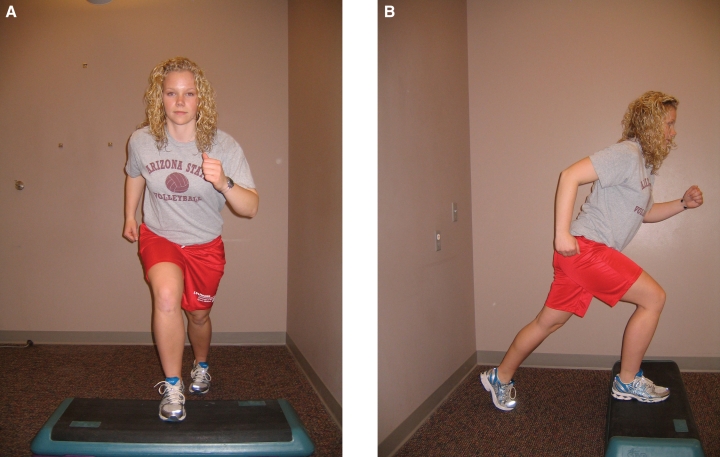
A & B. Step-back lower extremity closed chain functional strengthening exercise, frontal plane (A) and sagittal plane (B)
Figure 8.
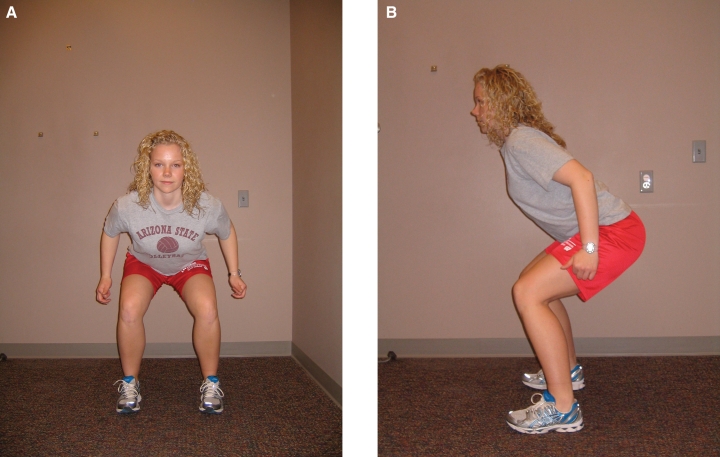
A & B. Plyometric exercise: jumping, frontal plane (A) and sagittal plane (B)
Surgical Management of CECS
Unlike acute compartment syndrome, the treatment of CECS is non-emergent. Furthermore, the surgical approach may be less extensive, involving only the involved compartments.12 The suggested resting pressure considered significant and that indicates the need for surgery for those with CECS is debated in the literature.12 Minimum elevated pressure for consideration of surgery is documented between 30 and 55 mmHg, but there is no clear consensus; one author has proposed that the interpretation should be associated with the individual's actual diastolic blood pressure.12
Currently surgical management is the treatment choice, after the failure of conservative care, in active patients with CECS; however, there is variety among the surgical techniques that are used. Various surgical decompression techniques have been described in the literature including: open fasciotomies with 1 or 2 incisions, minimally invasive subcutaneous fasciotomies through 1 or 2 incisions, fasciotomies with partial fasciectomies, and subcutaneous endoscopic fasciotomies with and without the use of balloon dissectors.3 An open fasciotomy involves 1-2 large incisions where fascial tissue is cut. A minimally invasive subcutaneous fasciotomy involves incision of fascia blindly via small skin incision(s). A fasciotomy with partial fasciectomy is when a portion of the fascia is removed. A subcutaneous endoscopic fasciotomy with the use of a balloon dissector creates an optical cavity to allow visualization of the fascia and space to perform the dissection with endoscopic equipment. There appears to be no consensus on a superior approach in terms of postoperative outcome. Since many athletes have bilateral CECS, bilateral fasciotomies may be considered. Bilateral simultaneous fasciotomies may be considered safe and effective with low complication rates and adequate ability to return to sport participation.20 Surgical treatment can be performed as an outpatient procedure under local anesthesia.12
Reports of improvement following anterior or lateral compartment release range between roughly 80-100%.5,10 Release of the deep posterior compartment has not been as successful with success being reported in only 50-65% of those who undergo the procedure.5,10 Complications following compartment release exist and include hemorrhage, hematoma, deep vein thrombosis, wound infection, skin breakdown, nerve entrapment or injury, swelling, vascular injury, residual weakness, and lymphocele.1,3,5,8,10 Incidence of complications ranges from 4.5-13%; recurrence of symptoms occurs in 7-17% of surgical cases.5
Results of surgical fasciotomy and rate of return to sport has not been consistent.12 A lower success rate has been documented for women following anterior compartment fasciotomy as well as following fasciotomy of the posterior compartment in patients of either sex.1 Follow-up regarding outcomes following compartment release is lacking in the literature. The lack of outcome assessment raises questions regarding the specifics of who does well long-term and who does not. Recurrence rates as high as 17% have been documented following various decompression techniques.3,5 However, 36% of patients had reoccurrence of compartment syndrome or development of a different lower leg compartment syndrome after partial fasciectomy of the anterior compartment, thereby causing a reduction in their ability to exercise.4 Recurrence may be due to incorrect diagnosis, inadequate release, failure to release a compartment thought to be asymptomatic, nerve compression by an unrecognized fascial hernia, and development of prolific scar tissue.3 Abnormal scarring of the fascia or overlying skin has been found to occur following surgical release.10 Approximately 10% of patients require a revision surgery.1 Of note, although a fasciotomy is often effective in eliminating the pathological increase in compartment pressure, it does not treat the elusive initial cause of the syndrome.1
Rehabilitation
The proposed treatment algorithm in Figure 2 suggests initially attempting conservative treatment for 6-8 weeks in all cases of CECS. Conservative management of CECS may include: reduction in or modification of activity, massage, other specific soft tissue mobilization and manipulation techniques, including myofascial stretching, taping, orthotic inserts, footwear modification, stretching, and nonsteroidal anti-inflammatory medications.10 In theory soft tissue mobilization, for example myofascial stretching, would facilitate increased fascial compliance which would address the proposed pathophysiology involving increased facial thickness and stiffness and increased pressure as a source for pain receptor stimulation in the fascia. There is inconsistency in the literature regarding efficacy of all types of conservative treatment. Many authors argue that conservative treatment will not bring definitive relief of symptoms, however most research on CECS has been conducted by surgeons.8,12 In a pilot study by Blackman, et al involving a 5-week course of massage for those with CECS of the anterior compartment, there was no significant difference in post-exercise compartment pressure following treatment. However, there was a significant increase in dorsiflexion work able to be performed prior to the onset of pain, measured using a Cybex isokinetic dynamometer.21
A carefully planned and implemented rehabilitation program is important for a patient to achieve optimal functional outcomes post-operatively.22 The proposed guidelines for rehabilitation following compartment release include scientific rationale based on tissue healing, muscle loading, scar tissue formation, and consideration of all tissues contained in the involved compartment. The recommendations given are based on the initial use of “PRICE” (protection, rest, ice, compression, and elevation), with progression to re-establishing range of motion (ROM) and soft tissue mobility, incorporating stretching, neurodynamic mobilizations, strengthening, and finally, incorporating biomechanical analysis of the athlete during sport specific activity.
Protection, Rest, Ice, Compression, and Elevation:
This approach is used during the proliferation phase of tissue repair which typically lasts approximately 3-20 days. During this phase, the tensile strength of the tissue can be as low as 15% of normal tissue, making protection of the tissue essential.23 Fasciotomy may cause significant edema and hematoma formation,14 therefore, rest, ice, compression, and elevation are crucial for swelling control.
Range of Motion and Soft Tissue Mobility:
During the proliferation phase of tissue repair, the injured area has the greatest amount of collagen; edema and excessive stress on the healing area may result in additional inflammation and deposition of collagen. The excessive collagen can result in excessive scarring which may limit functional outcome.23 Wound contraction begins at approximately 5 days after injury and peaks at about 2 weeks.23 The maturation phase occurs from day 9 onward and is the longest phase in the healing process. Several factors determine the rate of maturation as well as the final characteristics of the scar tissue, including fiber orientation and balance of collagen synthesis and lysis.23 Collagen in scar tissue is less organized than that of uninjured tissue. Internal and external stresses placed on tissue during the maturation phase can determine the final tissue structure.23 Muscle tension, joint movement, soft tissue loading, fascial gliding, temperature changes, and mobilization are forces proposed to affect collagen structure.23 Post-operative active and passive range of motion of the ankle and knee will assist in prevention of formation of hematoma and scar near the fasciotomy incision.13 Early motion is beneficial to prevent scarring and contracture that may occur with open decompression in addition to the existing tissue damage that occurs from CECS.12 Surgery may be ineffective if fascia heals in original position; recurrences have been attributed to restrictive scarring at the fasciotomy site.13,15
Sensitization (hyper sensitivity) is caused by strengthening of responses to perceived injurious stimuli. It can be short-term or long-term; mechanisms for long-term memory of sensitization involve the same cells as short-term memory but reflect structural changes in the cells.24 Animals with long-term sensitization had twice as many synaptic terminals, increased dendrites in postsynaptic cells, and a 40-65% increase in the number of active zones at synaptic terminals than untrained animals.24 The use of a desensitization approach may decrease anxiety and fear of touch over areas of scar tissue. A treatment progression involving brushing material over the skin could initially involve soft, light, fabric with progression toward stiffer, thicker fabric. Brushing is slow and purposeful, it should not damage the skin.
Stretching:
Passive stretching increases pain when pressures are elevated in the respective compartment10,12,14 which suggests that muscle stretching may also result in stretching of the compartment fascia. The intensity, frequency, timing, and duration of stretching are critical to obtain anti-fibrotic effects.25 Scar tissue responds to low load, long duration stretch for permanent change.23 There are several types of cutaneous receptors in the skin including mechanoreceptors, thermoreceptors, and nocioceptors. Mechanoreceptors include pacinian corpuscles, Merkel's discs, Meissner's corpuscles, Ruffini endings, and lanceolate endings around hair follicles. Ruffini endings detect tension deep in the skin and Merkel's discs detect sustained touch and pressure. Muscle spindles, which are found in the belly of skeletal muscles, send signals to the nervous system via afferent fibers and are controlled by the central nervous system via efferent fibers. The group Ia afferents have a low threshold to stretch and follow changes in length easily; they code the rate of stretch (dynamic response) and the length of muscle at the end of a stretch (static response).24 The American College of Sports Medicine (ACSM) recommended flexibility training parameters include a daily stretching dose of 180 seconds, with a frequency of 3 days/week. Active or passive technique and variation in single stretch duration (15, 30, or 45 seconds) resulted in no significant difference in flexibility after 12 weeks of stretching.26
Neurodynamic Mobilizations:
Butler27 proposed that stretch pain or paresthesia symptoms are a result of tension being placed on some component of the nervous system. Restriction of movement can be from inflammation and scarring between the nerve and tissue through which it runs.27 Treatment involving neurodynamic mobilizations including sliding and tensioning techniques are important to enhance nerve gliding and restore neural tissue mobility, and have been shown to be beneficial for ulnar nerve entrapment.19 Given the amount of inflammation and scarring which may be present within a compartment, this type of neurodynamic intervention may be beneficial in conservative treatment as well as following surgical compartment release for CECS. Intervention should be focused on the involved nerves within a specific lower extremity compartment. The focus of this clinical commentary is on the anterior compartment and therefore neurodynamic interventions are aimed at the deep peroneal nerve.
Strengthening:
Preoperatively, patients with CECS of the anterior compartment are weaker than control subjects during dorsiflexion isometric exercise, and exhibit lower isokinetic muscle strength in ankle dorsiflexors compared to normal individuals.8,16 Postoperatively, a decrease in muscle contractile force may occur due to altered biomechanics of the muscle following fascial incision.8 Decreased dorsiflexion strength pre-operatively may lead to recurrence of CECS of the anterior compartment post-operatively unless appropriate strengthening is implemented.8 Because intramuscular pressure at rest is elevated following eccentric muscular activity as compared to concentric muscular activity,1 eccentric muscle strengthening should initially be avoided postoperatively. The guidance of a skilled professional is recommended for methodical progression of strengthening exercises.
Biomechanical Analysis of Specific Activity:
Development of anterior compartment CECS has been described after implementation of a new skating method of cross-country skiing, when compared to the classic skiing technique. The skating technique involves kicking diagonally versus the classic skiing method in which movement remains primarily in the sagittal plane. This finding highlights the importance of lower extremity biomechanical assessment prior to return to any specific sport or activity, as the precise technique of a sport may involve biomechanical movement in multiple planes of motion.1 A forceful heel strike results in high pressure in the tibialis anterior muscle associated with eccentric muscle contraction during running.1 This further supports the need for biomechanical assessment of any activity that the athlete participates in, in order to avoid reoccurrence of symptoms. Given that the cause of CECS is thought to be multifactorial, instrinsic factors including leg length discrepancy, varus or valgus malalignment, foot biomechanics, and motor control patterns should be addressed postoperatively.8 Extrinsic factors including training volume, intensity, or frequency should also be considered in addition to activity technique, training surface, and footwear.8
It is vital to assess patient outcomes before and after a formal course of rehabilitation following surgical release of the anterior compartment, given that this is lacking in current literature. Following the proposed guidelines will assist in data collection to better assess outcomes following this type of procedure. The Foot and Ankle Ability Measure (FAAM) is the recommended outcome assessment instrument as it has proven to be a reliable, responsive, and valid measure of physical function for individuals with a broad range of musculoskeletal disorders of the lower leg, foot, and ankle.28 The FAAM consists of a 21-item activities of daily living subscale, as well as an 8-item sports subscale. The minimal clinically important differences are 8 and 9 points for the ADL and sports subscales, respectively.28
Proposed Scientifically-Based Rehabilitation Guidelines Following Open Fasciotomy with Anterior Compartment Release1,2,5,10,12,13,14,15,19,17,22,23,29,30,31
PHASE I: Protection and Mobility (2-3 weeks Post-operatively).
Table 1:
PHASE I: Protection and Mobility (Surgery to 2-3 weeks).
| Category | Information |
|---|---|
| Phase I Appointments | Physician appointment: 5-10 days post-operatively for suture removal, no drain used Rehabilitation appointments begin 5-7 days after surgery, continue 1 time every 5-10 days |
| Phase I Rehabilitation Goals | Administer FAAM (ADL and sport subscales) |
| Protection of the post-surgical compartment | |
| Minimize postoperative swelling; lower extremity circumference within 2 cm of uninvolved side | |
| Instruction in safe positioning and limb self-management | |
| Restore normal knee and ankle range of motion (ankle ROM with knee flexed: DF=20, PF=50, inversion=30-35, eversion=15-20. Knee ROM: ext=full, flex=130) | |
| Able to lift leg involved leg in all directions in standing without pain or compensation. | |
| Restore ability to control leg in open and closed kinetic chain during gait. | |
| Non-antalgic gait on level surface with full weight bearing and no assistive device at >2 mph with equal step length bilaterally. | |
| Phase I Precautions | Use axillary crutches for gait with progressive weight bearing as tolerated, keeping pain at or below 2/10 on visual analog scale (1-2 weeks). |
| Avoid any activity which causes increased swelling (for example: extended sitting or sitting with lower extremity in dependent position, tight clothing proximal to surgical release, and hot pack or bath. | |
| Avoid any friction on new scar formation (for example: crossing legs, tight clothing, pushing object with legs or using weight machine that presses into skin over incision site). | |
| Avoid any impact activity including running, jumping, or hopping (6-8 weeks). | |
| Phase I Suggested Therapeutic Exercise | Active ankle range of motion immediately to maintain ext ensibility of soft tissues as they heal to prevent postoperative contractures (include ankle PF, DF, inversion and eversion, knee flex and ext), begin with 10 repetitions in each direction, 1-2 times/day and progress number of repetitions as tolerated. May also consider initiation of open kinetic chain strengthening; begin with theraband level 1-2, 1-2 sets of 10 in each direction, 1 time/day, at 3 weeks post-op. Progress as tolerated. |
| Quadriceps sets for isometric strengthening. Begin with 5-10 second holds, 10 repetitions, 1-2 times/day. Progress hold time or repetitions followed by progression into short arc or long arc quad or straight leg raise. | |
| Leg lifts for hip strength: hip flexion, abduction and extension. Begin in supine, side lying and prone, respectively, and progress into standing. 1 set of 10 in each direction, 1-2 times/day. Progress as tolerated. | |
| Elevation of the operative extremity (above level of heart) begin with 30-40 minutes every 1-2 hours and modify as needed, ice 15-20 minutes with barrier between skin and icepack, and compression garment (Ace wrap or TED stocking), as needed, for swelling control | |
| Active muscle pumping exercises at distal/ankle joint while lower extremity is elevated on wall to assist with venous return and swelling. 1-2 minutes of active ankle pumping; 3-6 times/day or as needed for swelling control. (Figure 3) | |
| Gentle distal-to-proximal massage to assist with venous return and swelling. Can perform with leg elevated on wall. Avoid contact with incisions. 3-5 minutes, 1-2 times/day or as needed to assist with swelling control. | |
| Phase I Cardiovascular Exercise | Upper body circuit training or upper body ergometer, as able. Begin with 5-10 minutes, 1-2 times/day, and progress as able. |
| Progression Criteria to Phase II | Patient may progress to Phase II if they have met the above stated goals. |
PHASE II: Light Strengthening (Begin after meeting Phase I criteria, approximately 3-4 weeks following surgery).
Table 2:
PHASE II: Light Strengthening (begin after meeting Phase I criteria, approximately 3-4 weeks following surgery).
| Category | Information |
|---|---|
| Phase II Appointments | Rehabilitation appointments are 1 time per week on average |
| Phase II Rehabilitation Goals | Lower extremity circumference within 1 cm of uninvolved side |
| Incision is well healed | |
| Minimize muscle atrophy and flexibility deficits in anterior/involved compartment | |
| Single leg stance control with eyes open on unstable surface for 30-60 seconds | |
| Full flexibility of gastrocnemius (ankle DF with knee extended): 15-20 degrees | |
| Maintain motion and strength of uninvolved muscle groups, as well as cardiovascular endurance, as able | |
| Perform active or gentle resisted exercises of the hip of the operated lower extremity and resistance exercises of the upper extremities | |
| Proper lower extremity control and alignment with no pain during functional double leg squats | |
| Non-antalgic gait on level surface with full weight bearing and no assistive device, >3mph with equal step length bilaterally | |
| 8 point (or greater) improvement on FAAM (ADL portion) | |
| Phase II Precautions | Avoid over-stressing new scar formation by avoiding any friction over tissue (as per Phase I) |
| Avoid post-activity swelling by limiting prolonged weight bearing activity as appropriate. If swelling occurs, manage with rest, ice, elevation and compression (as per Phase I). | |
| Avoid eccentric loading with any impact activity. | |
| Phase II Suggested Therapeutic Exercise | Scar massage/mobility and desensitization (once incision is healed). Begin with 3-5 minutes, 1-2x/day and modify as needed. |
| Gentle stretching and nerve mobilizations to tissue in involved compartment. Stretch holds for 30-60 seconds, 2-3x/day. Nerve mobilizations begin with 5-8 reps, 3-5x/day; progress number of reps as tolerated. For nerve mobilizations, begin with supine positioning with the lower extremity in a straight-leg raise position; ankle plantarflexion with inversion places tension on the common peroneal tract. (Figure 4a and 4b) Progress by adding hip adduction or internal rotation while doing the straight-leg raise to increase tension on the nervous system. Passive neck flexion will also pull the spinal cord superiorly and places the entire nervous system on a stretch. | |
| Progress open kinetic chain ankle strengthening. 2-3 sets of 10; progress resistance, sets and reps as tolerated. | |
| Balance and proprioception exercises: initiate a progression of bilateral to unilateral balance activities first on a level, firm surface, then on a soft/unstable surface, such as dense foam or Bosu ball, and then on a balance board. Begin with eyes open; progress with head turns and eyes closed as able. Goal of 30-60 second holds; 2-3 repetitions, 1-2 times/day. | |
| Gait drills: begin with sagittal plane and progress to frontal and transverse planes. Examples include forward and backward marching (sagittal plane, Figure 5), sidestepping or side marching (frontal plane), and carioca/grapevine walking (transverse plane). Begin with 10-20 steps, 2-3 repetitions, 1-2 times/day. Progress as tolerated. | |
| Phase II Cardiovascular Exercise | Upper body circuit training, upper body ergometer (as per Phase I) |
| May begin stationary biking if wound is healed; begin with 5-10 minutes at a low resistance (for example, level 1-2 on a bike with 10 levels), and low cadence (for example 60-80 revolutions per minute). Progress time, cadence, and resistance as able. | |
| Begin treadmill or track walking if wound is healed; begin with 5-10 minutes at 2-3 mph and progress time and speed as able. | |
| May swim or water walk if wound is FULLY healed (do not risk infection); begin with 10-15 minutes and progress time and speed as able. | |
| Progression Criteria to Phase III | Patient may progress to Phase III if they have met the above stated goals |
PHASE III: Progression of Strengthening (Begin after meeting Phase II criteria, approximately 4-6 weeks following surgery).
Table 3:
PHASE III: Progression of Strengthening (begin after meeting Phase II criteria, approximately 4-6 weeks following surgery).
| Category | Information |
|---|---|
| Phase III Appointments | Rehabilitation appointments are 1x every 7-10 days on average |
| Phase III Rehabilitation Goals | Prevent post-operative recurrence of symptoms with all activity |
| Tolerate 15-30 minutes of continuous aerobic activity without the onset of symptoms/pain | |
| Reinforce self-monitoring and review signs of recurrence and complications. | |
| Full 5/5 pain free ankle strength with manual muscle testing of muscles in involved compartment | |
| Proper lower extremity control and alignment and no pain with single leg functional movements including squats and lunges | |
| No residual swelling 12-24 hours following all physical activity (including impact exercises) | |
| No pain 1-2 hours following physical activity (including impact exercises) | |
| Phase III Precautions | Avoid friction over scar tissue (as per phase I-II) |
| Avoid post-activity swelling (as per phase I-II) | |
| No strenuous activity until wound is fully healed | |
| Suggest no running until 6-8 weeks postoperatively (patient should be advised by physical therapist and MD prior to initiation of any running) | |
| Avoid pain with any exertional activity | |
| Phase III Suggested Therapeutic Exercise | Lower extremity stretching and nerve mobilizations as appropriate (per Phase II) |
| Lower extremity myofascial stretching/massage and “The Stick” or a foam roller (rolling lower extremities over the roller in long sitting or prone with legs internally rotated, for posterior (Figure 6a) or anterior (Figure 6b) legs) for rolling deep massage to improve flexibility and soft tissue mobility. Begin with 1-3 minutes 1x/day and progress as tolerated. | |
| Progression of lower extremity closed chain functional strengthening including lunges, step-backs (off of a standard step; Figure 7a and 7b), and single leg squats. Also consider double leg heel rise progressing to single leg heel rise with and without gait drills (such as marching), toe and heel walking. Begin with 2-3 sets of 10 reps, 1 time/day, and progress as tolerated. | |
| Initiate plyometric exercises (with focus on lower extremity control and alignment at hip, knee, and ankle) at 6 weeks. Begin with 2 feet to 2 feet (jumping; Figure 8a and 8b), progressing from 1 foot to other (leaping) and then 1 foot to same foot (hopping). Focus on proper landing/deceleration mechanics. Begin with 1-2 sets of 10 repetitions. Progress number of repetitions, sets, as well as height or distance as tolerated. | |
| Phase III Cardiovascular Exercise | Initiate or progress swimming or water walking if wound is fully healed (as per Phase II) |
| Progress walking time and speed (as per Phase II) | |
| May begin elliptical trainer for 10-15 minutes at low resistance (for example, level 1-2 on an elliptical with 10 levels). Progress time and resistance as able. | |
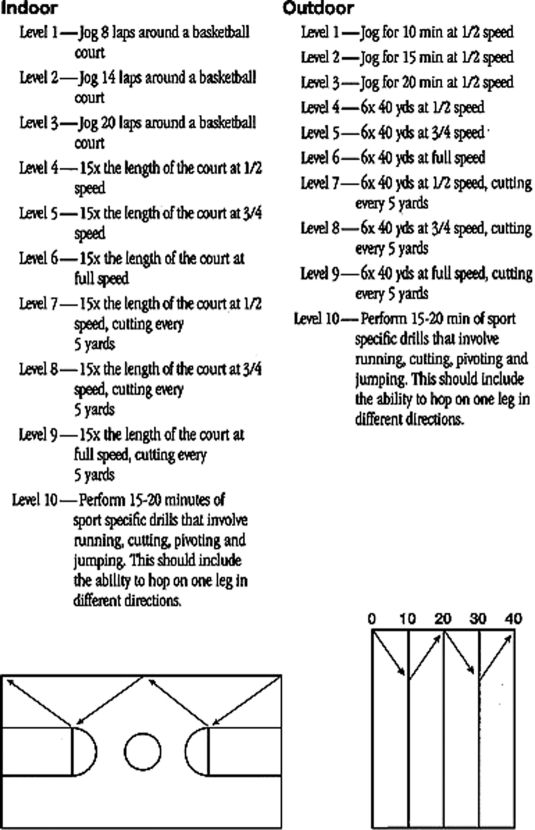
| Light jogging can be initiated at 6-8 weeks; initially begin on level surface while avoiding hills and speed work. For runners, consider 5 minute interval training involving walking (for example 1-2 minutes of jogging followed by 3-4 minutes of walking). Progress jog interval times, incline, and speed as appropriate for return to sport/activity goals. For those returning to multi-planar sport, consider progression outlined in Appendix 1. | |
| Progression Criteria to Phase IV | Patient may progress to Phase IV if they have met the above stated goals. |
PHASE IV: Impact/Sport Training (Begin after meeting Phase III criteria, approximately 8-12 weeks following surgery).
Table 4:
PHASE IV: Impact/Sport Training (begin after meeting Phase III criteria, approximately 8-12 weeks following surgery).
| Category | Information |
|---|---|
| Phase IV Appointments | Rehabilitation appointments are 1 time every 2-3 weeks |
| Phase IV Rehabilitation Goals | Administer FAAM (ADL and sport subscales) prior to discontinuation of therapy. |
| 9 point (or greater) improvement on FAAM (sport subscale portion) | |
| Proper dynamic neuromuscular control and alignment with eccentric and concentric multi-plane activities (including impact) for return to work/sports, without pain, instability or swelling | |
| Within 90% of pain free strength (compared to uninvolved side) on leg press (heel raise/PF) or isokinetic biodex testing (PF and DF) | |
| Phase IV Precautions | Avoid pain with any exertional activity |
| Avoid post-activity swelling (as per phase 1-III) | |
| Phase IV Suggested Therapeutic Exercise | Biomechanical assessment of specific sport activity with video analysis as needed (running, biking, etc) |
| Instruct in proper return to activity progression (incremental running, biking, etc) | |
| Progressive strengthening exercises using higher stability, and neuromuscular control with increased loads and speeds and combined movement patterns; begin with low velocity, single plane activities and progressing to higher velocity, multi-plane activities. Begin with forward and backward, progress to side-to-side, diagonals and transverse plane movements | |
| Integrate movements and positions into exercises that simulate functional activities. Sport-specific training beginning with low-intensity simulated movements. | |
| Phase IV Cardiovascular Exercise | Replicate sport or work specific energy demands |
| Return To Sport/Work Criteria | Patient may return to sport/work if they have met the above stated goals and have physician and rehabilitation specialist approval. |
| Precautions to reduce the risk of re-injury when returning to sports or high-demand activities as appropriate. If collision/contact sport, may consider protective padding over area of scar tissue. |
SUMMARY
Adequate long-term follow-up of outcomes following compartment release for CECS is lacking in the current literature. No specific rehabilitation guidelines following surgical compartment release for lower extremity CECS were found in the literature search utilized by the author of this clinical commentary. Limited time-based rehabilitation guidelines were found, although there was inconsistency in the timelines for treatment, treatment progression, and return to activity/sport. None of the guidelines specifically considered time requirements for tissue healing, demands of eccentric muscle loading, effects of scar tissue formation, and consideration of all tissues (particularly nerve) contained in a compartment. The proposed scientifically-based guidelines for rehab following compartment release presented in this clinical commentary include consideration of tissue healing, muscle loading, scar tissue formation, and consideration of all tissues contained in the involved compartment. Following these proposed guidelines, may reduce risk of reoccurrence and optimize outcomes following this type of procedure. In addition, it may allow for additional research to be performed that can effectively assess outcomes following this type of procedure. Furthermore, use of these guidelines may allow better understanding regarding why some patients do well following surgical decompression and others fail.
Acknowledgements:
Suzanne Brown, MPH, PT, PhD, David Bernhardt, MD, Tamara Scerpella, MD
Appendix 1:
Sample running progression for return to multi-planar sport. Property of UW Health Sports Medicine Clinic, used with permission
REFERENCES
- 1. Styf J. Definitions and terminology. Etiology and pathogenesis of chronic compartment syndrome. In: Compartment syndromes: diagnosis, treatment, and complications. 2004. Boca Raton, FL: CRC Press LLC [Google Scholar]
- 2. Wilder RP. Exertional compartment syndrome. Clin Sports Med. 2010;29:429–435 [DOI] [PubMed] [Google Scholar]
- 3. Wittstein J, Moorman CT, III, Levin LS. Endoscopic compartment release for chronic exertional compartment syndrome. Am Jour Sports Med. 2010;20:1–6 [DOI] [PubMed] [Google Scholar]
- 4. Slimmon D, Bennell K, Brukner P, et al. Long-term outcome of fasciotomy with partial fasciectomy for chronic exertional compartment syndrome of the lower leg. Am J Sports Med. 2002;30:581–588 [DOI] [PubMed] [Google Scholar]
- 5. Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Ortho Surg. 2003;11:268–276 [DOI] [PubMed] [Google Scholar]
- 6. Gray's Anatomy Website Anterior compartment of leg. 30 June 2010. Available at http:www.en.wikipedia.org/wiki/Anterior_compartment_of_leg. Accessed Aug 13, 2010
- 7. Lecocq J, Isner-Horobeti ME, Dupeyron A, et al. Exertional compartment syndrome. Ann Readapt Med Phys. 2004;47:334–345 [DOI] [PubMed] [Google Scholar]
- 8. Anuar K, Gurumoorthy P. Systematic review of the management of chronic compartment syndrome in the lower leg. Physiotherapy Singapore. 2006;9:2–15 [Google Scholar]
- 9. Turnipseed WD, Hurschler C, Vanderby R Jr. The effects of elevated compartment pressure on tibial arteriovenous flow and relationship of mechanical and biochemical characteristics of fascia to genesis of chronic anterior compartment syndrome. J Vasc Surg. 1995;21:810–816 [DOI] [PubMed] [Google Scholar]
- 10. Brennan FH, Jr, Kane SF. Diagnosis, treatment options, and rehabilitation of chronic lower leg exertional compartment syndrome. Curr Sport Med Report. 2003;2:247–250 [DOI] [PubMed] [Google Scholar]
- 11. Birtles DB, Rayson MP, Fones DA, et al. Effect of eccentric exercise on patients with chronic exertional compartment syndrome. Eur J Appl Physiol. 2003;88:565–571 [DOI] [PubMed] [Google Scholar]
- 12. Hutchinson MR, Lloyd Ireland M. Common compartment syndromes in athletes: treatment and rehabilitation. Sports Med. 1994;17:200–208 [DOI] [PubMed] [Google Scholar]
- 13. Shah SN, Miller BS, Kuhn JE. Chronic exertional compartment syndrome. Am Jour Ortho. 2004;335–341 [PubMed] [Google Scholar]
- 14. Gill CS, Halstead ME, Matava MJ. Chronic exertional compartment syndrome of the leg in athletes: evaluation and management. Physician and Sportsmed. 2010;38:1–7 [DOI] [PubMed] [Google Scholar]
- 15. Verleisdonk EJMM. The exertional compartment syndrome: a review of the literature. Ortopedia Traumatologia Rehabilitacja. 2002;5:626–631 [PubMed] [Google Scholar]
- 16. Birtles DB, Minden D, Wickes SJ, et al. Chronic exertional compartment syndrome: muscle changes with isometric exercise. Med Sci Sports Exer. 2002;1900–1906 [DOI] [PubMed] [Google Scholar]
- 17. Edmundsson D, Toolanen G, Sojka P. Chronic compartment syndrome also affects non-athletic subjects. Acta Orthopaedica. 2007;78:136–142 [DOI] [PubMed] [Google Scholar]
- 18. Bong MR, Polatsch DB, et al. Chronic exertional compartment syndrome: diagnosis and management. Bulletin of NYU Hosp for Jt Diseases. Winter-Spring; 2005 [PubMed] [Google Scholar]
- 19. Oskay D, Meric A, Kirdi N, et al. Neurodynamic mobilization in the conservative treatment of cubital tunnel syndrome: long-term follow-up of 7 cases. J Manipulative Physio Ther. 2010;33:156–163 [DOI] [PubMed] [Google Scholar]
- 20. Raikin SM, Rapuri VR, Vitanzo P. Bilateral simultaneous fasciotomy for chronic exertional compartment syndrome. Foot Ank Intern. 2005;12:20–29 [DOI] [PubMed] [Google Scholar]
- 21. Blackman PG, Reid Simmons L, Crossley KM. Treatment of chronic exertional compartment syndrome with massage: a pilot study. Clin Jour Sport Med. 1998;8:14–17 [DOI] [PubMed] [Google Scholar]
- 22. Kisner C, Colby LA. Surgical interventions and postoperative management, the ankle and foot. In: Therapeutic Exercise: Foundations and Techniques. 5th Edition 2007. Philadelphia, PA: F. A. Davis Company [Google Scholar]
- 23. Cameron MH. Inflammation and tissue repair. In: Physical Agents in Rehabilitation: From Research to Practice. 2nd Edition 2003. St. Louis, MO: Elsevier Science [Google Scholar]
- 24. Shumway-Cook A, Woollacott MH. Physiology of motor control, physiological basis of motor learning and recovery of function. In: Motor Control: Theory and Practical Applications. 2nd Edition 2001. Baltimore, MD: Lippincott Williams & Wilkins [Google Scholar]
- 25. Bouffard NA, Cutroneo KR, Badger GJ, et al. Tissue stretch decreases soluble TGF-B1 and type-1 procollagen in mouse subcutaneous connective tissue: evidence from ex vivo and in vivo models. Jour Cell Phys. 2007;10:389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sainz de Baranda P, Ayala F. Chronic flexibility improvement after 12 week of stretching program utilizing the ACSM recommendations: hamstring flexibility. Int J Sports Med. 2010; 389–396 [DOI] [PubMed] [Google Scholar]
- 27. Butler DS. Mobilization of the Nervous System. 1991. New York, NY: Churchill Livingstone [Google Scholar]
- 28. Martin RL, Irrgang JJ, Burdett RG, et al. Evidence of validity for the foot and ankle ability measure (FAAM). Foot Ank Intern. 2005; 968–983 [DOI] [PubMed] [Google Scholar]
- 29. Norkin CC, White DJ. The ankle and foot. In: Measurement of Joint Motion: A Guide to Goniometry. 2003. Philadelphia, PA: F.A. Davis Company [Google Scholar]
- 30. Carcia CR, Martin RL, Drouin JM. Validity of the foot and ankle ability measure in athletes with chronic ankle instability. J Athl Train. 2008;43:179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stephenson K, Saltzman CL, Brotzman SB. Foot and ankle injuries. In: Brotzman SB, Wilk KE. Clinical Orthopaedic Rehabilitation. 2nd Edition 2003. Philadelphia, PA: Mosby [Google Scholar]