Abstract
New methods are needed for the nondestructive measurement of tooth demineralization and remineralization to monitor the progression of incipient caries lesions (tooth decay) for effective nonsurgical intervention and to evaluate the performance of anticaries treatments such as chemical treatments or laser irradiation. Studies have shown that optical coherence tomography (OCT) has great potential to fulfill this role since it can be used to measure the depth and severity of early lesions with an axial resolution exceeding 10 µm, it is easy to apply in vivo and it can be used to image the convoluted topography of tooth occlusal surfaces. In this paper, a review of the use of polarization-sensitive-OCT for the measurement of tooth demineralization is provided along with some recent results regarding improved methods for the detection of caries lesions in the earliest stages of development. Automated methods of analysis were used to measure the depth and severity of demineralized bovine enamel produced using simulated caries models that emulate demineralization in the mouth. Significant differences in the depth and integrated reflectivity from the lesions were detected after only a few hours of demineralization. These results demonstrate that cross-polarization-OCT is ideally suited for the nondestructive assessment of early demineralization.
Index Terms: Dental caries, polarization-sensitive-optical coherence tomography (PS-OCT), tooth demineralization
I. INTRODUCTION
New methods are needed for the nondestructive measurement of tooth demineralization and remineralization to monitor the progression of incipient caries lesions (tooth decay) for effective nonsurgical intervention and to evaluate the performance of anticaries treatments such as chemical treatments or laser irradiation. A nondestructive, quantitative method of monitoring demineralization and remineralization “in vivo” with high sensitivity would be invaluable for use in short-term clinical trials for various anticaries agents such as fluoride dentifrices and antimicrobials, particularly in high risk areas of the tooth such as the pits and fissures of the occlusal surfaces. Optical coherence tomography (OCT) is uniquely capable of this task since it provides a measure of the reflectivity from each layer of the lesion and is able to show the formation of a zone of increased mineral density and reduced light scattering due to remineralization. OCT is not only valuable as a nondestructive tool for the assessment of anticaries agents in vivo but is also valuable for in vitro studies as well since it does not require thin sectioning, and it can be carried out rapidly.
Several studies have demonstrated that OCT can be used to nondestructively measure the severity of subsurface demineralization in enamel and dentin [1]–[7]. The first images of hard tissue structures of the oral cavity and dental caries were acquired by Colston et al. [8]–[10]. Soon after, Baumgartner et al. [11]–[13] presented the first polarization-resolved images of dental caries and Feldchtein et al. [14] presented the first in vivo images of enamel and dentin caries and restorations. Wang et al. [15] used polarization-sensitive (PS)-OCT to measure the birefringence in dentin and enamel. In 1999, Everett et al. [16] acquired polarization-resolved images by using a high power 1310-nm broadband source and a bulk optic PS-OCT system and changes in the mineral density of tooth enamel were resolvable to depths of 2–3 mm into the tooth. Later measurements demonstrated that polarization sensitivity provides considerable advantages for the measurement of lesion severity and for tracking lesion progression [3]–[7], [16], [17].
Although the first OCT images of dental hard tissues included images of caries lesions that clearly indicated that there was increased reflectivity in the areas of decay, it was not until a few years later that serious attempts were made to quantify the severity of caries lesions from OCT images. Since areas of demineralization appear with increased reflectivity in the OCT images the most obvious approach is to directly measure the reflectivity from the lesion area and use that as a measure of lesion severity. However, the high refractive index of dental hard tissue produces a strong reflection at the tooth surface. Even with a high-resolution OCT system with an axial depth resolution of less than 10 µm, the strong surface reflection can dominate the reflectively at greater depths from the surface. Amaechi et al. [1], [2], [18] demonstrated that the loss of penetration depth in conventional OCT images correlated well with the mineral loss measured with microradiography for shallow artificial lesions on flat surfaces. However, since the penetration depth depends on many factors it is problematic to define, and therefore, difficult to use as a quantitative measurement, particularly on convoluted surfaces and for lesions that are not uniform.
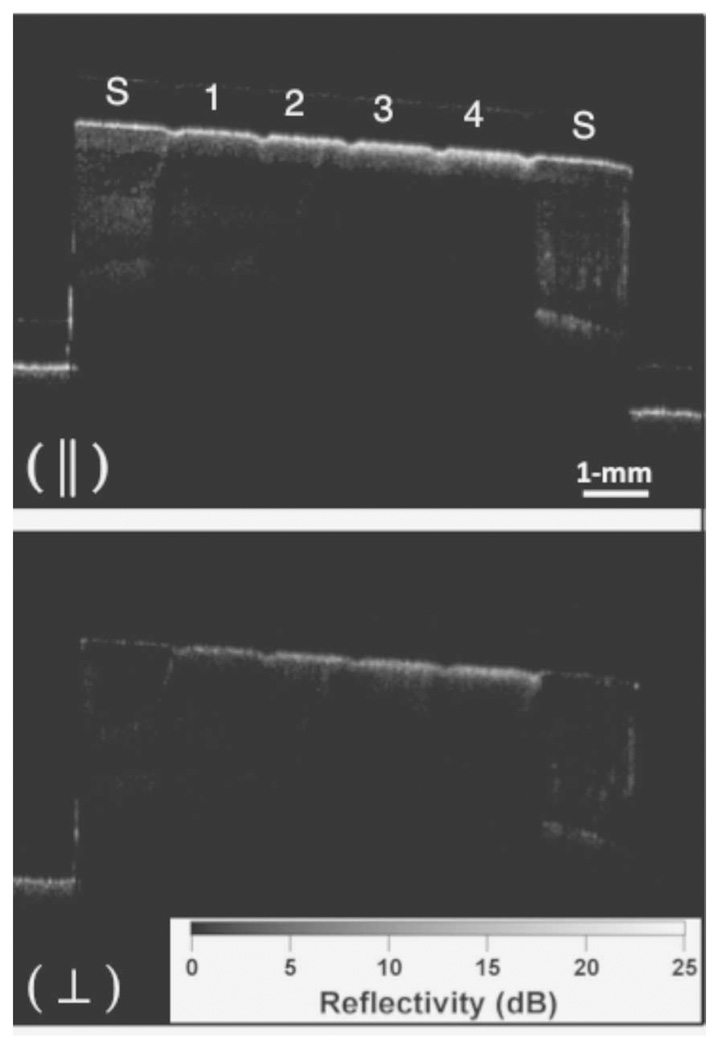
In our approach to quantifying demineralization, we deliver polarized light to the tooth surface and measure the reflectivity in both orthogonal polarizations states [3], [4] using a PS-OCT system. The reflection from the tooth surface can be reduced by 20–30 dB in the cross-polarization image. Moreover, the reflectivity from sound enamel is suppressed and there is higher contrast between sound and demineralized enamel in the cross-polarization image (see Fig. 1). In most implementations of PS-OCT, the incident light is circularly polarized to reduce the influence of the tissue orientation and the reflected light is measured in both orthogonal polarizations (left and right circularly polarized light) [12], [13], [16], [19]. The reflectivity from both polarization states are subsequently combined to yield either intensity, phase retardation or degree of polarization images that are calculated from the images of both orthogonal polarizations. Most groups using PS-OCT are interested in the measurement of tissue birefringence and have focused their efforts on images of phase retardation. More sophisticated configurations have also been implemented to measure the Stokes parameters and the full Mueller matrix [20], [21]. Since we are more interested in increasing the contrast of the lesions and decreasing the strong reflection at the tooth surface, the cross-polarization image or the image in the orthogonal polarization to the light incident on the tooth is most useful.
Fig. 1.
PS-OCT b-scan images of one of the samples after 8-h period of demineralization created using the surface softened lesion model. The sound areas on each end of the samples are marked (S) and the four areas of increasing demineralization separated by the laser incisions are labeled 1–4. Only the orthogonal polarization image or cross polarization (⊥) was used for analysis in these studies.
The gold standard for quantifying lesion severity and tooth surface and subsurface demineralization is transverse microradiography (TMR) [22]–[26]. The lesion severity is typically reported as relative mineral loss, representing the vol.% mineral loss versus depth, ΔZ (vol.% × µm). Therefore, it is advantageous to be able to report a similar measure using optical methods. The reflectivity from the lesion area in the cross-polarization image provides an analogous measurement to mineral loss (ΔZ). We call that cross-sectional area ΔR (reflectivity (dB units) × µm) a measurement analogous to ΔZ (vol.% × µm) [4], [27].
OCT has been applied to the longitudinal monitoring of enamel erosion and demineralization employing in vitro caries-like lesion models [1]–[4], [7], [18], [28]–[32]. It has also been employed successfully to measure the inhibition of decay by various agents for delivering fluoride and after thermal modification of enamel and dentin by laser irradiation [31], [33]. In the most basic model, the erosion model, a severe acid challenge erodes away the tooth surface. However, simple models employing rapid demineralization do not realistically represent natural caries lesions that slowly develop in the mouth and typically maintain an intact surface while a subsurface lesion forms. The surface softened model is more realistic and produces subsurface demineralization without erosion of the surface [4], [32]. The pH cycling model employs a cycle of demineralization and remineralization that better replicates what transpires in vivo, and creates a greater surface zone. Another advantage of OCT is that analysis of demineralization can be automated and algorithms can be applied to automatically identify the lesion depth and the lesion severity [32]. Similar studies have also been carried out on dentin and cementum surfaces [34]–[36].
One of the most exciting applications of OCT is the measurement of the remineralization or repair of existing lesions. Not only is it capable of measuring a decrease in reflectivity due to the increased mineral content that results from new mineral deposition, it is also capable of acquiring images of the outer layer of high mineral content that may be formed as the mineral is preferentially deposited in the outer most layer of the lesion [5], [6], [37], [38]. Surfaces of arrested lesions are typically hard and shiny with less light scattering, in contrast to the soft and chalky surface of active lesions. The presence of a substantial surface zone of reduced light scattering in the OCT image indicative of remineralization may indicate the lesion is arrested. PS-OCT images are particularly valuable for resolving these highly mineralized surface zones since the image in the original incident polarization shows the position of the lesion surface while the cross-polarization image shows the thickness of the more transparent surface zone. Studies have shown that remineralization requires the presence of the residual partially dissolved crystals to serve as a template for growth [39]. Furthermore, remineralization has been observed to proceed from the outside of the lesion toward the lesion body, therefore, as the remineralization takes place in the surface zone of the lesion the diffusion pathways to the lesion body are blocked, thus preventing further remineralization of the lesion body. PS-OCT images of lesions in dentin exposed to a remineralization solution also show similar results to what was observed for enamel [36].
The purpose of the study presented in this paper was to develop improved methods for measurement of early enamel demineralization employing cross-polarization OCT. Two models of demineralization, the surface softened and pH cycling models were used to produce early lesions and automated methods of analysis were employed to measure the depth and severity of the demineralized bovine enamel to determine the earliest stage at which the lesions could be detected.
II. METHODS
A. Sample Preparation and Simulated Lesion Models
Enamel blocks, approximately 8–12 mm in length with a width of ~3-mm and a thickness of 2 mm of bovine enamel were prepared from extracted bovine tooth incisors acquired from a slaughterhouse. Each enamel sample was partitioned into six regions or windows (two sound and four lesion areas) by etching small incisions 1.4-mm apart across each of the enamel blocks using a laser (see Fig. 1). Incisions were etched using a transverse excited atmospheric pressure (TEA) CO2 laser operating at 9.3 µm, Impact 2500, GSI Lumonics (Rugby, U.K.). The incision area also has an increased resistance to acid dissolution that serves to more effectively isolate each group [33]. A thin layer of acid resistant varnish in the form of red nail polish, Revlon (New York, NY) was applied to protect the sound enamel control area on each end of the block before exposure to the demineralization solutions. The samples were immersed in demineralization solutions for different time periods to produce lesions of varying severity. Two dissolution models were employed. The first model the surface softened lesion model produces subsurface demineralization without erosion of the surface. The mineral loss profiles are fairly uniform in these lesions, and they emulate a very active lesion. The pH cycling better simulates the slowly developing lesions in the mouth and this model employs cycles of demineralization followed by remineralization to produce lesions that are less severe and contain an outer zone that is more highly mineralized than the body of the lesion. Surface softened lesions were produced on 30 enamel blocks. There were six windows on each sample and the blocks were exposed to a demineralization solution at pH 4.8 composed of a 40-mL aliquot of 2.0 mmol/L calcium, 2.0 mmol/L phosphate, and 0.075 mol/L acetate for either four 1-h periods (1–4 h), four 4-h periods (4–16 h), or four 8-h periods (8–32-h) with ten blocks used for each series. The groups overlapped each other by one period, so that they could be compared to ensure consistency.
Each sample was then placed into the demineralization solution and incubated at 37 °C. After each period of demineralization, one region of each sample was covered with a thin layer of the same acid resistant varnish to prevent further demineralization. After the fourth period, the samples were removed from the demineralization solution, and the acid resistant varnish was removed using acetone. Each sample was then stored in 0.1% thymol solution to prevent fungal and bacterial growth.
Lesions were also produced on ten bovine blocks using the pH cycling model [40]. Samples were cycled for four 1-day periods. The procedures were identical to those described earlier with the exception that during each 1-day period the samples were placed in the same demineralization solution described earlier at the same pH 4.8 for 6 h each day, rinsed and then placed for 17 h in a remineralization solution. The remineralization solution was composed of 1.5-mmol/L calcium, 0.9-mmol/L phosphate, 150-mmol/L KCl, and 20-mmol/L cacodylate buffer maintained at pH 7.0 and 37 °C.
B. PS-OCT System
An all-fiber-based optical coherence domain reflectometry (OCDR) system with polarization maintaining (PM) optical fiber, high-speed piezoelectric fiber-stretchers and two balanced InGaAs receivers that was designed and fabricated by Optiphase, Inc., Van Nuys, CA. This two-channel system was integrated with a broadband superluminescent diode (SLD) Denselight (Jessup, MD) and a high-speed XY-scanning system (ESP 300 controller and 850G-HS stages, National Instruments, Austin, TX) for in vitro optical tomography. This system is based on a polarization-sensitive Michelson white light interferometer. The high power (15 mW) polarized SLD source operated at a center wavelength of 1317 nm with a spectral bandwidth full-width at half-maximum (FWHM) of 84 nm was aligned using polarization controller to deliver 15 mW into the slow axis of the PM fiber of the source arm of the interferometer. This light was split into the reference and sample arms of the Michelson interferometer by a 50/50 PM-fiber coupler. The sample arm was coupled to an AR-coated fiber-collimator to produce a 6-mm in diameter, collimated beam. That beam was focused onto the sample surface using a 20-mm focal length AR-coated planoconvex lens. This configuration provided axial and lateral resolution of approximately 20 µm with a signal to noise ratio of greater than 40–50 dB. Both orthogonal polarization states of the light scattered from the tissue are coupled into the slow and fast axes of the PM fiber of the sample arm. A quarter wave plate set at 22.5° to horizontal in the reference arm rotated the polarization of the light by 45° upon reflection. After being reflected from the reference mirror and the sample, the reference beams were recombined by the PM fiber coupler. A polarizing cube splits the recombined beam into its horizontal and vertical polarization components or “slow” and “fast” axis components, which were then coupled by single mode fiber optics into two detectors. The light from the reference arm was polarized at 45°, and therefore, split evenly between the two detectors. Readings of the electronically demodulated signal from each receiver channel represent the intensity for each orthogonal polarization of the backscattered light. Neutral density filters are added to the reference arm to reduce the intensity noise for shot limited detection. The all-fiber-based OCDR system is described in [41]. The PS-OCT system is completely controlled using Labview software (National Instruments, Austin, TX). Acquired scans are compiled into b-scan files. Image processing was carried out using Igor Pro, data analysis software (Wavemetrics Inc., Lake Oswego, OR).
PS-OCT scans acquired from PM fiber-based PS-OCT systems typically contain artifacts (additional peaks) due to crosstalk and the limited extinction ratio of the fiber that may confound analysis. Automated removal of such artifacts can be carried out successfully with a few extra data alteration steps after data collection. A reference a-scan was acquired from a mirror prior to scanning the samples. The reference a-scan contains several weak artifact signals along with the primary reflection. A smaller 400-point a-scan array was extracted from the 2000-point reference a-scan containing the principal artifacts. The reference array was normalized to the intensity of the point of interest and subtracted to selectively remove the artifacts.
C. Calculation of Integrated Reflectivity and Lesion Depth
The integrated reflectivity ΔR in units of (dB × µm) was calculated for each of the five windows (one sound, four demineralized) for every sample. Line profiles were taken from cross-polarization OCT images in each of the five regions, and the reflectivity was integrated from the enamel surface to various depths, yielding the integrated reflectivity ΔR of the regions in units of decibels per micrometer. Previous studies have shown that ΔR can be correlated with the integrated mineral loss (vol.% mineral × µm) called ΔZ [4, 27].
An initial background subtraction was carried out for each OCT scan and a 2 × 2 convolution filter was applied to remove speckle noise. In the edge-detection approach, the enamel edge and the lower lesion boundary were determined by applying an edge locator. Two passes were required for each a-scan to locate each respective boundary with each pass starting from opposite ends of the a-scan and identifying the first pixel that exceeds the threshold of e−2 of the maximum value. The minimum threshold values for edge detection were previously experimentally determined by comparison of lesion depths measured using polarized light microcopy with measurements using OCT in order to avoid overestimation of lesion depth due to weak signals caused by birefringence in sound enamel [32]. Distance (micrometer) per pixel conversion factor was obtained experimentally by system calibration. The two cutoff points for the lesion surface and endpoint represent the calculated lesion depth and the integration between these two positions represents the integrated reflectivity. A 1-mm square area was chosen for analysis in the center of each of the 1.4-mm by 3-mm areas demarcating each group on each sample. Therefore, 400 a-scans were analyzed for each group.
Typically there are large variation in the depth and integrated mineral loss from sample-to-sample for these types of demineralization experiments resulting in large standard deviations for each group. Sample groups were compared using repeated measures analysis of variance (ANOVA) with a Tukey–Kramer post hoc multiple comparison test. Having all the study groups (five) for each series on each sample allowed us to decrease intersample variability. InStat from GraphPad software (San Diego, CA) was used for statistical calculations.
III. RESULTS
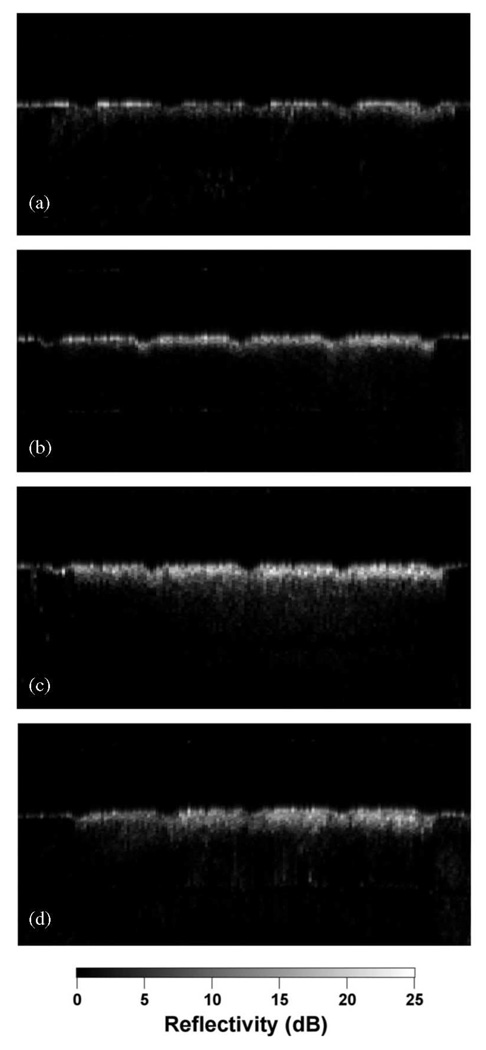
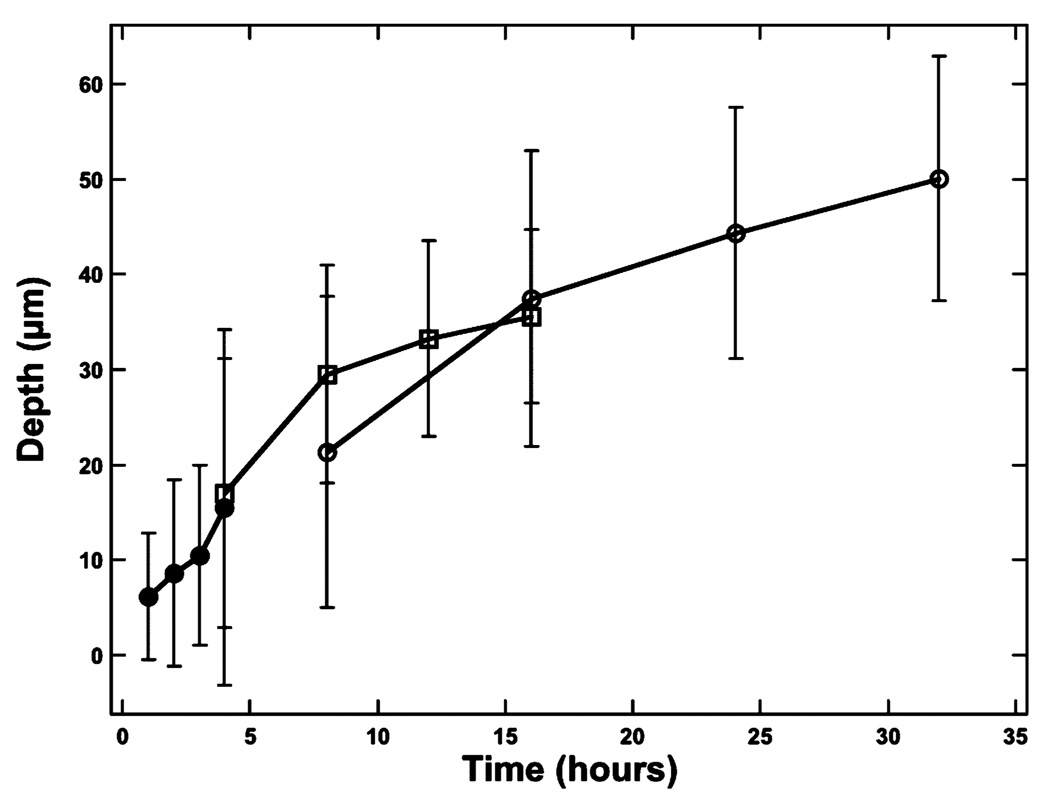
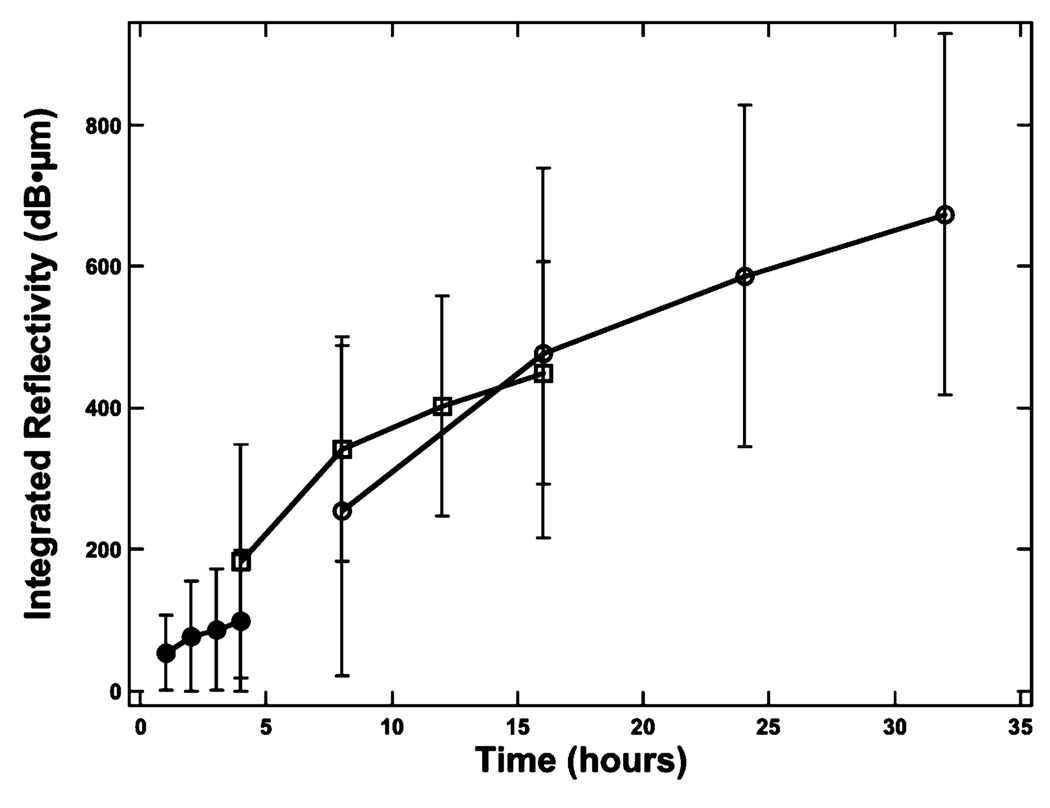
Fig. 1 shows PS-OCT images from one of the samples containing lesions produced in 8-h intervals. Linearly polarized light was incident on the sample and the reflected light was measured in the same polarization (‖) to the incident light and also in the orthogonal polarization (⊥) to the incident light. It is much harder to see the lesions in the original (‖) polarization than in the orthogonal polarization or cross-polarization image due to the intense reflectivity at the surface. Fig. 2 shows sample cross-polarization images from all four demineralization groups. With the exception of the first group representing only 1-h intervals of demineralization, differences in the depth of the lesions in each group are visible in the individual scans. PS-OCT was able to detect significant differences between most of the groups in each demineralization series with the exception of the 1-h demineralization period as can be seen in Table I. The lesion and depth and the integrated reflectivity were plotted for the three “softened surface” groups representing the 1-, 4-, and 8-h intervals in Figs. 3 and 4. PS-OCT was able to detect significant differences in the integrated reflectivity from the lesion area only after 2 h of demineralization and significant differences in lesion depth between the sound and demineralized groups only after 4 h of demineralization. Analysis of the cross-polarization OCT scans suggests that lesions as small as 10–20 µm were detected. Such small lesions are much smaller than the lesions previously measured with OCT.
Fig. 2.
Cross-polarization images of samples from each of the four different series of demineralization. (a) Softened surface (SS) model with 1-h periods of demineralization (1–4 h). (b) SS model with 4-h periods of demineralization (4–16 h). (c) SS model with 8-h periods of demineralization (8–32 h). (d) pH cycling model with 1-day cycling periods of 6-h demineralization followed by 17 h of remineralization.
TABLE I.
Depth and integrated reflectivity for each group
| Group | Depth (µm) Mean (S.D.) |
Integrated Reflectivity (dB × µm) Mean (S.D.) |
|---|---|---|
| SSG1 | ||
| 0 | 0.52 (0.82) a | 4.68 (8.08) a |
| 1 | 6.23 (6.63) a, b | 53.4 (63.6) a, b |
| 2 | 8.61 (9.76) a, b | 78.2 (93.0) b |
| 3 | 10.6 (9.50) a, b | 86.1 (90.7) b |
| 4 hr | 15.6 (18.6) b | 99.4 (105) b |
| SSG2 | ||
| 0 | 1.95 (3.84) a | 20.9 (41.2) a |
| 4 | 17.1 (14.1) b | 183 (164) b |
| 8 | 29.5 (11.4) c | 343 (159) c |
| 12 | 33.2 (10.2) c | 402 (155) c, d |
| 16 hr | 35.6 (9.10) c | 449 (158) d |
| SSG3 | ||
| 0 | 1.49 (3.07) a | 17.0 (35.6) a |
| 8 | 21.4 (16.3) b | 254 (233) b |
| 16 | 37.5 (15.6) c | 477 (261) c |
| 24 | 44.4 (13.1) c, d | 586 (242) c, d |
| 32 hr | 50.0 (12.8) d | 674 (255) d |
| pH Cycled | ||
| 0 | 0.47 (0.85) a | 5.08 (9.04) a |
| 24 | 9.58 (10.4) a, b | 95.0 (145) a, b |
| 48 | 16.4 (12.8) b, c | 174 (141) b |
| 72 | 19.0 (13.5) c | 191 (140) b |
| 96 hr | 20.4 (14.5) c | 201 (145) c |
Surface softened (SS): Groups from each of the four series that were statistically simillar (p > 0.05) have the same letter.
Fig. 3.
Plot of the mean ± S.D. lesion depth for each sample group calculated for the surface softened lesions. The solid circles represent the 1-h periods, the open square represents the 4-h periods, and the open circles represent the 8-h periods of demineralization.
Fig. 4.
Plot of the mean ± S.D. integrated reflectivity for each sample group calculated for the surface softened lesions. The solid circles represent the 1-h periods, the open square represents the 4-h periods, and the open circles represent the 8-h periods of demineralization.
It is more difficult to detect differences between the different groups exposed to demineralization in the pH cycling model since lesions are less severe and the surface zone is more highly mineralized in the lesions presenting less contrast with sound tissues. Each day of pH cycling involves 6 h of demineralization followed by 17 h of remineralization. The mean lesion depth as measured by OCT was on the order of 10 µm; therefore, these lesions are comparable in severity to the straight 1-h demineralization samples. Significant differences in both the lesion depth and integrated reflectivity were visible after two days of pH cycling suggesting similar performance to the “surface softened” lesions.
IV. DISCUSSION
These measurements demonstrate that a PS-OCT system operating with an axial resolution of 9 µm in air (SLD band width 84 nm) and 6 µm in enamel at 1317 nm is capable of detecting extremely shallow lesions. The use of algorithms to automatically calculate the depth and integrated reflectivity from the lesion area were essential since the edge detection approach enabled reliable calculation of the lesion depth and the automatic processing allowed analysis of large areas on each sample area. In previous PS-OCT studies, the severity of lesion areas was accessed by integration of single a-scans [3]–[7], [16], [17]. Better discrimination of lesion areas is possible if the entire lesion cross-section area or volume is integrated as opposed to a single a-scan since such lesions are seldom uniform. Since this approach involves large volumes of data, it is only practical with the development of algorithms for automated processing. This becomes even more important for the efficient implementation of fast Fourier domain (FD) OCT systems that are capable of the acquisition of entire lesion volumes, e.g., 2 × 2 × 3 mm3 volumes, at video rates.
Being able to analyze the entire lesion area provides a larger effective sample size and also reduces variability caused by variations in the lesion severity over each sample window. It is important to point out that we have yet to analyze these samples using transverse microradiography and polarized light microscopy that will provide a gold standard for comparison. This analysis requires complete destruction of the samples, and we have withheld this analysis since the samples are also being analyzed using other imaging methods.
Two simulated lesion models were utilized, which produce subsurface lesions while maintaining an intact surface without erosion. The “surface softened” model simulates a highly active incipient lesion without a highly mineralized outer layer. The “pH cycling” model simulates a more slowly developing lesion by replicating periods of demineralization followed by remineralization. This is what occurs naturally in the mouth and this model produces lesions with a highly mineralized outer layer since the outer pores of the lesion produced by the demineralization periods are filled preferentially by mineral during the remineralization cycle. Thus, the pH cycling model produces less dramatic lesion gradients that are more difficult to detect.
V. CONCLUSION AND FUTURE DIRECTIONS
PS-OCT was effective for both lesion models and using the automated analysis, lesions were detected far earlier than was achieved in early PS-OCT imaging studies [3], [4], [32]. It is feasible to apply this approach in vivo for clinical studies to evaluate the efficacy of various anticaries agents such as different formulations of fluoride or laser treatments.
High speed Fourier domain systems (FD-OCT) are now available that can be operated with or without polarization sensitivity. Such systems are capable of scanning at real time video rates and are capable of acquiring images of the entire tooth or a large area of interest without motion artifacts. This is a major step forward for demineralization and remineralization studies since it is now possible to monitor the entire lesion and greatly facilitates matching OCT images to histology. These systems have already been investigated for imaging dental caries [42]–[45]. Systems employing either spectral domain or swept laser approaches were used to acquire images with high resolution and can be modified to also have large scan ranges even though most of the turn key systems currently available have a scan range limited to under 4 mm, which is a problem for scanning dental hard tissue due to the occlusal surface topography and the high refractive index of dental enamel 1.63.
Pilot studies on both artificial lesions and natural caries lesions suggest that it is also feasible to combine OCT with laser ablation for highly selective image guided ablation, namely a CAD/CAM system for removing dental caries [46], [47]. OCT images of dental decay were employed to program a scanned laser beam to remove only decay. Images were acquired of demineralized enamel surfaces in which specific lesion patterns were generated and those images were used to program a CO2 laser to selectively remove them. OCT images were acquired before and after removal, and the scans demonstrate the highly selective removal.
Acknowledgments
This work was supported in part by the National Institute of Health under Grant R01-DE17869.
Biographies
Hobin Kang received the B.S. degree in mechanical engineering from the University of California, Berkeley, in 2008.
He is currently a Staff Research Associate in the Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco. His research interests include optical imaging techniques and dental biomaterials.
Jian J. Jiao is currently working toward the four year undergraduate degree in integrative biology at the University of California, Berkeley.
She is also a Laboratory Assistant in the Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco. Her research interests include dentistry, biology, and optical imaging techniques.
Chulsung Lee received the B.Sc. degree in bioengineering from the University of California, Berkeley. He is currently working toward the D.D.S. and Ph.D. degrees at the University of California, San Francisco.
He was a Staff Research Associate in the Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco. His research interests include optical imaging techniques and biomaterials.
Michael H. Le received the B.Sc. degree in electrical engineering and computer science, and bioengineering from the University of California, Berkeley, in 2007. He is currently working toward the D.D.S. and Ph.D. degrees in oral and craniofacial sciences, Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco.
His current research interests include optical imaging techniques.
Cynthia L. Darling received the Ph.D. degree in physical chemistry from Wayne State University, Detroit, MI, in 1993.
She is currently an Assistant Professor in the Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco. She has been a Researcher in the field of biophotonics for the past seven years and has made contributions to the development of digital microradiography for the assessment of enamel and dentin mineral content, near-IR imaging for caries detection, and polarization resolved optical property measurements. Her thesis work during the Ph.D. degree involved electronic structure theory in quantum mechanics.
Daniel Fried received the Ph.D. degree in physical chemistry from Wayne State University, Detroit, MI, in 1992.
He is currently a Professor in the Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco. He has been a Researcher in the field of biophotonics for the past 15 years and his contributions in this field include fundamental measurements of the optical properties of dental hard tissues from the ultraviolet to the infrared (IR), studies of the interaction of carbon dioxide lasers with dental hard tissues for laser ablation of caries and the surface modification of enamel for caries prevention, the use of lasers for the selective removal of caries and composite restorative materials, the assessment of demineralization and remineralization with polarization sensitive optical coherence tomography, and the development of near-IR imaging for caries detection.
Contributor Information
Hobin Kang, Email: hobin.kang@ucsf.edu, Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco, CA 94143-0758 USA.
Jian J. Jiao, Email: jiaojj@berkeley.edu, University of California, Berkeley, CA 94720-1776 USA, and also with the Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco, CA 94143-0758 USA.
Chulsung Lee, Email: ChulSung.Lee@ucsf.edu, Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco, CA 94143-0758 USA.
Michael H. Le, Email: michael.Le@ucsf.edu, Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco, CA 94143-0758 USA.
Cynthia L. Darling, Email: cynthia.darling@ucsf.edu, Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco, CA 94143-0758 USA.
Daniel Fried, Email: daniel.fried@ucsf.edu, Division of Biomaterials and Bioengineering, Department of Preventative and Restorative Dental Sciences, San Francisco School of Dentistry, University of California, San Francisco, CA 94143-0758 USA.
REFERENCES
- 1.Amaechi BT, Higham SM, Podoleanu A, Rodgers JA, Jackson DA. Use of optical coherence tomography for assessment of dental caries. J. Oral Rehabil. 2001;vol. 28(no. 12):1092–1093. doi: 10.1046/j.1365-2842.2001.00840.x. [DOI] [PubMed] [Google Scholar]
- 2.Amaechi BT, Podoleanu A, Higham SM, Jackson DA. Correlation of quantitative light-induced fluorescence and optical coherence tomography applied for detection and quantification of early dental caries. J. Biomed. Opt. 2003;vol. 8(no. 4):642–647. doi: 10.1117/1.1606685. [DOI] [PubMed] [Google Scholar]
- 3.Fried D, Xie J, Shafi S, Featherstone JDB, Breunig T, Lee CQ. Early detection of dental caries and lesion progression with polarization sensitive optical coherence tomography. J. Biomed. Opt. 2002;vol. 7(no. 4):618–627. doi: 10.1117/1.1509752. [DOI] [PubMed] [Google Scholar]
- 4.Jones RS, Darling CL, Featherstone JDB, Fried D. Imaging artificial caries on occlusal surfaces with polarization sensitive optical coherence tomography. Caries Res. 2004;vol. 40(no. 2):81–89. doi: 10.1159/000091052. [DOI] [PubMed] [Google Scholar]
- 5.Jones RS, Darling CL, Featherstone JDB, Fried D. Remineralization of in vitro dental caries assessed with polarization sensitive optical coherence tomography. J. Biomed. Opt. 2006;vol. 11(no. 1):014016-1–014016-9. doi: 10.1117/1.2161192. [DOI] [PubMed] [Google Scholar]
- 6.Jones RS, Fried D. Remineralization of enamel caries can decrease optical reflectivity. J. Dent. Res. 2006;vol. 85(no. 9):804–808. doi: 10.1177/154405910608500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong SL, Darling CL, Fried D. Nondestructive measurement of the inhibition of demineralization on smooth surfaces using polarization-sensitive optical coherence tomography. Lasers Surg. Med. 2007;vol. 39(no. 5):422–427. doi: 10.1002/lsm.20506. [DOI] [PubMed] [Google Scholar]
- 8.Colston B, Everett M, Da Silva L, Otis L, Stroeve P, Nathel H. Imaging of hard and soft tissue structure in the oral cavity by optical coherence tomography. Appl. Opt. 1998;vol. 37(no. 19):3582–3585. doi: 10.1364/ao.37.003582. [DOI] [PubMed] [Google Scholar]
- 9.Colston BW, Everett MJ, Da Silva LB, Otis LL. Proc. Coherence Domain Opt. Methods Biomed. Sci. Clin. Appl. II. vol. 3251. San Jose, CA: 1998. Optical coherence tomography for diagnosis of periodontal diseases; pp. 52–58. [Google Scholar]
- 10.Colston BW, Sathyam US, DaSilva LB, Everett MJ, Stroeve P. Dental OCT. Opt. Exp. 1998;vol. 3(no. 3):230–238. doi: 10.1364/oe.3.000230. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner A, Hitzenberger CK, Dicht S, Sattmann H, Moritz A, Sperr W, Fercher AF. Proc. Lasers Dent. IV. vol. 3248. San Jose, CA: 1998. Optical coherence tomography for dental structures; pp. 130–136. [DOI] [PubMed] [Google Scholar]
- 12.Dicht S, Baumgartner A, Hitzenberger CK, Sattmann H, Robi B, Moritz A, Sperr W, Fercher AF. Proc. Lasers Dent. V. vol. 3593. San Jose, CA: 1999. Polarization-sensitive optical coherence tomography of dental structures; pp. 169–176. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner A, Dicht S, Hitzenberger CK, Sattmann H, Robi B, Moritz A, Sperr W, Fercher AF. Polarization-sensitive optical coherence tomography of dental structures. Caries Res. 2000;vol. 34:59–69. doi: 10.1159/000016571. [DOI] [PubMed] [Google Scholar]
- 14.Feldchtein FI, Gelikonov GV, Gelikonov VM, Iksanov RR, Kuranov RV, Sergeev AM, Gladkova ND, Ourutina MN, Warren JA, Reitze DH. In vivo OCT imaging of hard and soft tissue of the oral cavity. Opt. Exp. 1998;vol. 3(no. 3):239–251. doi: 10.1364/oe.3.000239. [DOI] [PubMed] [Google Scholar]
- 15.Wang XJ, Zhang JY, Milner TE, d. Boer JF, Zhang Y, Pashley DH, Nelson JS. Characterization of dentin and enamel by use of optical coherence tomography. Appl. Opt. 1999;vol. 38(no. 10):586–590. doi: 10.1364/ao.38.002092. [DOI] [PubMed] [Google Scholar]
- 16.Everett MJ, Colston BW, Sathyam US, Silva LBD, Fried D, Featherstone JDB. Proc. Lasers Dent. V. vol. 3593. San Jose, CA: 1999. Non-invasive diagnosis of early caries with polarization sensitive optical coherence tomography (PS-OCT) pp. 177–183. [Google Scholar]
- 17.Hirasuna K, Fried D, Darling CL. Near-IR imaging of developmental defects in dental enamel. J. Biomed. Opt. 2008;vol. 13(no. 4):044011-1–044011-7. doi: 10.1117/1.2956374. [DOI] [PubMed] [Google Scholar]
- 18.Amaechi BT, Podoleanu AG, Komarov G, Higham SM, Jackson DA. Quantification of root caries using optical coherence tomography and microradiography: A correlational study. Oral Health Prev. Dent. 2004;vol. 2(no. 4):377–382. [PubMed] [Google Scholar]
- 19.Hee MR, Huang D, Swanson EA, Fujimoto JG. Polarization-sensitive low-coherence reflectometer for birefringence characterization and imaging. J. Opt. Soc. Amer. B. 1992;vol. 9:903–908. [Google Scholar]
- 20.de Boer JF, Milner TE, Nelson JS. Stokes parameters imaging of light reflected from biological tissue using polarization sensitive optical cohernence tomography. Proc. Coherence Domain Opt. Methods Biomed. Sci. Clin. Appl. III. 1999;vol. 3598:140–144. [Google Scholar]
- 21.Wang LV, Wu H. Biomedical Optics: Principles and Imaging. Hoboken, NJ: Wiley-Interscience; 2007. [Google Scholar]
- 22.Darling CL, Featherstone JDB, Le CQ, Fried D. Proc. Lasers Dent. VX. vol. 7162. San Jose, CA: 2009. An automated digital microradiography system for assessing tooth demineralization; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Josselin de Jong E, ten Bosch JJ, Noordmans J. Optimized microcomputer-guided quantitative microradiography on dental mineralized tissue slices. Phys. Med. Biol. 1987;vol. 32(no. 7):887–899. doi: 10.1088/0031-9155/32/7/008. [DOI] [PubMed] [Google Scholar]
- 24.de Josselin de Jong E, van der Linden AHIM, Borsboom PCF, ten Bosch JJ. Determination of mineral changes in human dental enamel by longitudinal microradiography and scanning optical mintoring and their correlation with chemical analysis. Caries Res. 1988;vol. 22:153–159. doi: 10.1159/000261098. [DOI] [PubMed] [Google Scholar]
- 25.de Josselin de Jong E, van der Linden AHIM, ten Bosch JJ. Longitudinal microradiography: A non-destructive automated quantitative method to follow mineral changes in mineralized tissue slices. Phys. Med. Biol. 1987;vol. 32(no. 10):1209–1220. doi: 10.1088/0031-9155/32/10/001. [DOI] [PubMed] [Google Scholar]
- 26.Hafstroem-Bjoerkman U, de Josselin de Jong E, Oliveby A, Angmar-Mansson B. Comparison of laser fluorescence and longitudinal microradiography for quantitative assessment of in vitro enamel caries. Caries. Res. 1992;vol. 26:241–247. doi: 10.1159/000261446. [DOI] [PubMed] [Google Scholar]
- 27.Ngaotheppitak P, Darling CL, Fried D. Polarization optical coherence tomography for the measuring the severity of caries lesions. Lasers Surg. Med. 2005;vol. 37(no. 1):78–88. doi: 10.1002/lsm.20169. [DOI] [PubMed] [Google Scholar]
- 28.Amaechi BT, Podoleanu A, Higham SM, Jackson DA. Correlation of quantitative light-induced fluorescence and optical coherence tomography applied for detection and quantification of early dental caries. J. Biomed. Opt. 2003;vol. 8(no. 4):642–647. doi: 10.1117/1.1606685. [DOI] [PubMed] [Google Scholar]
- 29.Amaechi BT, Podoleanu A, Komarov G, Higham SM, Jackson DA. Proc. Lasers Dent. VI. vol. 4610. San Jose, CA: 2002. Optical coherence tomography for dental caries detection and analysis; pp. 1–8. [Google Scholar]
- 30.Fried D, Featherstone JD, Le CQ, Fan K. Dissolution studies of bovine dental enamel surfaces modified by high-speed scanning ablation with a λ = 9.3 µm TEA CO2 laser. Lasers Surg. Med. 2006;vol. 38(no. 9):837–845. doi: 10.1002/lsm.20385. [DOI] [PubMed] [Google Scholar]
- 31.Hsu DJ, Lachica M, Darling CL, Fried D. Nondestructive assessment of the inhibition of enamel demineralization by CO2 laser treatment using polarization sensitive optical coherence tomography. J. Biomed. Opt. 2008;vol. 13(no. 5):1–9. doi: 10.1117/1.2976113. [DOI] [PubMed] [Google Scholar]
- 32.Le MH, Darling CL, Fried D. Lasers Dent. VX. vol. 7162. San Jose, CA: 2009. Methods for calculating the severity of demineralization on tooth surfaces; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Can AM, Darling CL, Ho CM, Fried D. Non-destructive assessment of inhibition of demineralization in dental enamel irradiated by a λ = 9.3-µm CO2 laser at ablative irradiation intensities with PS-OCT. Lasers Surg. Med. 2008;vol. 40:342–349. doi: 10.1002/lsm.20633. [DOI] [PubMed] [Google Scholar]
- 34.Lee C, Darling CL, Fried D. Polarization sensitive optical coherence tomographic imaging of artificial demineralization on exposed surfaces of tooth roots. Dent. Mat. 2009;vol. 25(no. 6):721–728. doi: 10.1016/j.dental.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manesh SK, Darling CL, Fried D. Nondestructive assessment of dentin demineralization using polarization sensitive optical coherence tomography. J. Biomed. Mater. Res. B. 2009;vol. 90(no. 2):802–812. doi: 10.1002/jbm.b.31349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manesh SK, Darling CL, Fried D. Polarization sensitive optical coherence tomography for the nondestructive assessment of the remineralization of dentin. J. Biomed. Opt. 2009;vol. 14(no. 4):1–6. doi: 10.1117/1.3158995. [DOI] [PubMed] [Google Scholar]
- 37.Can AM, Darling CL, Fried D. Proc. Lasers Dent. XIV. vol. 6843. San Jose, CA: 2008. High-resolution PS-OCT of enamel remineralization; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee C, Hsu DJ, Le MH, Darling CL, Fried D. Proc. Lasers Dent. XV. vol. 71620. San Jose, CA: 2009. Non-destructive measurement of demineralization & remineralization in the occlusal pits and fissures of extracted 3rd molars with PS-OCT; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ten Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977;vol. 11(no. 5):277–286. doi: 10.1159/000260279. [DOI] [PubMed] [Google Scholar]
- 40.Featherstone JDB, Barrett NA, Conners MG, Shariati M. A pH cycling model for assessing fluoride effects on root caries. J. Dent. Res. 1989;vol. 68(no.):995. (Abstract) [Google Scholar]
- 41.Bush J, Davis P, Marcus MA. All-fiber optic coherence domain interferometric techniques. Fiber Opt. Sens. Technol. II. 2000;vol. 4204:71–80. [Google Scholar]
- 42.Madjarova VD, Yasuno Y, Makita S, Hori Y, Voeffray JB, Itoh M, Yatagai T, Tamura M, Nanbu T. Investigations of soft and hard tissues in oral cavity by spectral domain optical coherence tomography. Proc. Coherence Domain Opt. Methods Opt. Coherence Tomogr. Biomed. X. 2006;vol. 6079(no. 1):1–7. [Google Scholar]
- 43.Seon YR, Jihoon N, Hae YC, Woo JC, Byeong HL, Gil-Ho Y. Realization of fiber-based OCT system with broadband photonic crystal fiber coupler. Proc. Coherence Domain Opt. Methods Opt. Coherence Tomogr. Biomed. X. 2006;vol. 6079(no. 1):1–7. [Google Scholar]
- 44.Yamanari M, Makita S, Violeta DM, Yatagai T, Yasuno Y. Fiber-based polarization-sensitive Fourier domain optical coherence tomography using B-scan-oriented polarization modulation method. Opt. Exp. 2006;vol. 14(no. 14):6502–6515. doi: 10.1364/oe.14.006502. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa H, Hiro-Oka H, Amano T, DongHak C, Miyazawa T, Yoshimura R, Shimizu K, Ohbayashi K. Proc. Coherence Domain Opt. Methods Opt. Coherence Tomogr. Biomed. X. vol. 6079. San Jose, CA: 2006. Reconstruction of three-dimensional structure of an extracted tooth by OFDR-OCT; pp. 1–7. [Google Scholar]
- 46.Tao YC, Fan K, Fried D. Proc. Lasers Dent. XIII. vol. 6425. San Jose, CA: 2007. Near-infrared image-guided laser ablation of artificial caries lesions; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao YC, Fried D. Proc. Lasers Dent. XIV. vol. 6843. San Jose, CA: 2008. Selective removal of natural occlusal caries by coupling near-infrared imaging with a CO2 laser; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]