Abstract
The complement system serves many biologic functions, including the eradication of invasive pathogens and the removal of damaged cells and immune-complexes. Uncontrolled complement activation causes injury to host cells, however, so adequate regulation of the system is essential. Control of the complement system is maintained by a group of cell surface and circulating proteins referred to as complement regulatory proteins. The expression of the cell surface complement regulatory proteins varies from tissue to tissue. Furthermore, specific cell types can up-regulate or down-regulate the expression of these proteins in response to a variety of signals or insults. Altered regulation of the complement regulatory proteins can have important effects on local complement activation. In some circumstances this can be beneficial, such as in the setting of certain infections. In other circumstances, however, this can be a cause of complement-mediated injury of the tissue. A full understanding of the mechanisms by which the complement system is modulated at the local level can have important implications for how we diagnose and treat a wide range of inflammatory diseases.
Keywords: complement, complement regulatory protein, inflammation
Introduction
The complement system is an ancient and integral part of the innate immune system. Individuals with congenital deficiencies in proteins involved in complement activation are susceptible to infections (1), highlighting the importance of this system in protecting the host from pathogens. In contrast, congenital deficiency of complement regulatory proteins renders hosts susceptible to several inflammatory diseases (2). These two extremes – inadequate complement activation versus uncontrolled complement activation – delineate the central task of the complement system. It must readily eliminate pathogens while causing minimal injury to host cells.
Complement is activated by immunoglobulin, but it also has an intrinsic ability to discriminate pathogens from healthy cells. Mannose binding lectin (MBL), for example, binds to mannose groups displayed on the surface of some bacteria and activates the complement system (3). The alternative pathway of complement is spontaneously activated in the fluid phase and is amplified on surfaces that do not express complement regulatory proteins. The expression of proteins that control the complement system on host cells is equally important to the expression of proteins that activate the system on pathogens. Adequate regulation of the complement system on self-cells is thus a central component of the system’s ability to discriminate self from pathogens.
Every cell on the body expresses complement regulatory proteins (2). Some of these proteins are expressed on the cell membrane and some are soluble proteins that circulate in plasma. The complement regulatory proteins can act at different points in the complement cascade (Figure 1). The repertoire of complement regulatory proteins expressed on different tissues is also distinct. This may be due to the inflammatory challenges that each tissue has evolved to handle. It is notable that systemic defects in the complement regulatory proteins are associated with tissue specific diseases, demonstrating that some tissue types are more dependent than others on protection by a given complement regulatory protein. A number of different studies have also revealed that the expression of complement regulatory proteins by some tissues is dynamic. The expression levels can be increased or decreased in response to certain stressors or signals.
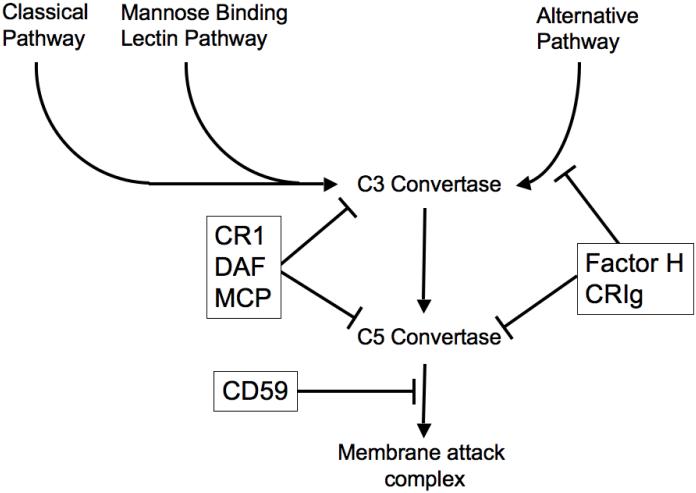
Figure 1. Regulation of complement activation.
Three complement activation pathways - the classical, mannose binding lectin and alternative pathways - can form C3 convertases. Cleavage of C3 then enables formation of C5 convertases, which ultimately generate the membrane attack complex. The complement regulatory proteins (in boxes) block this cascade at several steps.
One important consequence of this is that tissues can modulate local activation of the complement system through alterations in the density of complement regulatory proteins. Some of the complement regulatory proteins have other functions too, however, so altered expression of complement regulatory proteins may have effects outside of the complement system. Complement regulatory proteins are involved in cell signaling, they can function as portals of entry into cells by viruses, they affect neutrophil migration, and they influence thymic development. Although more studies are needed in order to fully understand the causes and consequences of altered expression of complement regulatory proteins, the work done to date suggests that this is an important mechanism whereby cells may foster or suppress local inflammation.
Complement regulatory proteins
Several soluble and cell bound complement regulatory proteins are expressed throughout the body (Table 1) (2). The expression pattern of the different complement regulatory proteins varies from tissue to tissue (4-6). This, by itself, indicates that control of the complement system is not simply a binary problem of distinguishing self from non-self. In other words, the repertoire of complement regulatory proteins expressed at various anatomical locations may confer different resistance to complement activating stimuli. Endothelial cells, for example, are in continual contact with high levels of circulating complement proteins. Endothelial cells express multiple complement regulatory proteins including membrane cofactor protein (MCP or CD46), decay accelerating factor (DAF or CD55), complement receptor 1 (CR1), and CD59, and factor H interacts with the endothelial cell surface to provide further surface regulation (7, 8).
Table 1. Complement regulatory proteins.
| Membrane Bound Complement Inhibitor |
Mechanism of action |
|---|---|
| CR1 (CD35) | Cofactor for factor I mediated cleavage of C3b and C4b; accelerates decay of the C3 and C5 convertases. |
| CRIg | Binds Beta chain of C3b and inhibits alternative pathway C3 and C5 convertases. |
| Membrane cofactor protein (MCP; CD46) |
Cofactor for factor I mediated cleavage of C3b and C4b. |
| Decay accelerating factor (DAF; CD55) |
Accelerates the decay of C3 convertase. |
| CD59 | Prevents formation of the membrane attack complex. |
| Crry (rodent only) | Cofactor for factor I mediated cleavage of C3b and C4b; accelerates the decay of the C3 convertases. |
| Factor I | Soluble protein that mediates inactivating cleavage of C3b and C4b. |
| Factor H | Soluble protein that can interact with host cell surfaces and inhibit alternative pathway C3 convertase. Factor H accelerates the decay of C3 convertase and also serves as a cofactor for factor I mediated cleavage of C3b. |
The complement regulatory proteins restrict complement activation at several stages of the complement cascade and through several biochemical effects (2). Consequently, the different complement regulatory proteins may limit the production of some activation fragments while permitting generation of others. Cells expressing surface CD59 may resist lysis by the membrane attack complex (MAC), for example, but may be readily opsonized with C3b (Figure 1). It is worth noting that factor H, although generated as a soluble protein, is believed to protect cells by binding to the cell surface (9, 10). Although the current work focuses on regulated expression of cell surface complement regulatory proteins, the interaction of circulating complement regulatory proteins such as factor H with cells may also be altered by changes in the cell surface.
Congenital deficiency of complement regulatory proteins predisposes to inflammatory injury of specific tissues
Complement activation in the fluid phase is continuous and spontaneous. Consequently, all cells in the body must adequately control complement activation on the cell surface in order to prevent nucleation of pathogenic complement activation. Several diseases are associated with mutations in complement regulatory proteins, highlighting the importance of adequate control of this system. One example of such a disease is paroxysmal nocturnal hemoglobinuria (PNH). In this disease, mutations in the gene for phosphatidylinositol glycan-complementation class A (PIGA) prevent the linkage of DAF and CD59 to cell surfaces (11). Although this defect also prevents DAF and CD59 expression on other cell types, the disease primarily manifests as lysis of erythrocytes. Furthermore, erythrocyte lysis increases during periods of systemic stress caused by infection, surgery, or alcohol intake, strenuous exercise, or other conditions that increase the state of complement activation (12). The episodic and inducible nature of this disease demonstrates that the requirement of complement regulation on the cell surface is dynamic and varies from tissue to tissue.
Mutations and polymorphisms in factor H are associated with several diseases, including dense deposit disease (a rare form of glomerulonephritis), atypical hemolytic uremic syndrome (another rare form of renal disease), and age-related macular degeneration (AMD) (13). Like PNH, dense deposit disease and atypical hemolytic uremic syndrome are episodic. It is also notable that these systemic defects in complement regulation cause focal inflammation in specific target organs. These findings suggest that when the body is faced by transient stresses, some tissues will be more reliant upon particular complement regulatory proteins than others.
Expression of complement regulatory proteins by cancer cells
Immune surveillance is an important mechanism by which the body eliminates cancer cells. Complement activation has been detected in several different types of cancer, suggesting that the complement system is engaged within the tumor microenvironment. Many types of cancer express complement regulatory proteins (14, 15), and over-expression of complement regulatory proteins by cancer cells is associated with greater invasiveness (16). The expression of complement regulatory proteins is a logical mechanism by which cancer cells may evade elimination by the complement system. In addition to controlling endogenous complement activation within the tumor, high expression of complement regulatory proteins may confer resistance against antibody-mediated anti-tumor therapies. Consequently, investigators have sought methods of inactivating complement regulatory proteins as a means of sensitizing tumors to antibody-mediated therapies (17-19).
Although activation of the complement system within tumors may be cytotoxic to the cancer cells and may facilitate an immune response against tumor, there is also evidence that complement activation fragments promote tumor growth (20). C5a, one of the fragments, is believed to suppress the anti-tumor T cell response. In this light, complement activation within the tumor may not simply have an anti-tumor effect but may also alter local immune activation to the advantage of tumor growth.
Complement regulatory protein expression is down-regulated on infected cells
Complement regulatory proteins can serve as receptors for various infectious organisms. MCP, for example, is a receptor for a large variety of intracellular pathogens including Measles virus, Herpes virus, and Neisseria species (21). DAF is also a ligand for some viruses and for E. coli (22). Complement regulatory proteins are down-regulated in several different cell types in response to infection. Hepatitis B lowers CD59 levels in the liver (23), and the expression of complement regulatory proteins on the surface of hepatocytes decreases after infection with vaccinia virus (24). MCP on the surface of cervical epithelial cells can serve as a receptor for piliated neisseria. After exposure of cervical epithelial cells to piliated neisseria the surface levels of MCP are actively reduced through shedding of the protein (25). Exposure of the cells to non-piliated neisseria, however, does not cause shedding of surface MCP. Reduced expression of the complement regulatory proteins may limit the spread of infection by fostering local complement activation, but this may also contribute to inflammatory injury of the tissue. complement regulatory protein expression can increase on some cell types during infection. CD59 and MCP are increased in T cells infected by Herpes virus 7, for example, potentially helping the virus to evade the immune system (26).
Complement regulatory protein expression is down-regulated on apoptotic cells
In addition to opsonizing pathogens and facilitating their eradication, the complement system also aids in the clearance of injured cells host cells (27). This is evident in C1q-deficient mice, where there is an increase in prevalent apoptotic cells (28). For complement to be selectively activated on apoptotic cells, the surface expression of some complement regulatory proteins on these cells decreases relative to expression in healthy cells. In a detailed series of experiments, Elward et al. demonstrated that surface expression of MCP is down-regulated on apoptotic cells (29). The decrease in MCP expression was associated with increased complement activation when the cells were exposed to serum.
Apoptotic cells induce less inflammation than necrotic cells, and some complement regulation on apoptotic cells is necessary. Otherwise, uncontrolled complement activation would cause apoptotic cells to become necrotic, generate anaphylatoxins, attract inflammatory cells, and injure bystander cells. In order to opsonize apoptotic cells with C3 but limit the insertion MAC into the cells, regulators of early complement regulation are reduced while CD59 (a regulator of MAC formation) persists (29). Soluble factors also bind to the cells to limit the terminal stages of complement activation. C-reactive protein, for example, simultaneously augments classical pathway activation on the apoptotic cell surface while restricting alternative pathway amplification (30). This protein-cell interaction causes deposition of C3 fragments on the cell surface and increased phagocytosis of the apoptotic cells by macrophages. Binding of C-reactive protein to the cells, however, does not trigger complement-mediated cell lysis. Concurrent regulation of alternative pathway activation by C-reactive protein is achieved through its interactions with factor H which limits complement deposition to the level of C3 (30). C-reactive protein does not have these effects on necrotic cells. Regarded together, these findings indicate that reduced expression of complement regulatory proteins on apoptotic cells permits complement activation on the cell surface and aids in their clearance. This is a tightly regulated process, however, that permits the complement system to discriminate between healthy, apoptotic, and necrotic cells.
Altered complement regulatory proteins expression by viable cells modulates local complement activation
The above discussion considers examples of decreased complement regulation on altered cells. Viable, untransformed cells may also alter surface expression of complement regulatory proteins. Active modulation of complement inhibition on the cell surface may be an adaptive response to various environmental challenges. By increasing complement regulation the cells may protect themselves from bystander injury. By decreasing complement regulation the cells may foster a local environment better suited for the elimination of pathogens or damaged tissue and debris.
Kidney
It has been known for decades that immune-complex glomerulonephritis is characterized by glomerular complement activation. The recent association of some renal diseases with mutations in complement regulatory proteins has rekindled interest in how the complement system is regulated within the kidney. DAF, MCP, CR1, and CD59 are all expressed within the glomeruli of normal kidneys (Figure 2) (31-33), suggesting that the function of these proteins is overwhelmed or somehow impeded during the development of glomerulonephritis.
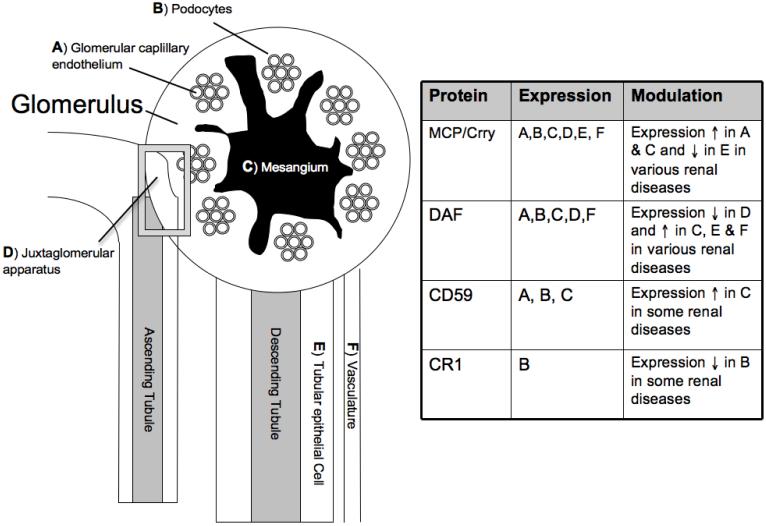
Figure 2. Expression of complement regulatory proteins in the kidney.
Even within a single organ, different cell types will express a different repertoire of complement regulatory proteins. Within the kidney, the expression of different complement regulatory proteins is increased or decreased in various diseases. The localization to different structures within the kidney is also altered (see text for greater detail). Crry expression in the kidneys of rodents is similar to that of MCP in humans. We have categorized them as having the same pattern of expression, although this has not be rigorously examined.
In normal kidneys, DAF expression is concentrated in the vascular pole of the juxta-glomerular apparatus (31). DAF expression at this location is decreased in patients with some types of renal diseases, however, including hemolytic uremic syndrome, lupus nephritis, and interstitial nephritis. Conversely, DAF expression is increased in the mesangium, tubulointerstitium, and vasculature of patients with renal disease, and DAF expression at these locations correlates with the presence of tissue C3. One exception to this finding is lupus nephritis. Almost all of the lupus patients in this study demonstrated glomerular C3 but had persistently low level of mesangial DAF expression (31). DAF expression on mesangial cells in culture increases after complement from serum is activated on the cell surface, indicating that mesangial cells can actively modulate surface expression of DAF in response to complement activation (34).
Like DAF, MCP is heavily expressed in the juxtaglomerular apparatus of normal kidneys, but it is also detected in the glomerular capillaries and tubulointerstitial arterioles, the mesangium, the podocytes, and the tubules (33, 35). MCP expression in the mesangium and glomerular capillaries is increased in several renal diseases, including membranous disease, lupus nephritis, and membranoproliferative glomerulonephritis (32). As was seen with DAF, the increase in MCP expression corresponded to increased deposition of C3 within glomeruli. Mesangial cells in culture express MCP at baseline (34). Unlike what is seen with DAF, however, complement activation on the mesangial cell surface does not change MCP levels (34).
CD59 is expressed by all of the resident glomerular cell types. Mesangial cells increase the expression of CD59 in response to complement activation, an effect that appears to require MAC formation. Increased CD59 expression has been observed in patients with lupus nephritis (36). Alternatively, the levels of CD59 are decreased in some patients with membranous nephropathy, and shed CD59 has been detected in the urine of these patients (37). CR1 expression in the kidney is limited to the podocytes, and several diseases characterized by podocyte injury are associated with decreased CR1 expression at this location (37).
In rodent kidneys Crry (a homologue of human MCP and DAF) is the only complement regulatory protein expressed on the tubular epithelial cells, and its expression is polarized to the basolateral surface of the cells (38). The polarized expression of Crry is lost in hypoxic cells, permitting alternative pathway complement activation on the basolateral surface of the tubules. Complement activation on the hypoxic tubules promotes further inflammation and tissue injury. When tubular epithelial cells in culture were made hypoxic the overall level of surface Crry also decreased (unpublished results). Oxidative stress, on the other hand, did not alter the surface levels of Crry (39). Antibody mediated neutralization of Crry in rat kidneys resulted in C3 activation in the glomeruli, tubules, and peri-vascular capillaries, further demonstrating the critical role that this complement regulatory protein plays in the rodent kidney (40).
Eye
Age related macular degeneration is associated with polymorphisms in factor H and factor B, suggesting that impaired control of the alternative pathway contributes to injury in this disease (41, 42). Patients with dense deposit disease may also develop retinal lesions similar to those seen in AMD, further supporting a link between impaired complement activation and injury of the retina. The polymorphisms in factor H associated with AMD are located in regions of the protein that mediate surface binding, suggesting that it is deficient complement regulation by factor H on retinal surfaces that predisposes to disease (42). Protection of retinal epithelial cells in culture appears to be lost when the cells are subjected to oxidative stress, indicating that alterations in the cell surface can reduce complement regulation by factor H (43).
Retinal epithelial cells express MCP, DAF, and CD59. In rodents, Crry is expressed instead of MCP. In a study in rats, the intra-ocular injection of inhibitory antibodies to Crry caused the development of severe anterior uveitus, demonstrating that adequate complement regulation in the eye is necessary to prevent injury (44). The levels of the complement regulatory proteins expressed on retinal epithelial cells decreases when the cells are subjected to oxidative stress, increasing the degree of complement activation seen when the cells are subsequently exposed to serum (43). These finding indicate that environmental insults may decrease local complement regulation by the retinal epithelial cell layer and contribute to chronic retinal injury.
The complement system also plays a pathogenic role in other models of eye disease, including experimental autoimmune uveoretinitis (EAU) (45, 46) and experimental autoimmune anterior uveitis (EAAU) (47). Mice that are deficient in DAF develop more severe EAU than wild-type mice, demonstrating that endogenous DAF limits disease (48). The administration of additional soluble DAF protects mice from EAU, however, indicating that the endogenous levels are inadequate to fully prevent injury. When EAAU was induced in Lewis rats, it was found that the expression of Crry and CD59 in the eyes increased (49). The suppression of Crry and/or CD59 expression exacerbated the disease, demonstrating that increased expression of these complement regulatory proteins is an adaptive, protective response. Complement depleted rats are protected in this model (47), indicating that even the increased expression of Crry and CD59 is insufficient to fully prevent complement mediated injury.
Liver
Many of the complement proteins are primarily synthesized in the liver, so resident liver cells must tolerate high local concentrations of complement proteins. Expression of the complement regulatory proteins varies among the different cell types (50, 51). Intra-hepatic complement activation during transplantation is associated with hepatocellular injury, although this does not appear to be due to altered complement regulatory protein expression in response to ischemia (50, 52). As mentioned above, however, complement regulatory protein levels in the liver do decrease in response to certain viral infections, sensitizing the cells to complement mediated injury (23). In vitro experiments demonstrate that the addition of complement sufficient serum slows viral spread during infection of hepatic cells, suggesting increased complement on the cells may have a salutary effect. (24). Alcohol also causes decreased expression of complement regulatory proteins within the liver and is associated with intra-hepatic complement activation. With aseptic insults such as alcohol this complement-activating response may be maladaptive and increase tissue injury. Complement activation fragments have also been shown to aid in liver cell survival, however, and they may be important for liver regeneration (53). Altered local regulation of the complement system may, therefore, be an important method by which the liver initiates recovery after both infectious and aseptic insults.
Brain
Complement activation has been observed in several neurologic diseases, including Alzheimer’s disease, multiple sclerosis and stroke (54). There is not a clear relationship between alterations in complement regulatory protein expression and these neurologic diseases. For example there have been conflicting reports regarding the expression of CD59 in the brains of patients with Alzheimer’s disease (55, 56). Expression of the complement regulatory proteins is decreased in a model of multiple sclerosis (57), and appears to be increased in the brains of patients with Huntington’s disease (58). Bacterial meningitis is associated with increased expression of CD55 and CD59 (59). On the other hand, CD59 expression was decreased in the brains of two patients with diabetic ketoacidosis who died of cerebral edema (60). Interestingly, injury can also cause down-regulation of factor H expression by neural cells (61). Although most factor H is generated by the liver and arrives to cells via the circulation, it is possible that changes in the production of this protein by tissues could affect local complement regulation, particularly in privileged tissues such as the brain. Thus, although the physiologic effect of altered complement regulatory protein expression in these diseases is not yet clear, there does appear to be dynamic regulation of the complement system within the brain.
Hematopoietic cells
complement regulatory protein expression on hematopoietic cells has been shown to change in several clinical settings. For example, recent work has shown that expression of DAF by T cells can be regulated. The production of complement proteins by T cells and antigen presenting cells is believed to increase the T cell response to antigen (61). Concurrent with the release of activating proteins such as C3 and factor B, the T cells decrease surface DAF expression (62). This response likely potentiates local complement activation and increases its effect on T cell activation.
In addition to regulating complement, CR1 expressed on erythrocytes is critical for clearance of immune complexes. Erythrocyte CR1 is decreased in several diseases associated with high levels of circulating immune complexes, including systemic lupus erythematosus (63), rheumatoid arthritis (64), and pulmonary tuberculosis (65). Decreased CR1 may be due to consumption of the protein during immune-complex clearance. Consequently, low levels may be an indicator of effective immune-complex clearance rather than a cause of immune-complex disease (66).
Cardiovascular system
The complement system is activated in the ischemic myocardium. The expression of CD59 is decreased in ischemic human myocardium compared to normal tissue, possibly due to shedding of the protein (67). The loss of CD59 is associated with increased MAC deposition, suggesting that decreased CD59 expression permits increased MAC formation within the tissue. Statins increase the expression of DAF on endothelial cells in vitro, and it has been proposed that this may be a mechanism by which this class of drugs protects against inflammatory injury (68).
Implications for therapy
As highlighted by the above discussion, many tissues are capable of altering the local expression of complement regulatory proteins. Decreased complement regulation by tissues can contribute to autologous injury by the complement system, but it can also have beneficial effects. Increased complement activation may help eradicate infection, may increase the adaptive immune response, and complement activation products may foster protection and regeneration of organs such as the liver. Although the complement proteins circulate at high concentrations, the effects of complement activation are typically localized. The altered expression of complement regulatory proteins by tissues is, therefore, an important mechanism by which the complement system can be turned on or off in a tissue-specific fashion.
Given the localized effects of complement activation in many diseases, therapies to modulate complement activation – i.e. to either increase or decrease its effects – may work best when they can be delivered locally. Several strategies have been employed in recent years to achieve local complement regulation. One complement inhibitor is comprised of the complement regulatory region of CR1 linked to a membrane-binding moiety (69). When rat kidneys were perfused with this agent it bound to endothelial cells and tubules, and protected the kidneys from injury during reperfusion. Another effective targeting strategy is to link the iC3b/C3d binding region of CR2 to a complement regulatory protein (70-73). These agents deliver the complement inhibitor to sites of complement activation and may carry less risk of infection than untargeted agents (71). Antibodies to renal-specific antigens have also been used to deliver complement inhibitors to the kidney in a rat model of renal disease (74).
Conclusions
The local tissue expression of complement regulatory proteins is altered in many different diseases. These alterations in complement regulatory protein expression can cause a variety of different physiologic consequences, including autologous injury of host cells by the complement system itself. Indeed, all complement-mediated injury can be regarded, in one sense, as inadequate local complement regulation. A better understanding of the mechanisms by which various tissues regulate the complement system at the local level will improve our understanding of disease pathogenesis, and may lead to the development of improved therapies. Numerous agents have been developed to prevent complement mediated injury (75), and one of these agents, eculizumab, has recently entered clinical use (76). Tissue-targeted complement inhibitors may be able to restore control of the complement system specifically to the tissue sites where this control has been lost. Such agents may reverse the causes of inflammatory tissue injury while minimizing the systemic side-effects of complement inhibition.
Abbreviations
- AMD
age-related macular degeneration
- CR1
complement receptor 1
- DAF
decay accelerating factor
- EAAU
experimental autoimmune anterior uveitis
- EAU
experimental autoimmune uveoretinitis
- MBL
mannose binding lectin
- MAC
membrane attack complex
- MCP
membrane cofactor protein
- PNH
paroxysmal nocturnal hemoglobinuria
- PIGA
phosphatidylinositol glycan-complementation class A
Footnotes
Disclosure
JMT is a stockholder in and consult for Taligen Therapeutics, Inc.
References
- 1.Tedesco F. Inherited complement deficiencies and bacterial infections. Vaccine. 2008;26(Suppl 8):I3–8. doi: 10.1016/j.vaccine.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 3.Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10(1):50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 4.Lin F, Fukuoka Y, Spicer A, Ohta R, Okada N, Harris CL, et al. Tissue distribution of products of the mouse decay-accelerating factor (DAF) genes. Exploitation of a Daf1 knock-out mouse and site-specific monoclonal antibodies. Immunology. 2001;104(2):215–225. doi: 10.1046/j.0019-2805.2001.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin X, Miwa T, Aktas H, Gao M, Lee C, Qian YM, et al. Genomic structure, functional comparison, and tissue distribution of mouse Cd59a and Cd59b. Mamm Genome. 2001;12(8):582–589. doi: 10.1007/s00335-001-2060-8. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Sallee C, Dehoff M, Foley S, Molina H, Holers VM. Mouse Crry/p65. Characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J Immunol. 1993;151(8):4295–4305. [PubMed] [Google Scholar]
- 7.Moutabarrik A, Nakanishi I, Namiki M, Hara T, Matsumoto M, Ishibashi M, et al. Cytokine-mediated regulation of the surface expression of complement regulatory proteins, CD46(MCP), CD55(DAF), and CD59 on human vascular endothelial cells. Lymphokine Cytokine Res. 1993;12(3):167–172. [PubMed] [Google Scholar]
- 8.Jokiranta TS, Cheng ZZ, Seeberger H, Jozsi M, Heinen S, Noris M, et al. Binding of complement factor H to endothelial cells is mediated by the carboxy-terminal glycosaminoglycan binding site. Am J Pathol. 2005;167(4):1173–1181. doi: 10.1016/S0002-9440(10)61205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira VP, Pangburn MK, Cortes C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 47(13):2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander JJ, Quigg RJ. The simple design of complement factor H: Looks can be deceiving. Mol Immunol. 2007;44(1-3):123–132. doi: 10.1016/j.molimm.2006.07.287. [DOI] [PubMed] [Google Scholar]
- 11.Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73(4):703–711. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky RA. Paroxysmal Nocturnal Hemoglobinuria. In: Hoffman R, Furie B, McGlave P, Silberstein LE, Shattil SJ, Benz EJ, et al., editors. Hoffman: Hematology: Basic Principles and Practice. 5th edn. Churchill Livingstone; Maryland Heights: 2008. [Google Scholar]
- 13.de Cordoba SR, de Jorge EG. Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol. 2008;151(1):1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajona D, Castano Z, Garayoa M, Zudaire E, Pajares MJ, Martinez A, et al. Expression of complement factor H by lung cancer cells: effects on the activation of the alternative pathway of complement. Cancer Res. 2004;64(17):6310–6318. doi: 10.1158/0008-5472.CAN-03-2328. [DOI] [PubMed] [Google Scholar]
- 15.Donin N, Jurianz K, Ziporen L, Schultz S, Kirschfink M, Fishelson Z. Complement resistance of human carcinoma cells depends on membrane regulatory proteins, protein kinases and sialic acid. Clin Exp Immunol. 2003;131(2):254–263. doi: 10.1046/j.1365-2249.2003.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikesch JH, Buerger H, Simon R, Brandt B. Decay-accelerating factor (CD55): a versatile acting molecule in human malignancies. Biochim Biophys Acta. 2006;1766(1):42–52. doi: 10.1016/j.bbcan.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Macor P, Tedesco F. Complement as effector system in cancer immunotherapy. Immunol Lett. 2007;111(1):6–13. doi: 10.1016/j.imlet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Macor P, Tripodo C, Zorzet S, Piovan E, Bossi F, Marzari R, et al. In vivo targeting of human neutralizing antibodies against CD55 and CD59 to lymphoma cells increases the antitumor activity of rituximab. Cancer Res. 2007;67(21):10556–10563. doi: 10.1158/0008-5472.CAN-07-1811. [DOI] [PubMed] [Google Scholar]
- 19.Yan J, Allendorf DJ, Li B, Yan R, Hansen R, Donev R. The role of membrane complement regulatory proteins in cancer immunotherapy. Adv Exp Med Biol. 2008;632:159–174. [PubMed] [Google Scholar]
- 20.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9(11):1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25(9):496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Lea S. Interactions of CD55 with non-complement ligands. Biochem Soc Trans. 2002;30(Pt 6):1014–1019. doi: 10.1042/bst0301014. [DOI] [PubMed] [Google Scholar]
- 23.Qu Z, Liang X, Liu Y, Du J, Liu S, Sun W. Hepatitis B virus sensitizes hepatocytes to complement-dependent cytotoxicity through downregulating CD59. Mol Immunol. 2009;47(2-3):283–289. doi: 10.1016/j.molimm.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Baranyi L, Okada N, Baranji K, Takizawa H, Okada H. Membrane-bound complement regulatory activity is decreased on vaccinia virus-infected cells. Clin Exp Immunol. 1994;98(1):134–139. doi: 10.1111/j.1365-2249.1994.tb06619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill DB, Koomey M, Cannon JG, Atkinson JP. Down-regulation of CD46 by piliated Neisseria gonorrhoeae. J Exp Med. 2003;198(9):1313–1322. doi: 10.1084/jem.20031159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takemoto M, Yamanishi K, Mori Y. Human herpesvirus 7 infection increases the expression levels of CD46 and CD59 in target cells. J Gen Virol. 2007;88(Pt 5):1415–1422. doi: 10.1099/vir.0.82394-0. [DOI] [PubMed] [Google Scholar]
- 27.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188(12):2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19(1):56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 29.Elward K, Griffiths M, Mizuno M, Harris CL, Neal JW, Morgan BP, et al. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J Biol Chem. 2005;280(43):36342–36354. doi: 10.1074/jbc.M506579200. [DOI] [PubMed] [Google Scholar]
- 30.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192(9):1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosio FG, Sedmak DD, Mahan JD, Nahman NS., Jr. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 1989;36(1):100–107. doi: 10.1038/ki.1989.167. [DOI] [PubMed] [Google Scholar]
- 32.Endoh M, Yamashina M, Ohi H, Funahashi K, Ikuno T, Yasugi T, et al. Immunohistochemical demonstration of membrane cofactor protein (MCP) of complement in normal and diseased kidney tissues. Clin Exp Immunol. 1993;94(1):182–188. doi: 10.1111/j.1365-2249.1993.tb05998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichida S, Yuzawa Y, Okada H, Yoshioka K, Matsuo S. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 1994;46(1):89–96. doi: 10.1038/ki.1994.247. [DOI] [PubMed] [Google Scholar]
- 34.Cosio FG, Shibata T, Rovin BH, Birmingham DJ. Effects of complement activation products on the synthesis of decay accelerating factor and membrane cofactor protein by human mesangial cells. Kidney Int. 1994;46(4):986–992. doi: 10.1038/ki.1994.358. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi I, Moutabarrik A, Hara T, Hatanaka M, Hayashi T, Syouji T, et al. Identification and characterization of membrane cofactor protein (CD46) in the human kidneys. Eur J Immunol. 1994;24(7):1529–1535. doi: 10.1002/eji.1830240711. [DOI] [PubMed] [Google Scholar]
- 36.Tamai H, Matsuo S, Fukatsu A, Nishikawa K, Sakamoto N, Yoshioka K, et al. Localization of 20-kD homologous restriction factor (HRF20) in diseased human glomeruli. An immunofluorescence study. Clin Exp Immunol. 1991;84(2):256–262. doi: 10.1111/j.1365-2249.1991.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nangaku M. Complement regulatory proteins in glomerular diseases. Kidney Int. 1998;54(5):1419–1428. doi: 10.1046/j.1523-1755.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 38.Thurman JM, Ljubanovic D, Royer PA, Kraus DM, Molina H, Barry NP, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest. 2006;116(2):357–368. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thurman JM, Renner B, Kunchithapautham K, Holers VM, Rohrer B. Aseptic injury to epithelial cells alters cell surface complement regulation in a tissue specific fashion. Adv Exp Med Biol. 664:151–158. doi: 10.1007/978-1-4419-1399-9_18. [DOI] [PubMed] [Google Scholar]
- 40.Nomura A, Nishikawa K, Yuzawa Y, Okada H, Okada N, Morgan BP, et al. Tubulointerstitial injury induced in rats by a monoclonal antibody that inhibits function of a membrane inhibitor of complement. J Clin Invest. 1995;96(5):2348–2356. doi: 10.1172/JCI118291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284(25):16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci. 2000;41(11):3492–3502. [PMC free article] [PubMed] [Google Scholar]
- 45.Copland DA, Hussain K, Baalasubramanian S, Hughes TR, Morgan BP, Xu H, et al. Systemic and local anti-C5 therapy reduces the disease severity in experimental autoimmune uveoretinitis. Clin Exp Immunol. 159(3):303–314. doi: 10.1111/j.1365-2249.2009.04070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Read RW, Szalai AJ, Vogt SD, McGwin G, Barnum SR. Genetic deficiency of C3 as well as CNS-targeted expression of the complement inhibitor sCrry ameliorates experimental autoimmune uveoretinitis. Exp Eye Res. 2006;82(3):389–394. doi: 10.1016/j.exer.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Jha P, Sohn JH, Xu Q, Nishihori H, Wang Y, Nishihori S, et al. The complement system plays a critical role in the development of experimental autoimmune anterior uveitis. Invest Ophthalmol Vis Sci. 2006;47(3):1030–1038. doi: 10.1167/iovs.05-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An F, Li Q, Tu Z, Bu H, Chan CC, Caspi RR, et al. Role of DAF in protecting against T-cell autoreactivity that leads to experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2009;50(8):3778–3782. doi: 10.1167/iovs.08-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jha P, Sohn JH, Xu Q, Wang Y, Kaplan HJ, Bora PS, et al. Suppression of complement regulatory proteins (CRPs) exacerbates experimental autoimmune anterior uveitis (EAAU) J Immunol. 2006;176(12):7221–7231. doi: 10.4049/jimmunol.176.12.7221. [DOI] [PubMed] [Google Scholar]
- 50.Scoazec JY, Delautier D, Moreau A, Durand F, Degott C, Benhamou JP, et al. Expression of complement-regulatory proteins in normal and UW-preserved human liver. Gastroenterology. 1994;107(2):505–516. doi: 10.1016/0016-5085(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 51.Halme J, Sachse M, Vogel H, Giese T, Klar E, Kirschfink M. Primary human hepatocytes are protected against complement by multiple regulators. Mol Immunol. 2009;46(11-12):2284–2289. doi: 10.1016/j.molimm.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Scoazec JY, Borghi-Scoazec G, Durand F, Bernuau J, Pham BN, Belghiti J, et al. Complement activation after ischemia-reperfusion in human liver allografts: incidence and pathophysiological relevance. Gastroenterology. 1997;112(3):908–918. doi: 10.1053/gast.1997.v112.pm9041253. [DOI] [PubMed] [Google Scholar]
- 53.Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, et al. The regulation of liver cell survival by complement. J Immunol. 2009;182(9):5412–5418. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanamadala V, Friedlander RM. Complement in neuroprotection and neurodegeneration. Trends Mol Med. 16(2):69–76. doi: 10.1016/j.molmed.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang LB, Li R, Meri S, Rogers J, Shen Y. Deficiency of complement defense protein CD59 may contribute to neurodegeneration in Alzheimer’s disease. J Neurosci. 2000;20(20):7505–7509. doi: 10.1523/JNEUROSCI.20-20-07505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasojima K, McGeer EG, McGeer PL. Complement regulators C1 inhibitor and CD59 do not significantly inhibit complement activation in Alzheimer disease. Brain Res. 1999;833(2):297–301. doi: 10.1016/s0006-8993(99)01514-0. [DOI] [PubMed] [Google Scholar]
- 57.Mead RJ, Neal JW, Griffiths MR, Linington C, Botto M, Lassmann H, et al. Deficiency of the complement regulator CD59a enhances disease severity, demyelination and axonal injury in murine acute experimental allergic encephalomyelitis. Lab Invest. 2004;84(1):21–28. doi: 10.1038/labinvest.3700015. [DOI] [PubMed] [Google Scholar]
- 58.Singhrao SK, Neal JW, Morgan BP, Gasque P. Increased complement biosynthesis by microglia and complement activation on neurons in Huntington’s disease. Exp Neurol. 1999;159(2):362–376. doi: 10.1006/exnr.1999.7170. [DOI] [PubMed] [Google Scholar]
- 59.Canova C, Neal JW, Gasque P. Expression of innate immune complement regulators on brain epithelial cells during human bacterial meningitis. J Neuroinflammation. 2006;3:22. doi: 10.1186/1742-2094-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffman WH, Cudrici CD, Zafranskaia E, Rus H. Complement activation in diabetic ketoacidosis brains. Exp Mol Pathol. 2006;80(3):283–288. doi: 10.1016/j.yexmp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Vieyra MB, Heeger PS. Novel aspects of complement in kidney injury. Kidney Int. 77(6):495–499. doi: 10.1038/ki.2009.491. [DOI] [PubMed] [Google Scholar]
- 62.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201(10):1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birmingham DJ, Gavit KF, McCarty SM, Yu CY, Rovin BH, Nagaraja HN, et al. Consumption of erythrocyte CR1 (CD35) is associated with protection against systemic lupus erythematosus renal flare. Clin Exp Immunol. 2006;143(2):274–280. doi: 10.1111/j.1365-2249.2005.02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar A, Malaviya AN, Sinha S, Khandekar PS, Banerjee K, Srivastava LM. C3b receptor (CR1) genomic polymorphism in rheumatoid arthritis. Low receptor levels on erythrocytes are an acquired phenomenon. Immunol Res. 1994;13(1):61–71. doi: 10.1007/BF02918226. [DOI] [PubMed] [Google Scholar]
- 65.Senbagavalli P, Geetha ST, Karunakaran K, Banu Rekha VV, Venkatesan P, Ramanathan VD. Reduced erythrocyte CR1 levels in patients with pulmonary tuberculosis is an acquired phenomenon. Clin Immunol. 2008;128(1):109–115. doi: 10.1016/j.clim.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Kavai M. Immune complex clearance by complement receptor type 1 in SLE. Autoimmun Rev. 2008;8(2):160–164. doi: 10.1016/j.autrev.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Vakeva A, Laurila P, Meri S. Regulation of complement membrane attack complex formation in myocardial infarction. Am J Pathol. 1993;143(1):65–75. [PMC free article] [PubMed] [Google Scholar]
- 68.Mason JC, Ahmed Z, Mankoff R, Lidington EA, Ahmad S, Bhatia V, et al. Statin-induced expression of decay-accelerating factor protects vascular endothelium against complement-mediated injury. Circ Res. 2002;91(8):696–703. doi: 10.1161/01.res.0000038151.57577.19. [DOI] [PubMed] [Google Scholar]
- 69.Pratt JR, Jones ME, Dong J, Zhou W, Chowdhury P, Smith RA, et al. Nontransgenic hyperexpression of a complement regulator in donor kidney modulates transplant ischemia/reperfusion damage, acute rejection, and chronic nephropathy. Am J Pathol. 2003;163(4):1457–1465. doi: 10.1016/S0002-9440(10)63503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atkinson C, Qiao F, Song H, Gilkeson GS, Tomlinson S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J Immunol. 2008;180(2):1231–1238. doi: 10.4049/jimmunol.180.2.1231. [DOI] [PubMed] [Google Scholar]
- 71.Atkinson C, Song H, Lu B, Qiao F, Burns TA, Holers VM, et al. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115(9):2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181(11):8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song H, He C, Knaak C, Guthridge JM, Holers VM, Tomlinson S. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J Clin Invest. 2003;111(12):1875–1885. doi: 10.1172/JCI17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He C, Imai M, Song H, Quigg RJ, Tomlinson S. Complement inhibitors targeted to the proximal tubule prevent injury in experimental nephrotic syndrome and demonstrate a key role for c5b-9. J Immunol. 2005;174(9):5750–5757. doi: 10.4049/jimmunol.174.9.5750. [DOI] [PubMed] [Google Scholar]
- 75.Qu H, Ricklin D, Lambris JD. Recent developments in low molecular weight complement inhibitors. Mol Immunol. 2009;47(2-3):185–195. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25(11):1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]