Abstract
The leaching of nickel from the surface of porous Nitinol (PNT) is mainly dependent on its surface characteristics, which can be controlled by appropriate surface treatments. In this investigation, PNT was subjected to two surface treatments, namely, water-boiling and dry-heating passivations. Phosphate buffer saline (PBS) solutions obtained from cyclic potentiodynamic polarization tests on PNT were employed to assess the cytotoxicity of Ni contained therein on osteoblast cells by Sulforhodamine B (SRB) assay. In addition, similar concentrations of Ni were added exogenously to cell culture media to determine cytotoxic effects on osteoblast cells. The morphologies of the untreated and the surface-treated PNTs were examined using SEM and AFM. Furthermore, growth of human osteoblast cells was observed on the PNT surfaces.
Keywords: biomaterials, cytotoxicity, nitinol, surface engineering
1. Introduction
Implants prepared from equiatomic Ni-Ti alloy are known for their psuedoelasticity, shape memory effect, superior corrosion resistance, and increased osteointegration properties in bone and dental implant applications (Ref 1–3). Porous Nitinol (PNT) of 65% porosity is one such promising alloy. However, there are concerns regarding the toxic and allergenic effects of Ni release after implantation (Ref 4–7). Since PNT has a larger surface area than a solid Nitinol implant of the same size, the possibility of Ni ions releasing form the former is of greater concern (Ref 5, 8, 9). Ni ion release is also dependent on other factors such as initial composition of Nitinol and the non-equiatomic chemical species present on its surface, such as Ti2Ni, Ni3Ti, and Ni (Ref 10). The concentration of Ni released in biological media in experiments by other researchers range between 130 and 150 ppb under static and dynamic conditions (Ref 7) in respect of the Ni concentration in human blood (25–67 ppb) (Ref 11). Systemic accumulation of Ni ions takes place mainly in the kidneys and lungs of the human body (Ref 12). In addition, the presence of Ni ions in the human body poses problems for the immune, cardiovascular, blood, and respiratory systems (Ref 13). Furthermore, nickel compounds, such as NixSy, NixSey, and NixAsy (x and y are integers) act as carcinogenic agents (Ref 14), which could result in the formation of tumors. One method for reducing Ni release from PNT consists of improving the surface chemistry of the passivating oxide layer in such a way that the Ni release is minimized. In this study, PNT was subjected to a combination of various surface treatments such as water boiling, passivation, and dry heating (Ref 1, 4, 5).
Surface characteristics, such as morphology, roughness (Ref 15–19), surface energy (Ref 18), corrosion resistance, and surface layer chemical composition (Ref 19, 20), play an important role in the interaction between Nitinol implants and cellular adhesion and their proliferation in the human body (Ref 16, 21). It was also observed that when Nitinol was subjected to various surface treatments, the concentration of nickel in the surface oxide layers was found to vary appreciably (Ref 8, 22). As a result, surface treatment of Nitinol before implantation can be employed to modify its chemical, physical, and biological interaction properties (Ref 23). In this investigation, a combination of three treatments was employed to modify the surface of PNT.
2. Materials
PNT material (Actipore, Biorthex, Montreal, QC, Canada) used in this research was prepared by Self Propagating High Temperature Synthesis (SHS). The open porosity of the PNT was about 65 ± 5% with a pore size of approximately 230 ± 130 μm, which lies in the conventional tissue integration range of 50–500 μm (Ref 24). The specimens used were in the form of circular disks with thicknesses of 1.0 ± 0.25 mm and were cut from cylindrical ingots of Ø = 12.5 ± 0.25 mm and L = 30.0 ± 0.1 mm using a high-speed saw.
3. Methodology
3.1 Water Boiling
PNT was boiled in distilled water at 100 °C for 30 min. During the water-boiling process, Ni ions are leached out, and a TiO2 layer is produced on its surface (Ref 25). Simultaneously, as a result of water boiling, the ratio of Ti/Ni on Nitinol’s surface increases substantially compared with an untreated surface (Ref 26).
3.2 Dry Heating
The PNT was dry heated in a tube furnace at 132 °C with a continuous supply of air. Dry heating enhances the formation of a protective titanium oxide and the thickness of the surface oxide layer (Ref 27).
3.3 Passivation
Passivation is a chemical dissolution process, where an oxidant removes exogenous atoms present on the surface of a metal (Ref 28–30). PNT samples were passivated in a 20% Conc. HNO3 medium at 80 °C for 20 min. Passivation of Nitinol results in the reduction of elemental Ni and NiO from the surface of the alloy, and increases the amount of TiO2 (Ref 28).
3.4 Pre-Cleaning
Localized corrosion of Nitinol can be triggered by the presence of impurities and foreign particles attached to its surface (Ref 31). This can be reduced by ultrasonic cleaning with reagents such as acetone, ethanol, etc. (Ref 21, 23, 32). The PNT samples were cleaned ultrasonically in both acetone and ethanol for 5 min, to ensure that the hydrocarbon contaminants were completely removed. Finally, the samples were washed with distilled water for 5 min and air dried. All the PNT samples were pre-cleaned before conducting surface treatments, corrosion, and osteointegration studies.
3.5 SRB Assay
The PBS collected after corrosion tests were analyzed using inductively coupled plasma mass spectroscopy (ICP-MS), ELAN DRC-II for Ni content. The cytotoxicity of Ni ions present in the PBS on human osteoblast cells (hFOB 1.19 cells, ATCC, Manassas, VA, USA) was determined by performing SRB assays. This is a simple, effective, and reproducible method to determine the cytotoxicity of a given liquid on the growth of a particular adherent cell line. It involves the absorption of a negatively charged dye, sulforhodamine B (SRB), by amino acids present in living cells. A measurement of the optical density of the absorbed dye is directly proportional to the number of proliferated cells in the given media or to the cell density. In an effort to further assess the cytotoxicity of the Ni on human osteoblast cells, various concentrations of NiCl2·6H2O powder were added exogenously to the cell culture media. Cytotoxic assays were then performed as previously described.
3.6 Cell Growth
Human osteoblast cells, at a concentration of 40000 cells/mL, were placed on pre-cleaned PNT samples and incubated at 37°C and 5% CO2 for 48 h. After two days, cells were washed with PBS (GIBCO 14190–144, Carlsbad, CA, USA) and then fixed with 25% formalin solution (1:3 formalin and PBS, Thermo-Shandon, 990244, USA) for SEM analysis (Ref 33).
3.7 AFM
The surface roughness of PNT was analyzed using a Veeco Multimode Nanoscope IIIa Atomic Force Microscope (AFM). Scan areas of 10 μm × 10 μm were analyzed on randomly selected surfaces on the rims around the pores.
3.8 Ni Release from PNT Corrosion
The ASTM F 2129–08 (Ref 34) standard was utilized to determine the corrosion resistance of PNT. A typical three-electrode (working, counter, and reference) corrosion cell interphased with a potentiostat (GAMRY) was used, in which the PNT (working electrode) was placed in de-aerated PBS. A saturated Calomel electrode was used as the reference electrode, and carbon as the counter electrode.
An ICP-MS (Perkin Elmer Sciex, model ELAN DRC-II) was employed to determine the concentration of Ni in PBS solution after each corrosion test. 5 mL of PBS was collected for each analysis, and the average concentration of Ni in three replicates of each sample was determined using ICP-MS, and standard deviation (SD) was calculated.
4. Results and Discussion
Untreated (UNT) PNT was subjected to two surface treatments, namely, water-boiling passivation (WB-PA) and dry-heating passivation (DH-PA). Passivation was not conducted as a stand-alone method; it was conducted after dry heating and/or water boiling. Figure 1 shows the variation in the surface roughness of PNT that was subjected to the aforementioned surface treatments. The average roughness of UNT PNT was 40 nm, whereas that of the surface-treated PNT increased to 44 nm for WB-PA PNT and 64 nm for DH-PA PNT. It should be noted that low surface roughness of Nitinol alloys correlates well with a high resistance to pitting and crevice corrosion (Ref 35).
Fig. 1.
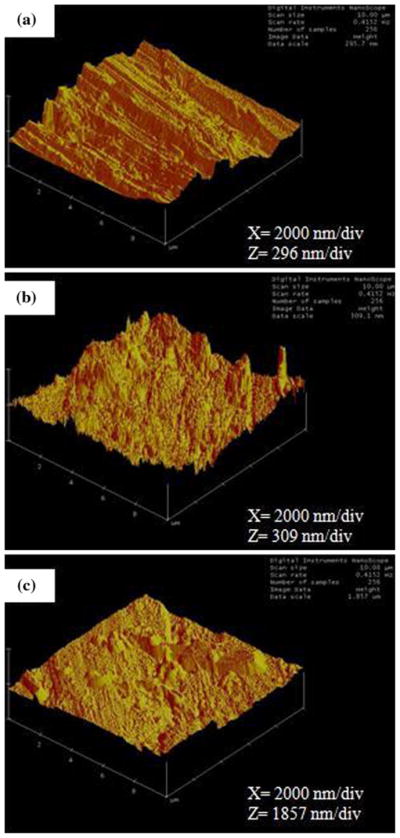
AFM pictures of different surface-treated PNTs: (a) UNT PNT, (b) WB-PA PNT, and (c) DH-PA PNT
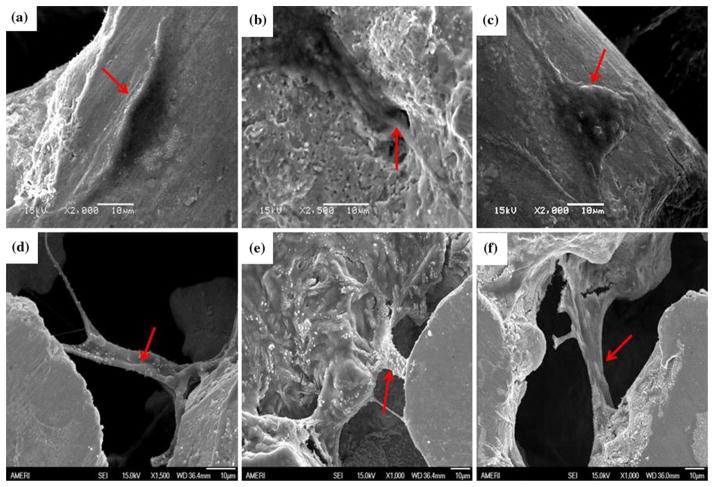
The increase in surface roughness after surface treatment is depicted in the SEM photomicrographs Fig. 2(a)–(c). Furthermore, the surface of the PNTs became pitted after corrosion (Fig. 2d–f). It should be noted that the surfaces depicted in Fig. 1 and 2 correspond to the ridges around the pores of the PNT. The concentration of Ni in the PBS collected after each corrosion test as per our previous study (Ref 36) is shown in Fig. 3. Although the WB-PA PNT appears to be highly pitted, the amount of Ni released (75 ppb) was less than that released from DH-PA and UNT PNTs. This was attributed to the leaching of Ni from the surface of the PNT during WB-PA, which resulted in the formation of a Ni-free passivating oxide layer. XPS analysis of Nitinol alloys subjected to WB-PA (Ref 7, 37) also confirmed the formation of a Ni-free passive layer. In another research (Ref 26), aging of solid Nitinol in boiling water promoted the diffusion of Ni atoms from the oxidized Ti-based surface layers into the surface/water interface and provided suitable conditions for enabling the Ni release.
Fig. 2.
SEM photomicrographs of surface-treated PNTs before corrosion: (a) UNT PNT; (b) WB-PA PNT; (c) DH-PA PNT and after corrosion (d) UNT PNT; (e) WB-PA PNT; and (f) DH-PA PNT
Fig. 3.
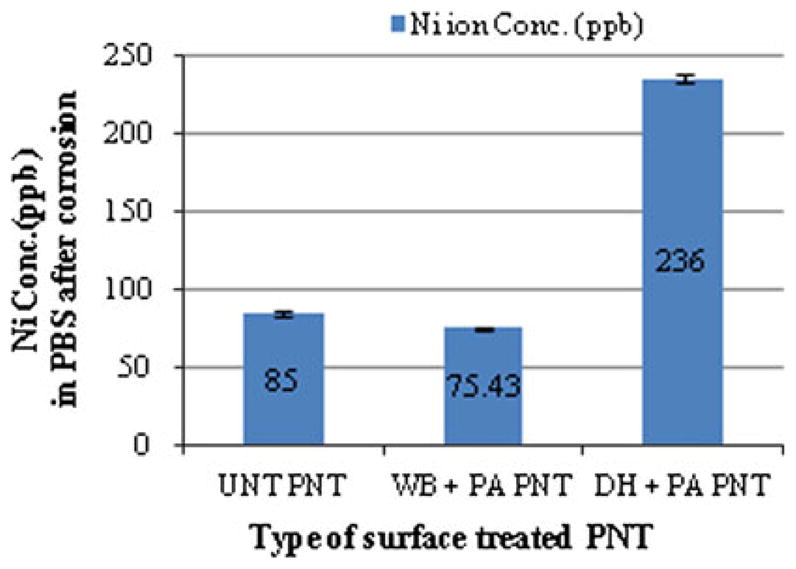
Concentration of Ni released from surface-treated PNT
It has been reported (Ref 29) that dry heating increases the surface concentration of Ni through diffusion at higher temperatures (Ref 28), whereas passivation results in the removal of exogenous atoms from the surface of PNT. In an effort to reduce the release of Ni after implantation, a combination of these two processes would enable the removal of a Ni rich outer layer that is produced by dry heating. Therefore, the high concentration of Ni (236 ppb) released from DH-PA PNT indicates that the passivation time of 20 min at 80 °C was insufficient to remove the Ni rich surface layer from PNT. The high concentration of Ni released from the corrosion of DH-PA PNT may be attributed to the high surface area of PNT as compared with that (70 ppb) from the solid Nitinol (Ref 35).
The concentration of Ni released from the untreated PNT (manufactured by CF-HIP process) in FBS (fetal bovine serum) in immersion tests (Ref 8) over a period of two weeks was found to be in the range of 400–550 ppb. Therefore, Ni release from Nitinol is dependent on its manufacturing process, the type of surface treatment as well as the environment in which it is placed.
The PBS solutions collected after corrosion tests were then exposed to human osteoblast cells to assess Ni cytotoxicity by SRB assay. A comparison of the cytotoxic effect of different concentrations of Ni on human osteoblast cells is shown in Fig. 4. On the X-axis, the highest concentration of Ni to which the osteoblast cells were exposed ranged from 236, 75, and 85 ppb (in the PBS collected after corrosion of DH-PA PNT, WB-PA PNT and UNT PNT respectively, as depicted in Fig. 3 and labeled 100% C in Fig. 4) to zero ppb in the solution labeled 100% P. The Y-axis of Fig. 4 corresponds to the fraction of live osteoblast cells relative to the quantity that would be present in the absence of Ni in solution. It should be noted that the superior response exhibited by WB-PA PNT was because of the lower concentration of Ni released in the PBS after corrosion (75 ppb).
Fig. 4.
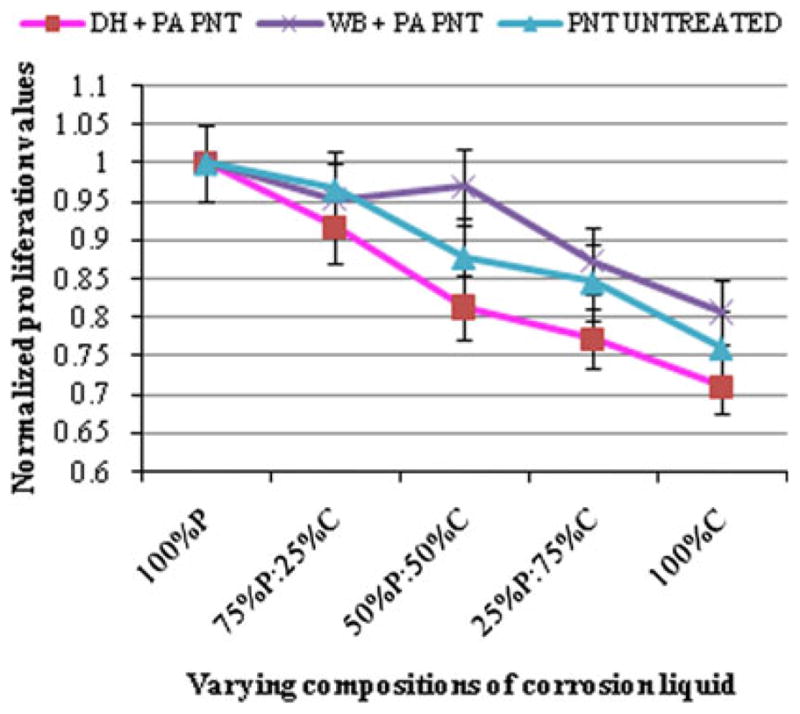
Osteoblast cell viability (normalized fraction of live cells) in. PBS and mixtures of PBS and solutions collected after corrosion of PNTs: P (PBS before corrosion), and C (PBS after corrosion)
Figure 5 shows the SRB assay results, where an increase in Ni concentration produced a decrease in viable cell growth, i.e., an increase in Ni cytotoxicity. In general, the cytotoxic effect of Ni on human osteoblast cells was dependent on the concentration of Ni to which they were exposed. It should be noted that a similar trend in osteoblast cell viability was obtained regardless of whether cells were exposed to Ni in PBS or Ni in cell culture media. For example, when cells were exposed to 100% C (Ni concentration between 75 and 236 ppb as shown in Fig. 4) as well as to cell culture media containing exogenously added Ni (75–200 ppb as shown in Fig. 5), a normalized cell proliferation of about 0.70–0.85 was obtained.
Fig. 5.
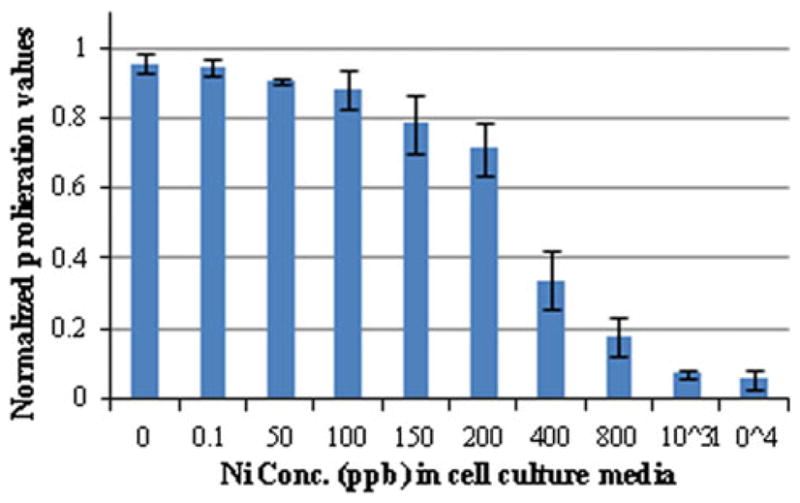
Osteoblast cell viability (normalized fraction of live cells) vs. Ni concentration in cell culture media
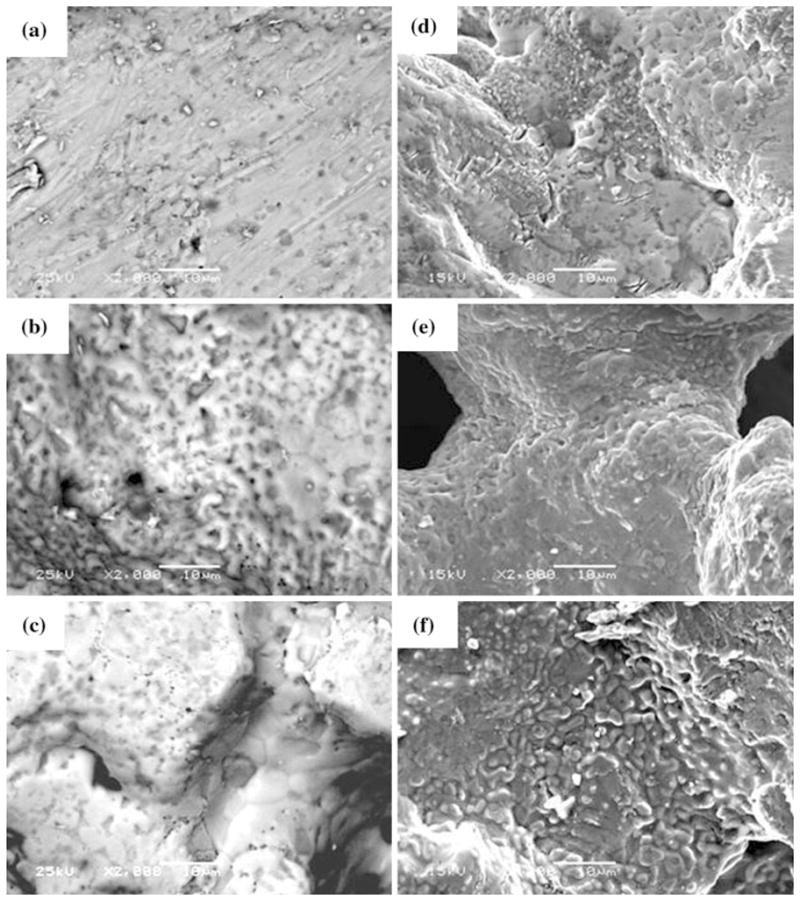
Human osteoblast cells were successfully cultivated on untreated and surface-treated PNTs as shown in Fig. 6. These cells are mononucleate cells that are responsible for bone formation. However, no discernable difference in cell attachment was observed on the different surface-treated PNTs after a 48-h-growth period. Elongated cell growth with the presence of extended fibers between the pores of PNT can be observed in Fig. 6. In order to gain an in-depth understanding of this cell line response to different PNT surfaces, future study needs to be performed on the cellular attachment rates.
Fig. 6.
(a) WB-PA PNT without cell growth; (b) WB-PA PNT with cell growth; (c) Osteoblast cells on WB-PA PNT at lower magnification; Osteoblast cells on (d) UNT PNT; (e) WB-PA PNT; and (f) DH-PA PNT
5. Conclusions
The surface treatments conducted on PNT resulted in an increase of surface roughness. Accelerated corrosion tests produced pits on the surface of the PNT. The WB-PA PNT exhibited the least Ni release during corrosion tests followed by the UNT PNT. Ni exhibited some toxicity on human osteoblast cells at concentrations greater than 50 ppb. A significant toxicity was observed at a Ni concentration greater than 200 ppb. In general, cell viability was independent of the source of Ni, but was dependent on Ni concentration exposure.
Acknowledgments
This research was supported by the Award number SC3GM084816 from the National Institute of General Medical Science.
Footnotes
This article is an invited paper selected from presentations at Shape Memory and Superelastic Technologies 2010, held May 16–20, 2010, in Pacific Grove, California, and has been expanded from the original presentation.
References
- 1.Ryhanen J, Niemie E, Serlo W, Niemela E, Sandvik P, Pernu H, Salo T. Biocompatibility of Nickel-Titanium Shape Memory Metal and Its Corrosion Behavior in Human Cell Cultures. J Biomed Mater Res. 1997;35(4):451–457. doi: 10.1002/(sici)1097-4636(19970615)35:4<451::aid-jbm5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Shabalovskaya SA. Physicochemical and Biological Aspects of Nitinol as a Biomaterial. Int Mater Rev. 2001;46(5):233–250. [Google Scholar]
- 3.Kapanen A, Ivesaro J, Danilov A, Ryhänen J, Lehenkari P, Tuukkanen J. Behaviour of Nitinol in Osteoblast-like ROS-17 Cell Culture. Biomaterials. 2002;23(3):645–650. doi: 10.1016/s0142-9612(01)00143-0. [DOI] [PubMed] [Google Scholar]
- 4.Shabalovskaya SA, Rondelli G, Rettenmayr M. Nitinol Surfaces for Implantation. J Mater Eng Perform. 2009;18(5–6):470–474. [Google Scholar]
- 5.Ho JPY, Wu SL, Poon RWY, Chung CY, Tjong SC. Oxygen Plasma Treatment to Restrain Nickel Out-Diffusion from Porous Nickel Titanium Orthopedic Materials. Surf Coat Technol. 2007;201:4893–4896. [Google Scholar]
- 6.Hallab N, Merritt K, Jacobs JJ. Metal Sensitivity in Patients with Orthopaedic Implants. J Bone Joint Surg Am. 2001;83A(3):428–436. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Haider W, Munroe N, Tek V, McGoron AJ, Gill PKS, Pulletikurthi C, Pandya S. An Assessment of Metal Ions Release from Ternary Nitinol Alloys Under Static and Dynamic Conditions. Proceedings of SMST 2010; May 16–20, 2010; Pacific Grove, CA. 2010. [Google Scholar]
- 8.Wu S, Liu X, Chan YL, Chu PK, Chung CY, Chu C, Yeung KWK. Nickel Release Behavior and Surface Characteristics of Porous NiTi Shape Memory Alloy Modified by Different Chemical Processes. J Biomed Mater Res A. 2009;89:483–489. doi: 10.1002/jbm.a.32008. [DOI] [PubMed] [Google Scholar]
- 9.Bansiddhi A, Sargeant TD, Stupp SI, Dunand DC. Porous NiTi for Bone Implants: A Review. Acta Biomater. 2008;4:773–782. doi: 10.1016/j.actbio.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu L, Lin PH, Chung CY. Characterization of Transformation Behavior in Porous Ni-rich NiTi Shape Memory Alloy Fabricated by Combustion Synthesis. J Mater Sci. 2005;40:773–776. [Google Scholar]
- 11.Cluett ML, Yoe JH. Spectrophotometric Determination of Submicrogram Amounts of Nickel in Human Blood. Anal Chem. 1957;29:1265–1269. [Google Scholar]
- 12.Shen HM, Zhang QF. Risk Assessment of Nickel Carcinogenicity and Occupational Lung Cancer. Environ Health Perspect. 1994;102(1):275–282. doi: 10.1289/ehp.94102s1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cempel M, Polish GN. Nickel: A Review of Its Sources and Environmental Toxicology. J Environ Stud. 2006;15:375–382. [Google Scholar]
- 14.Sunderman FW, Aitio A. Nickel in the Human Environment. Proceedings of a Joint Symposium Held at IARC, International Agency for Research on Cancer; Lyon. March 8–11, 1983; p. 350. [Google Scholar]
- 15.Geetha M, Singh AK, Asokamani R, Gogia AK. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog Mater Sci. 2009;54:397–425. [Google Scholar]
- 16.Brunette DM. The Effects of Implant Surface Topography on the Behavior of Cells. Int J Oral Maxillofac Implants. 1988;3:231–246. [PubMed] [Google Scholar]
- 17.Ong JL, Raikar GN, Lucas LC. Effect of Surface Topography of Titanium on Surface Chemistry and Cellular Response. Implant Dent. 1996;5:83–88. [PubMed] [Google Scholar]
- 18.Bowers KT, Randolph BA, Wick DG, Michaels CM. Optimization of Surface Micromorphology for Enhanced Osteoblast Responses In Vitro. Int J Oral Maxillofac Implants. 1992;7:302–310. [PubMed] [Google Scholar]
- 19.Setzer B, Bachle M, Metzger MC, Kohal RJ. The Gene-Expression and Phenotypic Response of hFOB 1.19 Osteoblasts to Surface-Modified Titanium and Zirconia. Biomaterials. 2009;30:979–990. doi: 10.1016/j.biomaterials.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Gu YW, Li H, Tay BY, Lim CS, Yong MS, Khor KA. In Vitro Bioactivity and Osteoblast Response of Porous NiTi Synthesized by SHS Using Nanocrystalline Ni-Ti Reaction Agent. Surf Coat Technol. 2008;202:2458–2462. doi: 10.1002/jbm.a.30743. [DOI] [PubMed] [Google Scholar]
- 21.Sargeant A, Goswami T. Hip Implants: Paper V. Physiological Effect. Mater Des. 2006;27(4):287–307. [Google Scholar]
- 22.Trépanier C, Leung TK, Tabrizian M, Yahia LH, Bienvenue JG, Tanguay JF, Piron DL, Bilodeau L. Preliminary Investigation of the Effects of Surface Treatments on Biological Response to Shape Memory NiTi Stents. J Biomed Mater Res. 1999;48:165–171. doi: 10.1002/(sici)1097-4636(1999)48:2<165::aid-jbm11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Wirth C, Comte V, Lagneau C, Exbrayat P, Lissac M, Renault NJ, Ponsonnet L. Nitinol Surface Roughness Modulates In Vitro Cell Response: A Comparison Between Fibroblasts and Osteoblasts. Mater Sci Eng C. 2005;25:51–60. [Google Scholar]
- 24.Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC. The Optimum Pore Size for the Fixation of Porous-Surfaced Metal Implants by the Ingrowth of Bone. Clin Orthop Relat Res. 1980;150:263–270. [PubMed] [Google Scholar]
- 25.Shabalovskaya SA, Andreas GCR, Undisz L, Anderegg JW, Burleigh TD, Rettenmayr ME. The Electrochemical Characteristics of Native Nitinol Surfaces. Biomaterials. 2009;30:3662–3671. doi: 10.1016/j.biomaterials.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Shabalovskaya SA, Anderegg J, Laab F, Thiel PA, Rondelli G. Surface Conditions of Nitinol Wires, Tubing, and As-Cast Alloys, the Effect of Chemical Etching, Aging in Boiling Water, and Heat Treatment. J Biomed Mater Res B Appl Biomater. 2003;65(B):193–203. doi: 10.1002/jbm.b.10001. [DOI] [PubMed] [Google Scholar]
- 27.Shabalovskaya SA, Anderegg J, Humbeeck JV. Critical Overview of Nitinol Surfaces and Their Modifications for Medical Applications. Acta Biomater. 2008;4:447–467. doi: 10.1016/j.actbio.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Brien BO, Carroll WM, Kelly MJ. Passivation of Nitinol Wire for Vascular Implants—A Demonstration of the Benefits. Biomaterials. 2002;23(8):1739–1748. doi: 10.1016/s0142-9612(01)00299-x. [DOI] [PubMed] [Google Scholar]
- 29.Tepe G, Schmehl J, Wendel HP, Schaffner S, Heller S, Gianotti M, Claussen CD, Duda SH. Reduced Thrombogenicity of Nitinol Stents—In Vitro Evaluation of Different Surface Modifications and Coatings. Biomaterials. 2006;27:643–650. doi: 10.1016/j.biomaterials.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Bertheville B. Porous Single-Phase NiTi Processed Under Ca Reducing Vapor for Use as a Bone Graft Substitute. Biomaterials. 2006;27(8):1246–1250. doi: 10.1016/j.biomaterials.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Es-Souni M, Es-Souni M, Brandies HF. On the Properties of Two Binary NiTi Shape Memory Alloys. Effects of Surface Finish on the Corrosion Behaviour and In Vitro Biocompatibility. Biomaterials. 2002;23:2887–2894. doi: 10.1016/s0142-9612(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 32.Chrzanowski W, Neel EA, Armitage D, Knowles J. Effect of Surface Treatment on the Bioactivity of Nickel-Titanium. Acta Biomater. 2008;4:1969–1984. doi: 10.1016/j.actbio.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Terceroa JE, Namin S, Lahiri D, Balani K, Tsoukias N, Agarwal A. Effect of Carbon Nanotube and Aluminum Oxide Addition on Plasma-Sprayed Hydroxyapatite Coating’s Mechanical Properties and Biocompatibility. Mater Sci Eng C. 2009;29(7):2195–2202. [Google Scholar]
- 34.Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices, ASTM F2129 – 08Annual Book of ASTM Standards, vol. 13.01
- 35.Haider W. PhD dissertation. Florida International University; Miami, FL, USA: 2010. Enhanced Biocompatibility of NiTi (Nitinol) via Surface Treatment and Alloying. [Google Scholar]
- 36.Munroe N, Pulletikurthi C, Haider W. Enhaced Biocompatibility of Porous Nitinol. J Mater Eng Perform. 2009;18(5–6):765–767. doi: 10.1007/s11665-009-9454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haider W, Munroe N, Pulletikurthi C. A Comparative Biocompatibility Analysis of Ternary Nitinol Alloys. J Mater Eng Perform. 2009;18(5–6):760–764. doi: 10.1007/s11665-009-9435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]